Abstract
Free full text

Regional Blood Volume and Peripheral Blood Flow in the Postural Tachycardia Syndrome
Abstract
Variants of postural tachycardia syndrome (POTS) are associated with increased (“high flow” POTS, HFP), decreased (“low flow POTS”, LFP) and normal (“normal flow POTS”, NFP) blood flow measured in the lower extremities while supine. We propose that postural tachycardia is related to thoracic hypovolemia during orthostasis but that the patterns of peripheral blood flow relate to different mechanisms for thoracic hypovolemia. We studied 37 POTS patients aged 14-21 years: 14 LFP, 15 NFP and 8 HFP patients and 12 healthy control subjects. Peripheral blood flow was measured supine by venous occlusion strain gauge plethysmography of the forearm and calf in order to subgroup patients. Using indocyanine green techniques we showed decreased cardiac index (CI) and increased total peripheral resistance (TPR) in LFP, increased CI and decreased TPR in HFP, and unchanged CI and TPR in NFP while supine compared to control subjects. Blood volume tended to be decreased in LFP compared to control subjects. We used impedance plethysmography to assess regional blood volume redistribution during upright tilt. Thoracic blood volume decreased while splanchnic, pelvic and leg blood volumes increased for all subjects during orthostasis, but were markedly lower than control for all POTS groups. Splanchnic volume was increased in NFP and LFP. Pelvic blood volume was increased in HFP only. Calf volume was increased above control in HFP and LFP. The results support the hypothesis of [at least] three pathophysiologic variants of POTS distinguished by peripheral blood flow related to characteristic changes in regional circulations. The data demonstrate enhanced thoracic hypovolemia during upright tilt and confirm that POTS is related to inadequate cardiac venous return during orthostasis.
Introduction
The pathophysiology of chronic orthostatic intolerance comprises abnormalities in compensatory mechanisms that excessively restrict venous return to the heart during upright stance. As a result, reflex tachycardia is invariably present during upright posture and chronic orthostatic intolerance is often denoted postural tachycardia syndrome (POTS). POTS is usually defined by symptoms of orthostatic intolerance (e.g. lightheadedness, headache) associated with an excessive increase in heart rate early during orthostatic stress; typically in adults this is defined by an increase of >30 beats per minute. The presence of POTS does not indicate a specific cause for such orthostatic intolerance but rather signifies a common final pathway producing thoracic hypovolemia.
We therefore expect and observe pathophysiologic heterogeneity in POTS. Our initial studies of POTS focused on peripheral venous pressure (Pv) and found that there were [at least] two separate populations of patients present, which at that time we distinguished on the basis of supine leg venous pressure: a group of POTS patients with high Pv (exceeding control limits of 20mmHg) and a group with normal Pv. However, later when we examined supine peripheral blood flow in POTS patients prospectively grouped by calf Pv, as shown in figure 1 (a synthesis of data from previous papers), we found that the high Pv data were unimodal with decreased calf peripheral blood flow and increased peripheral resistance, but the normal Pv data were bimodal with increased supine calf blood flow and decreased supine arterial resistance in some patients, and normal supine calf blood flow and normal supine arterial resistance in others. This suggests that there are [at least] two subgroups within the normal Pv group: a group of patients with increased blood flow and a group of patients with unchanged blood flow compared to control.
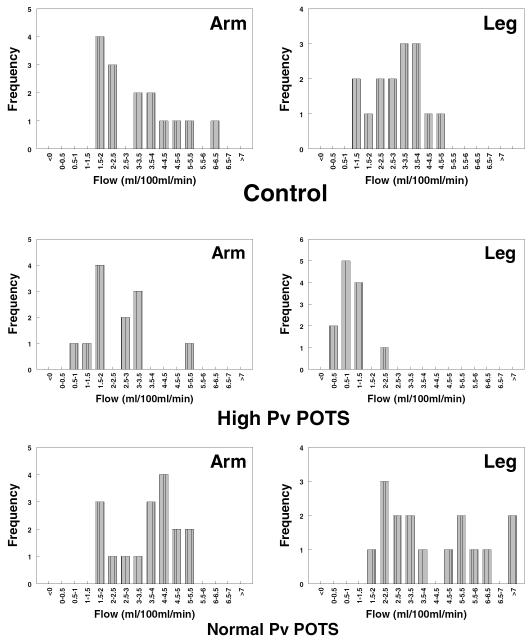
Frequency distribution of peripheral blood flow in patients with POTS with high Pv (middle panel), and normal Pv (lower panel) compared to control subjects (top panel). There was significantly decreased blood flow in POTS patients with high Pv (right middle panel) compared to control by X2 and significantly increased leg blood flow in normal Pv POTS patients (lower panels) compared to control subjects. However, the lower panel is bimodal containing patients with normal blood flow and patients with increased blood flow. There appear to be [at least] 2 populations of POTS patients with normal Pv distinguished by blood flow and peripheral arterial resistance.
We currently think that peripheral blood flow and arterial resistance furnish useful and physiologically important markers to classify POTS. We therefore subscribe to 3 groups of POTS patients distinguished by peripheral blood flow and peripheral arterial resistance.
They are:
A high blood flow, low arterial resistance group with normal to decreased Pv, now denoted “high flow POTS”.
A low blood flow, high arterial resistance, high Pv group now denoted “low flow POTS”.
A normal blood flow, normal arterial resistance group with normal Pv, currently denoted “normal flow POTS”.
We hypothesized that subsets of POTS have distinctive forms of thoracic hypovolemia accounting for postural tachycardia made apparent by orthostatic stress. Based on prior work we proposed that isolated lower extremity flow abnormalities would prevail in high flow POTS, widespread flow abnormalities or hypovolemia would prevail in low flow POTS and splanchnic regional redistribution would prevail in normal flow POTS during orthostatic challenge. The purpose of the current investigation is to study blood volume and the regional redistribution of blood volume in POTS during orthostatic stress.
Materials and Methods
Subjects and Experimental Outline
To test these hypotheses we studied 37 consecutive POTS patients aged 14-21 years (median=17.2 years, 11 male, 26 female) and 12 healthy volunteers aged 15-21 years (median=17.4 years, 5 male, 7 female).
POTS patients were referred to our center for symptoms of orthostatic intolerance lasting for longer than six months. Orthostatic intolerance was defined by the presence of lightheadedness, fatigue, headache, neurocognitive deficits, palpitations, nausea, blurred vision, abnormal sweating, and a sensation of shortness of breath or heat while upright with no other medical explanation for the symptoms. In all patients POTS was confirmed on a screening upright tilt table test at 70°. POTS was diagnosed by symptoms of orthostatic intolerance during the screening tilt test associated with an increase in sinus heart rate of greater than 30 beats per minute or to a rate of greater than 120 beats per minute during the first 10 minutes of tilt as defined in the adult literature (20; 29). We used mercury in silastic strain gauge plethysmography (SGP) to measure supine calf blood flow. Measurements were always made supine at the beginning of experiments and followed a 30-minute resting period. Occlusion cuffs were placed around the lower limb 10 cm above a strain gauge attached to a Whitney-type strain gauge plethysmograph (Hokanson, Inc). Blood flow was estimated while supine by standard venous occlusion methods (11) using rapid cuff inflation to a pressure below diastolic pressure to prevent venous egress. Briefly inflating a smaller secondary cuff to supra-systolic blood pressure prevented wrist or ankle blood flow. Arterial inflow in units of ml/ (100ml tissue)/min was estimated as the rate of change of the rapid increase in limb cross sectional area. We subdivided the POTS patients after the screening tilt test on the basis of calf blood flow. For normative purposes we have collected calf blood flow data from 42 control subjects spanning a number of prior research protocols. For purposes of this study, decreased calf blood flow was defined as less than 1.2ml/min/100mls of tissue, which was the smallest calf blood flow that we have measured in control subjects. Increased calf blood flow was defined as greater than 3.6 ml/min/100mls of tissue, which was the largest calf blood flow we have measured in control subjects. Therefore we defined low flow POTS as those POTS patients with calf blood flow less than 1.2ml/min/100mls of tissue, high flow POTS as those POTS patients with calf blood flow greater than 3.6ml/min/100mls of tissue, and normal flow POTS as those POTS patients with calf blood flow greater than or equal to 1.2ml/min/100mls of tissue and less than or equal to 3.6ml/min/100mls of tissue. Fourteen patients aged 15-22 years (median=17.4 years, all female) had low flow POTS, fifteen patients aged 13-19 years (median=16.5 years, 7 male, 8 female) had normal flow POTS, and eight patients aged 14-20 years (median=15.8 years, 2 male, 6 female) had high flow POTS.
The healthy control subjects comprised friends of POTS patients who were age matched with patients. They were free from all systemic illness and had normal electrocardiograms, echocardiograms and physical examinations. We excluded control subjects with a history of syncope or orthostatic intolerance.
There were no trained competitive athletes or bedridden subjects. No subjects were taking any medications for a minimum of two weeks preceding testing.
Informed consent was obtained and all protocols were approved by the Committee for the Protection of Human Subjects (IRB) of New York Medical College.
Protocol
Tests began in a temperature controlled room (24-26°C) after an overnight fast on a day other than the screening day. Following a 30 minute supine acclimatization period we measured blood volume by indocyanine dye dilution technique. Supine hemodynamic measurements were then made. Subjects were tilted upright tilt to 35° until a steady state was reached (approximately 15 minutes) and hemodynamic measurements were repeated. We and others have demonstrated that upright tilt to 35° produces autonomic changes due to orthostasis yet is tolerated by patients and control subjects (36).
We continuously measured blood pressure by arterial tonometry, heart rate by EKG, and estimated thoracic, splanchnic, pelvic, and calf blood segmental volumes (defined below) by impedance plethysmography throughout the entire experimental course.
We assessed peripheral blood flow, peripheral resistance, venous pressure and peripheral venous capacity while supine and at steady state while upright by venous occlusion strain gauge plethysmography.
Details of the Method
Strain Gauge Measurements - Peripheral Blood Flow, Venous Pressure (Pv), Peripheral Arterial Resistance, Venous Capacitance
We used venous occlusion strain gauge plethysmography to measure forearm and calf blood flow. Supine measurements were made while supine using occlusion cuffs placed around the upper and lower limbs approximately 10 cm above a strain gauge attached to a Whitney-type strain gauge plethysmograph (Hokanson, Inc). Blood flow was estimated while supine and while tilted to 35° using rapid cuff inflation to a pressure below diastolic pressure (e.g. 40 mmHg) to prevent venous egress (11). Briefly inflating a smaller secondary cuff to supra-systolic blood pressure prevented wrist and ankle blood flow. Systolic and diastolic blood pressures of the arm and leg were determined by oscillometry. Arterial inflow in units of ml/(100ml tissue)/min was estimated as the rate of change of the rapid increase in limb cross sectional area. Capacitance vessel pressure (venous pressure, Pv) was also assessed in the steady state: after strain gauge dimension returned to baseline following blood flow measurement, we measured Pv by gradually increasing the occlusion cuff pressure until an increase in limb volume occurred. Pv so measured closely approximates invasive catheter-based measurements in man (2). Peripheral resistance was calculated using the formula [(MAP-Pv)/Resting Flow] where MAP is the mean arterial pressure calculated as (systolic BP + 2*diastolic BP)/3. Peripheral resistance was assessed in the supine and upright position in all subjects using forearm and calf flow, MAP in the arm and the calf, and forearm and calf Pv in both positions.
We measured venous capacitance using our previously documented techniques(32; 34). In brief, while supine, the limb was gently raised above heart level until no further decrease in volume was obtained. After recovery, we used 10 mmHg steps in pressure, starting at the first multiple of 10 larger than Pv, to a maximum of 60 mmHg. This produced progressive limb enlargement. Pressure was maintained for 4 minutes until a steady state was achieved. At lower congestion pressures the limb size reaches a plateau representing venous filling alone. At higher pressures, a plateau is not reached. Instead, there are two components superimposed: a linear component representing microvascular filtration (33) which can be extracted from the total curve by least squares methods (27) and a residual curve that reaches a plateau which represents filling of capacitance vessels. Once the volume response is partitioned, capacitance is calculated from the sum of residual portions to which is added the estimate of supine venous volume obtained from raising the limb (32).
Heart Rate and Blood Pressure Monitoring
The electrocardiogram was monitored continuously. Right upper extremity blood pressure was continuously monitored with an arterial tonometer (Colin Instruments, San Antonio TX) placed on the right radial artery and recalibrated automatically every 5 minutes against oscillometric blood pressure. Leg blood pressure was measured intermittently by oscillometry on the calf contralateral to the strain gauge and used to calculate the calf mean arterial pressure. EKG, and tonometric pressure data were interfaced to a personal computer through an A/D converter (DataQ Ind, Milwaukee, Wi). These data were multiplexed with strain gauge and impedance data and were effectively synchronized.
Dye Dilution Measurement of Blood Volume, Cardiac Output and Total Peripheral Resistance
Indocyanine green dye dilution technique (1) employing a noninvasive spectrophotometric finger photosensor (DDG, Nihon-Kohden Inc) was used to estimate blood volume, cardiac output, and total peripheral resistance. This technique has been verified during clinical studies (12; 15). First pass kinetics were used to obtain cardiac output by Stewart’s classical area under the curve method (31). Cardiac index was obtained by dividing the cardiac output by the patient surface area computed from the formula of Dubois and Dubois (5): BSA(M2) = (weight0.425) • (height0.725) • 0.00718, with weight in kg and height in cm. The dye decay curve is a monoexponential representing clearance by the liver. Once the hematocrit is measured, we extrapolated the dye decay curve to the time of dye injection (t=0) yielding estimated blood volume. Total peripheral resistance was estimated by dividing the mean arterial blood pressure measured while supine in the right arm by the cardiac index.
Impedance Plethysmography (IPG) to Measure Changes in Segmental Blood Volume
Impedance plethysmography (IPG) has been used to detect internal volume shifts (25) including those produced during orthostatic stress (3; 6). We used a Tetrapolar High Resolution Impedance Monitor (THRIM) four-channel digital impedance plethysmograph (UFI, Inc) to measure volume shifts in four anatomic segments designated the thoracic segment, the splanchnic segment, the pelvic segment incorporating lower pelvis to upper leg, and the leg segment (23; 25; 39). Ag/AgCl EKG electrodes were attached to the left foot and left hand, which served as current injectors. Additional electrodes were placed in pairs representing anatomic segments as follows: ankle-upper calf just below the knee (the leg segment), knee-iliac crest (pelvic segment), iliac crest-midline xyphoid process (the splanchnic segment), and midline xyphoid process to supraclavicular area (the thoracic segment). The IPG introduces a high frequency (50 kHz), low amperage (0.1 mA RMS) constant current signal between the foot and hand electrodes. This is completely insensible to the subjects. Electrical resistance values were measured using the segmental pairs as sampling electrodes. Anatomic features were selected as the most appropriate locations for comparing changes within and across patients. This combination of electrodes gives highly repeatable changes in computed volume shifts and has been tested in a wide range of experiments by our group (22-24). The distance between the sampling electrodes (L) was measured carefully with a tape measure. We estimated the change in blood volume in each segment during the upright tilt from the formula:
Where ρ is electrical conductivity of blood estimated as 53.2*exp(Hct*.022) (Hct=hematocrit, or packed cell volume which we measured) given by Geddes and Sadler (10), R0 is the baseline resistance of a specific segment, and ΔR is change in resistance in a specific segment during the maneuver. Volume changes used for intergroup comparisons were calculated from maximum changes in ΔR during the orthostatic maneuver using the average value of R0 starting immediately preceding initiation of tilt and averaging over the entire change in resistance. ρ was regarded as constant during the maneuver. IPG measurements allow us to trace blood volume changes in the various segments during orthostasis.
Low Angle Tilt Table Testing
An electrically driven tilt table (Cardiosystems 600, Dallas, Texas) with a footboard was used. Data for arm and calf blood pressure, blood flow, Pv, and venous capacity were obtained supine. After supine vascular measurements were complete, the subjects underwent tilt to +35°. The angle +35° was chosen because we have observed orthostatic stress comparable to −30mmHg LBNP at which low and high pressure baroreceptors are unloaded (32-34). Subjects had no overt orthostatic intolerance or fainting over a 15 minute time period at these angles although lightheadedness was often reported by POTS patients. During these tilts arm occlusion cuffs were rapidly inflated to 45 mmHg to measure blood flow. Leg occlusion cuffs were inflated to a pressure just below diastolic pressure verified by BP measured on the contralateral calf. Upright tilt increases calf arterial BP due to hydrostatic forces. Forearm and calf flow were measured every 30 seconds for at least three recordings which were averaged. In practice flow and limb size reached a new steady state within approximately 2 minutes, which could be verified by observing the time course of impedance changes. Steady state was defined by no further change in limb flow, and a linear change in calf size at positive angles signifying complete capacitance vessel filling. We repeated measurements of forearm and calf Pv. We reapplied sequential 10mmHg pressure steps to compute the volume-pressure relation. The venous pressure at the strain gauge transducer was assumed to differ from the pressure at the cuff because of the hydrostatic column of blood between the cuff and the gauge. We corrected for the height of this column of blood at given angle of tilt by adding 0.776*D*sin(angle), where D is the distance between the edge of the cuff bladder and the strain gauge and the 0.776 is a conversion factor from cm of blood to mmHg.
Statistics
Tabular data concerning supine and upright blood flow, Pv, impedance blood volume shifts, capacitance, and peripheral resistance were compared by two-way analysis of variance comparing data before and after upright tilt. When significant interactions were demonstrated the ratio of F values was converted to a t distribution using Scheffe’s test and probabilities were thereafter determined. Thereafter unpaired t-tests corrected for multiple small samples were used for between-group comparisons, and paired t-tests corrected for multiple small samples were used for between-group comparisons during tilt. All results are reported as mean ± standard error of the mean. Significance was defined as a p value of <0.05. Unblinded data were collected and analyzed by the same investigator throughout.
Results
Results are depicted in figures 2--44 and in tables 1 and and2.2. All subjects were able to perform the 35° tilt.
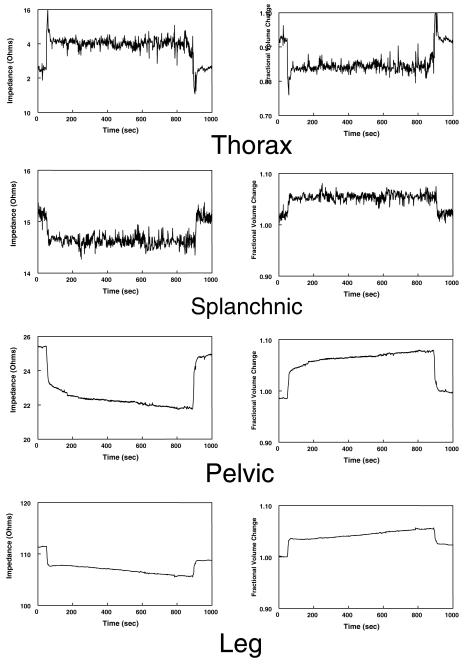
demonstrates representative changes in thoracic, splanchnic, pelvic, and leg impedances in the left hand panels and corresponding calculated fractional changes in volume in the right hand panels for a control subject. Impedance scales are not all the same. Thoracic impedance decreases while all other segmental impedances decrease with tilt up and revert towards control when tilted back down again.

shows changes in thoracic, splanchnic, pelvic, and leg percent volume changes during upright tilt averaged over subject groups. High flow POTS is red, low flow POTS is green, normal flow POTS is blue and control is black. Splanchnic changes dominate normal flow POTS. Low flow POTS patients have widespread blood collection. High flow POTS have blood pooling in the dependent body parts.
Table 1
Patient Dimensions and Supine Only Hemodynamic Data
| Control | POTS | |||
|---|---|---|---|---|
| Control | Low Flow | Normal Flow | High Flow | |
|
| ||||
| Body Surface Area (M2) | 1.75±.10 | 1.71±.05 | 1.76±.07 | 1.76±.07 |
| Weight (kg) | 62±3 | 57±4 | 64±5 | 61±7 |
| Height (cm) | 167±3 | 173±8 | 171±3 | 169±7 |
| Normalized Blood Volume (ml/ kg) | 70±5 | 58±4 | 69±4 | 74±4† |
| Cardiac Index (liter/min/ M2) | 4.3±0.4 | 3.1±0.3* | 3.7±0.6 | 5.1±0.7† |
| Total Peripheral Resistance (mmHg/liter/min/ M2) | 19±1 | 38±7* | 25±5 | 15±2* |
Table 2
Flow, Pressure and Heart Rate Data Before and During Tilt
| Control | POTS | ||||
|---|---|---|---|---|---|
| Control | Low Flow | Normal Flow | High Flow | ||
|
| |||||
| HR (beats/min) | |||||
| Supine | 67±3 | 81±5* | 62±3 | 76±6* | |
| Upright | 80±3 | 102±6*† | 93±4*† | 93±5*† | |
| MAP Right Arm (mmHg) | |||||
| Supine | 79±2 | 82±4 | 80±3 | 80±2 | |
| Upright | 80±2 | 83±5 | 85±4 | 77±8 | |
| MAP Right Leg (mmHg) | |||||
| Supine | 74±2 | 78±5 | 72±2 | 72±4 | |
| Upright | 113±4† | 112±5† | 105±3† | 106±3† | |
| Pv Forearm (mmHg) | |||||
| Supine | 9±1 | 10±1 | 11±1 | 11±1 | |
| Upright | 11±1 | 12±1 | 13±2 | 12±2 | |
| Pv Calf (mmHg) | |||||
| Supine | 12±1 | 19±2* | 13±2 | 11±1 | |
| Upright | 30±3† | 31±2† | 36±3† | 34±3† | |
| Forearm blood flow (ml/100ml/min) | |||||
| Supine | 2.6±0.3 | 2.0±0.3 | 2.7±0.3 | 3.9±0.3* | |
| Upright | 2.1±0.4† | 1.3±0.4*† | 2.3±0.6 | 4.6±0.8* | |
| Calf blood flow (ml/100ml/min) | |||||
| Supine | 2.8±0.4 | 1.0±0.1* | 2.6±0.2 | 4.5±0.3* | |
| Upright | 2.2±0.5† | 2.2±0.5† | 1.9±0.5† | 5.5±1.0* | |
| Forearm Arterial Resistance (ml/100ml/min/mmHg) | |||||
| Supine | 29±4 | 44±8 | 26±4 | 19±4 | |
| Upright | 50±10† | 40±8 | 58±14† | 17±7* | |
| Calf Arterial Resistance (ml/100ml/min/mmHg) | |||||
| Supine | 29±4 | 60±8* | 27±2 | 15±5* | |
| Upright | 46±9† | 39±5† | 60±10† | 20±4* | |
| Forearm Venous Capacity (ml/100ml) | |||||
| Supine | 4.5±0.4 | 4.5±0.3 | 5.2±0.5 | 4.8±0.3 | |
| Upright | 4.6±0.5 | 4.4±0.4 | 5.1±0.6 | 4.6±0.5 | |
| Calf Venous Capacity (ml/100ml) | |||||
| Supine | 5.0±0.4 | 3.3±0.3* | 4.8±0.2 | 4.6±0.4 | |
| Upright | 5.2±0.5 | 3.7±0.4* | 4.9±0.3 | 4.6±0.5 | |
Supine Systemic Hemodynamics (Table 1)
Cardiac index measured supine was decreased in low flow POTS compared to control (p<.01) and increased in the high flow patients compared to the low flow patients (p<.025). Total peripheral resistance was significantly increased above control in low flow POTS (p<.05), was not different from control in normal flow POTS, and was significantly lower than control in high flow POTS (p<.025). Blood volume was decreased in low flow POTS compared to HFP (p<.025) and tended to be decreased compared to control subjects (p=.08). Blood volume (see Table 1) was not different from control for normal flow POTS or for high flow POTS patients. Blood volumes of 58 to 74 ml/kg fall within the normal range with 58ml/kg being at the low end of normal (30).
Peripheral Hemodynamics Supine and Upright (forearm and calf blood flow, peripheral resistance, venous pressure and capacitance)
These are shown in Table 2.
Supine heart rate was increased in both high flow POTS (p<.025) and low flow POTS (p<.001) but was not different from control in normal flow POTS. Heart rate increased in all subjects during tilt and was significantly (P<.025) greater than control in all POTS patients. The highest heart rates tended to be recorded in low flow POTS patients.
Resting arterial blood pressure was not different among the groups. There was no significant change in arm blood pressure with upright tilt. Leg blood pressure increased in all subjects by similar amounts during upright tilt but there was no difference among subject groups. The increase in leg blood pressure was commensurate with the pressure predicted from gravitational considerations.
We measured venous pressure in forearms and calves while supine and upright. Forearm venous pressure was similar for all groups of subjects and was unchanged by tilt to 35°. Supine calf venous pressure was increased in low flow POTS compared to controls (p<.025). The magnitude of measured upright venous pressure was not commensurate with the pressure predicted from gravitational considerations. Thus, the change in venous pressure was significantly (p<.01) less than the change in arterial pressure for all subject groups during tilt.
Forearm blood flow decreased significantly with tilt (p<.05) for control subjects. Supine resting forearm blood flow in low flow patients tended to be decreased compared to control but this did not achieve significance. Upright forearm blood flow was significantly decreased (p<.05) in low flow patients compared to control. Supine forearm blood flow was significantly increased in high flow patients (p<.025) compared to control and remained increased when upright. Supine and upright data in normal flow POTS were similar to control. By design, calf blood flow was significantly decreased in low flow patients (p<.001) when supine but increased significantly (p<.01) when upright to flow rates similar to control subjects. Supine calf blood flow was increased in high flow patients (p<.0001) compared to control subjects and remained increased when upright.
Supine calf arterial resistance was significantly increased in low flow POTS (p<.025) but was not different from control when upright. Supine calf arterial resistance was significantly decreased in high flow POTS (p<.05) compared to control and did not change with tilt. There were no significant differences in calf arterial between control and normal flow POTS although upright resistance tends to be somewhat higher in the normal flow POTS group.
Venous capacitance was decreased only in the low flow POTS patients compared to controls (p<.025).
Impedance, and Segmental Blood Volume Changes during Upright Tilt
Representative Impedance and Volume Changes during Orthostasis
Figure 2 shows the changes in segmental impedance and corresponding calculated changes in segmental blood volumes during upright tilt in a typical representative normal control subject. There is an increase in thoracic impedance corresponding to a decrease in thoracic blood volume during orthostasis. Impedance decreases in splanchnic, pelvic, and leg segments during tilt corresponding to increased blood volume in these segments. Following the initial orthostatic volume shift, there is a gradual and nearly linear increase in pelvic and leg segments volume over time not observed in the splanchnic segment. Thorax and splanchnic impedance changes have superimposed respiratory signals accounting for their rougher configuration.
Segmental Volume Changes in POTS and Control
Figure 3 shows the calculated changes in segmental blood volumes during upright tilt in representative control, low flow POTS, normal flow POTS and high flow POTS subjects. There are larger decreases in fractional thoracic volume in all POTS subjects compared to the control subject and thus relative thoracic hypovolemia in POTS patients. There is marked enhancement in splanchnic volume during tilt in the normal flow POTS patient who has pelvic and leg volume changes that are otherwise similar to control. This contrasts with both low flow POTS and high flow POTS patients in whom segmental volume changes are largest in the leg segments, i.e. in the most dependent parts of the body. The high flow POTS patient shown also had marked increases in pelvic volume.

depicts representative changes in thoracic, splanchnic, pelvic, and leg fractional volume changes during upright tilt in high flow POTS (in red), low flow POTS (in green), normal flow POTS (in blue) and control subjects (in black). Thoracic impedance decreases to a greater extent in POTS patients than control subjects. Splanchnic blood accumulates to the largest extent in normal flow POTS, leg and pelvic blood accumulates in the high flow POTS patients and leg blood accumulates the most in low flow patients as shown in this figure.
Data averaged over all subjects are shown in figure 4. Thoracic volume decreased significantly for all subject groups during orthostasis but was significantly decreased in all POTS groups compared to the control group. The control volume decreased −12±3%, low flow POTS volume decreased −25±5%, p<.025, normal flow POTS volume decreased −32±4%, p<.001, and high flow POTS volume decreased −30±5%, p<.004.
Splanchnic segmental blood volume increased significantly for all subjects during orthostasis but was further significantly increased above control in normal flow (p<.005) and low flow subjects (p<.01). Control volume increased 16±2%, low flow POTS volume increased 29±3%, normal flow POTS volume increased 36±5%, and high flow POTS volume increased 17±8%.
Pelvic segmental blood volume increased significantly for all subjects during tilt. Pelvic volume was further significantly increased above control in high flow POTS only (p<.05) although there was a trend towards such an increase in low flow subjects (p=.09). Control volume increased 15±2%, low flow POTS volume increased 20±3%, normal flow POTS volume increased 15±5%, high flow POTS volume increased 21±1%.
Calf segmental blood volume increased significantly for all subjects during tilt. Calf volume was further significantly increased above control in high flow POTS (p<.025) and low flow POTS (p<.05). Control volume increased 12±2%, low flow POTS volume increased 18±2%, normal flow POTS volume increased 12±2%, high flow POTS volume increased 22±4%.
Discussion
General Discussion
The results support the hypothesis of [at least] three physiologic variants of POTS distinguished on the basis of peripheral blood flow and peripheral arterial resistance. The results suggest that the peripheral effects are associated with characteristic changes in regional blood volumes. The data emphasize the central importance of exaggerated thoracic hypovolemia during upright tilt in all POTS variants compared to control subjects and confirm that POTS is related to inadequate cardiac venous return during orthostasis. POTS physiology is the physiology of thoracic hypovolemia, which may explain tachycardia in terms of a reflex response.
Low Flow POTS
There appears to be a general deficit in blood flow regulation in low flow POTS that is most notable in the dependent parts of the body. Prior work suggests that there are defects in local blood flow regulation in these patients (35). Mild absolute hypovolemia is also probably present and further contributes to the orthostatic intolerant response. Decreased peripheral venous capacitance provides evidence for either venous remodeling or persistent peripheral leg venoconstriction, which should tend to allow for cephalad redistribution of blood under resting conditions.
Normal Flow POTS
Normal flow POTS is characterized by normal peripheral resistance supine and upright and specific venous pooling within the splanchnic vascular bed. The specific mechanism or mechanisms for such pooling remain undetermined.
High Flow POTS
High flow POTS is characterized by inadequate peripheral vasoconstriction both supine and upright. This enhances cardiac output as in other high output conditions.
Specific Discussion
Systemic Hemodynamics
Low Flow POTS
Heart rate is increased, supine cardiac index is decreased, blood volume tends to be decreased and total peripheral resistance is increased prior to orthostatic challenge. These data are consistent with hypovolemic circulatory insufficiency. Low flow POTS patients may be similar to hypovolemic orthostatic intolerance patients described by Fouad and by Jacob et al (7; 16; 18).
High Flow POTS
Heart rate is increased, supine cardiac index is increased, and total peripheral resistance is decreased prior to orthostatic challenge. These data are consistent with a high output circulatory state.
Normal Flow POTS
Supine heart rate is normal, cardiac index is normal, blood volume and total peripheral resistance are similar to control prior to orthostatic challenge. These data are consistent with normal global and regional circulations at supine rest.
Peripheral Hemodynamics (within the context of systemic hemodynamics)
Low Flow POTS
Peripheral resistance is markedly increased and blood flow markedly decreased in the lower extremity. Blood flow decreases while peripheral resistance increases in the arm consistent with autonomically mediated vasoconstriction during tilt (The arms are maintained at heart level during tilt). Blood flow increases while peripheral resistance decreases in the leg during tilt. This is inconsistent with simple hypovolemia but consistent with abnormal local flow regulatory mechanisms, which we previously demonstrated in these patients (35). Venous capacitance is decreased. Within the context of hypovolemia this suggests remodeling of the venous vasculature to accommodate reduced blood volume. Reduced venous volume might produce the increase in measured Pv which we regularly find in this group of patients: proportionately more blood would be contained within the arterial circulation (provided no important arterial volumetric changes occur) effectively “arterializing” the average pressure at which blood is stored.
High Flow POTS
Peripheral resistance is decreased and peripheral blood flow is increased compared to control while supine and upright. This is associated with increased resting cardiac output and suggests that the high output state occurs due to inappropriate peripheral vasodilation. This observation is consistent with decreased norepinephrine release (autonomic dysfunction) demonstrated by Jacob et al (17) and consistent with intact local flow regulation (13; 14) in similar POTS patients. The prevailing pathophysiologic theory in high flow patients is that they are “neuropathic”; they fit criteria for a long axon neuropathy with deficient norepinephrine release in the lower extremities and therefore ineffective postural vasoconstriction. They can, however, respond to exogenous alpha-1 adrenergic agonists with effective vasoconstriction (a 40-60% increase in resistance and decrease in flow), with venoconstriction (a 20% decrease in capacity), and with restoration of orthostatic tolerance in response to phenylephrine infusion (36). Recent data (33) demonstrates that increased microvascular filtration drives fluid collection in the lower extremities during orthostasis in high flow POTS.
Normal Flow POTS
There is no difference in supine systemic or peripheral hemodynamics in these patients compared to control subjects. Given postural tachycardia the data predict that regional volume redistribution is paramount in the pathophysiology of this group of POTS patients.
Segmental Blood Volume Changes during Upright Tilt
During upright tilt we observed that blood empties from the thoracic segment and fills all other segments. This answers a non-trivial question concerning splanchnic filling during orthostasis. The splanchnic bed is the only regional circulation with known active venoconstrictive as well as arterial vasoconstrictive capabilities (4; 28). Does it fill or empty during orthostatic challenge? We found that the splanchnic circulation fills during our experimental orthostatic conditions in all subjects including normal control subjects. Normal active and passive vascular changes are unable to prevent sequestration of blood within the splanchnic circulation during orthostasis even in normal subjects. At rest, the splanchnic venous reservoir is large and highly compliant (26). Thus, in control subjects subjected to orthostasis, we believe that the increase in splanchnic pooling is limited but not eliminated by vasoconstriction and venoconstriction.
Low Flow POTS
These data indicate that despite potential global hypovolemia, thoracic blood volume is decreased above control during orthostasis. Decreases persist even if normalized to a proportionately smaller overall blood volume. There appear to be increased blood volumes in all segments in low flow POTS, which, however, reach significance in splanchnic and calf circulations during current experimental conditions. These are unexpected circulatory responses from volume-contracted subjects in whom upright vasoconstriction would be expected to reduce dependent volume shifts. Prior work from our laboratory indicates markedly abnormal local blood flow regulation mechanisms producing paradoxical changes in venoarteriolar and perhaps myogenic responses to limb dependence (35) which may be of wider anatomic importance in low flow patients. A partial dusautonomia could also play a role here. Hypovolemia cannot account for the sum of all findings in low flow POTS.
High Flow POTS
High flow patients collect blood in the lower extremities. This is predicted by prior work (37) and on the work of others (17). Prior data indicate that there is enhanced microvascular filtration in these patients (33). Thus a fraction of the volumetric change is filtrate rather than blood, which would actually tend to underestimate the amount of limb sequestered fluid (blood+filtrate) in the dependent extremities.
Normal Flow POTS
Impedance data are most interesting for this group of POTS patients in whom only splanchnic pooling occurs. The segmental data suggest localized splanchnic vascular abnormality as opposed to the global vascular abnormalities of low flow POTS and the lower body abnormalities of high flow POTS. Increased splanchnic pooling may occur as the result of failure of venoconstriction and arterial vasoconstriction with or without an increase in venous resistance (21), or as the result of increased splanchnic vascular compliance. Current data cannot distinguish between these possibilities. However, prior work indicates that splanchnic arterial inflow is abnormal in POTS (38). Potential candidates for splanchnic dysfunction include partial (regional) dysautonomia or alternatively local regulatory dysfunction.
Limitations
Shortcomings of IPG
Segmental changes are reported as fractional volume changes. They are not quantitative accurate measures of absolute volume. They are, however, relatively good qualitative and directional measures of segmental volume changes, which, in our hands, give repeatable results. Nevertheless, drawing rigorous quantitative conclusions is probably not warranted.
Regional Circulations
We did not study all regional circulations. Past data has not indicated an important role for upper extremity circulation in POTS while cerebral blood flow is decreased in upright POTS patients (19). However, we did investigate the regional circulations most likely to account for large blood volume shifts in POTS.
Orthostatic Blood Flow
We do not report thoracic, splanchnic, or pelvic measures of vasoconstriction during orthostasis. While segmental blood flow measurements using impedance methods have been reported, they have not been completely validated against standard methods and therefore fall beyond the scope of the present work. Other techniques such as Doppler ultrasound may help in this regard. Clearly measurements of selective regional changes in blood flow and peripheral resistance are essential to determining mechanism.
Autonomic Changes
A direct measure of sympathetic activity such as muscle sympathetic nerve activity (MSNA) could have enhanced our ability to attribute flow and arterial resistance findings to autonomic dysfunction in high flow POTS patients. However, such instrumentation is often regarded as problematic in subjects of the age range used in our studies and was therefore not pursued. However, measurements of catecholamine spillover and MSNA (but not peripheral blood flow) have been intensively investigated by the Vanderbilt group in similar patients (8; 17).
Tilt angle
We chose to examine patients in the supine position and when tilted to 35°. Prior work illustrate that 35° upright tilt evokes the orthostatic response although to a lesser extent than standing. However, our patients are often unable to sustain standing developing rapidly unstable findings. Therefore a tolerable orthostatic stressor was chosen.
Patient Age
Age limitations to generalizability may exist. Young adults and adolescents may not perfectly represent findings for mature adults. However, cardiovascular structure and function is essentially mature by puberty and therefore results can be regarded as at least qualitatively similar to older age groups. Moreover, younger patients have the advantage of absence of confounding illness such as heart disease, renal disease, hypertension, and diabetes.
In summary, our data to date suggest that POTS depends on thoracic hypovolemia, which can occur by diverse mechanisms. Regional sequestration of blood occurs in normal flow and high flow variants while a degree of absolute hypovolemia and abnormal local blood flow regulation occurs in the low flow variant. While molecular mechanisms remain forthcoming, we hope the data presented may help focus the effort.
Acknowledgements
Supported by 1RO1HL66007 and 1R01HL074873 from the National Heart Lung and Blood Institute of the National Institutes of Health.
Reference List
Full text links
Read article at publisher's site: https://doi.org/10.1152/ajpheart.00086.2004
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc4515760?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/158306228
Article citations
Persistent Autonomic and Immunologic Abnormalities in Neurologic Post-Acute Sequelae of SARS-CoV2 Infection.
Neurology, 103(6):e209742, 22 Aug 2024
Cited by: 0 articles | PMID: 39173103 | PMCID: PMC11343584
Postural Orthostatic Tachycardia Syndrome Associated with COVID-19: A Narrative Review.
Medicina (Kaunas), 60(8):1325, 15 Aug 2024
Cited by: 0 articles | PMID: 39202605 | PMCID: PMC11356245
Review Free full text in Europe PMC
Deep abdominal breathing reduces heart rate and symptoms during orthostatic challenge in patients with postural orthostatic tachycardia syndrome.
Eur J Neurol, 31(10):e16402, 04 Jul 2024
Cited by: 1 article | PMID: 38962840 | PMCID: PMC11414806
Association of adolescent postural tachycardia syndrome classifications with anxiety: a cross sectional study.
Biopsychosoc Med, 18(1):2, 29 Jan 2024
Cited by: 0 articles | PMID: 38287400 | PMCID: PMC10823659
Post-COVID dysautonomias: what we know and (mainly) what we don't know.
Nat Rev Neurol, 20(2):99-113, 11 Jan 2024
Cited by: 5 articles | PMID: 38212633
Review
Go to all (64) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Persistent splanchnic hyperemia during upright tilt in postural tachycardia syndrome.
Am J Physiol Heart Circ Physiol, 290(2):H665-73, 02 Sep 2005
Cited by: 63 articles | PMID: 16143646 | PMCID: PMC4513355
Postural hypocapnic hyperventilation is associated with enhanced peripheral vasoconstriction in postural tachycardia syndrome with normal supine blood flow.
Am J Physiol Heart Circ Physiol, 291(2):H904-13, 24 Mar 2006
Cited by: 42 articles | PMID: 16565300 | PMCID: PMC4511478
Contrasting neurovascular findings in chronic orthostatic intolerance and neurocardiogenic syncope.
Clin Sci (Lond), 104(4):329-340, 01 Apr 2003
Cited by: 22 articles | PMID: 12653674
Microvascular filtration is increased in postural tachycardia syndrome.
Circulation, 107(22):2816-2822, 19 May 2003
Cited by: 30 articles | PMID: 12756156
Funding
Funders who supported this work.
NHLBI NIH HHS (4)
Grant ID: 1R01HL-074873
Grant ID: 1R01HL-66007
Grant ID: R01 HL074873
Grant ID: R01 HL066007




