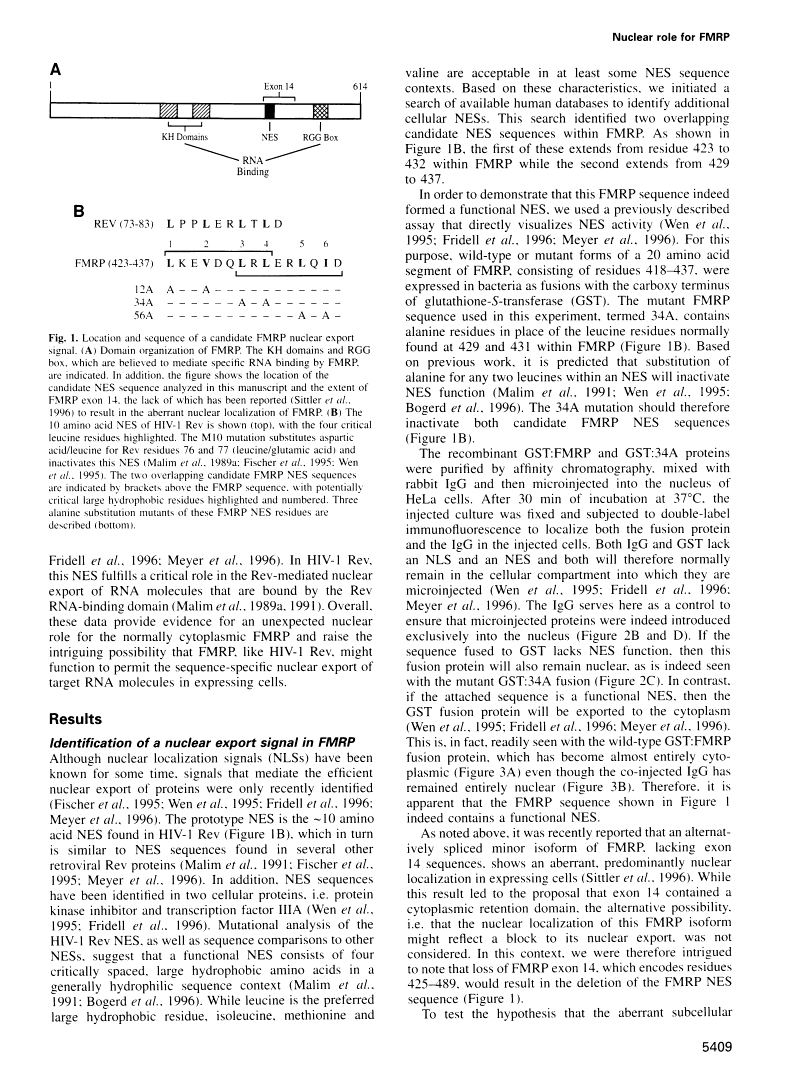
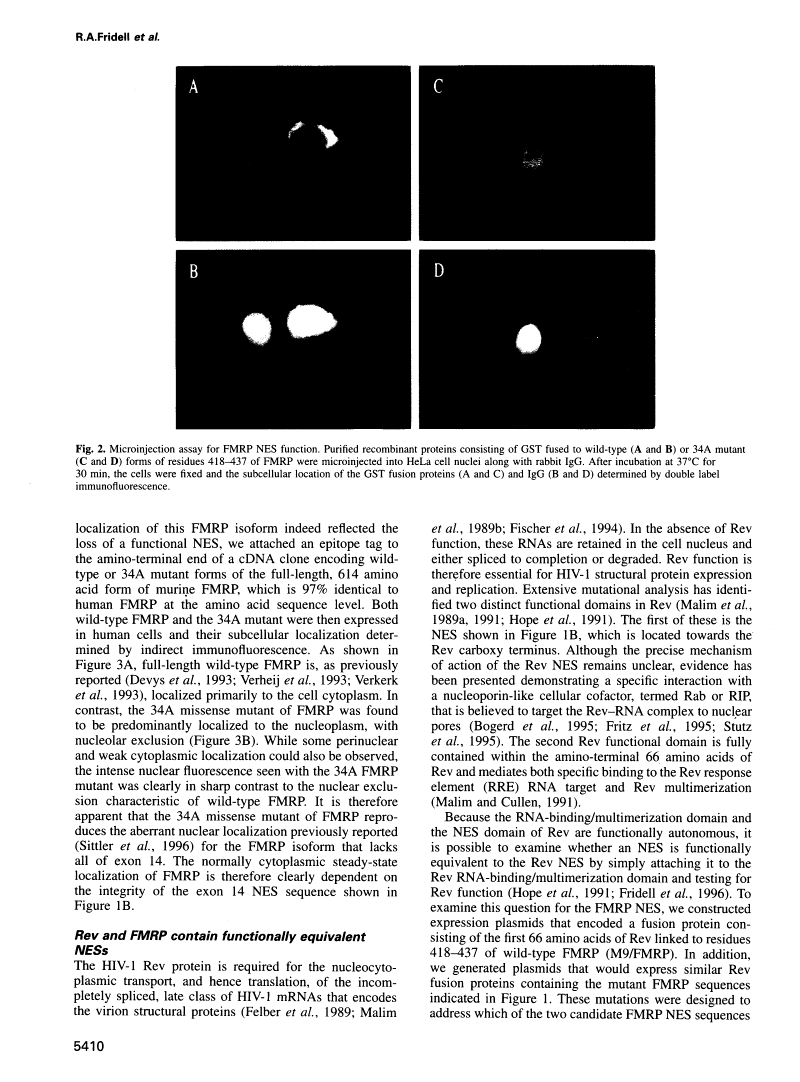
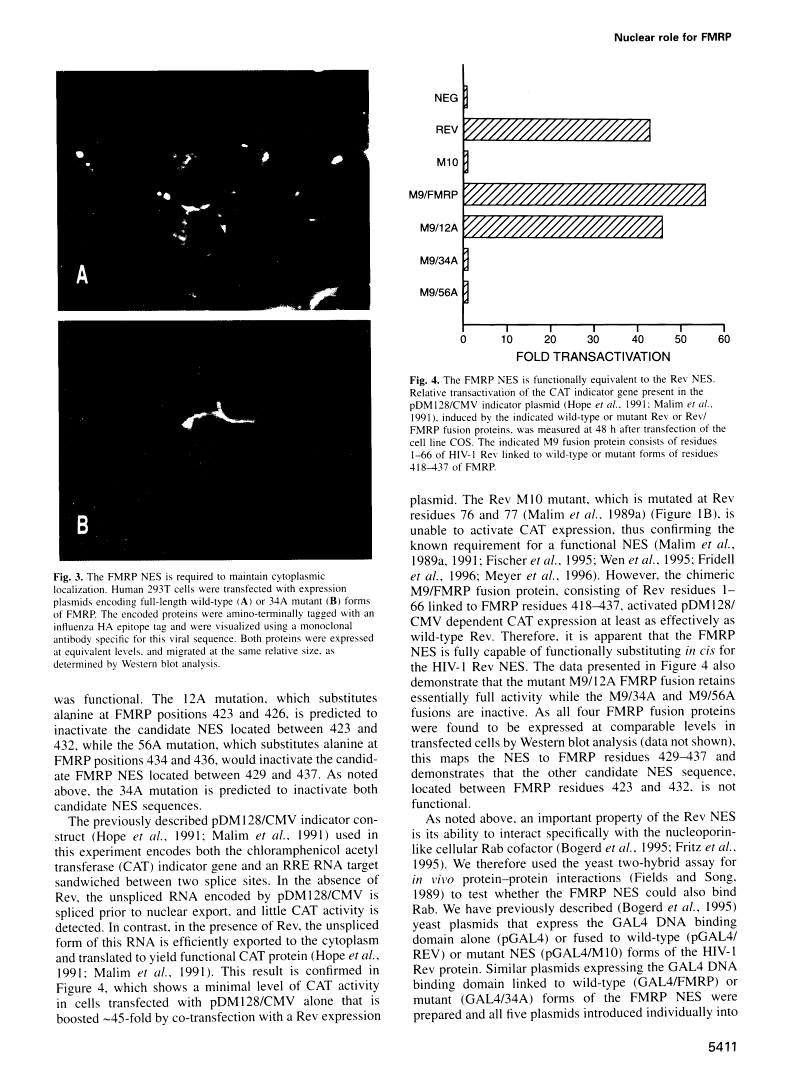
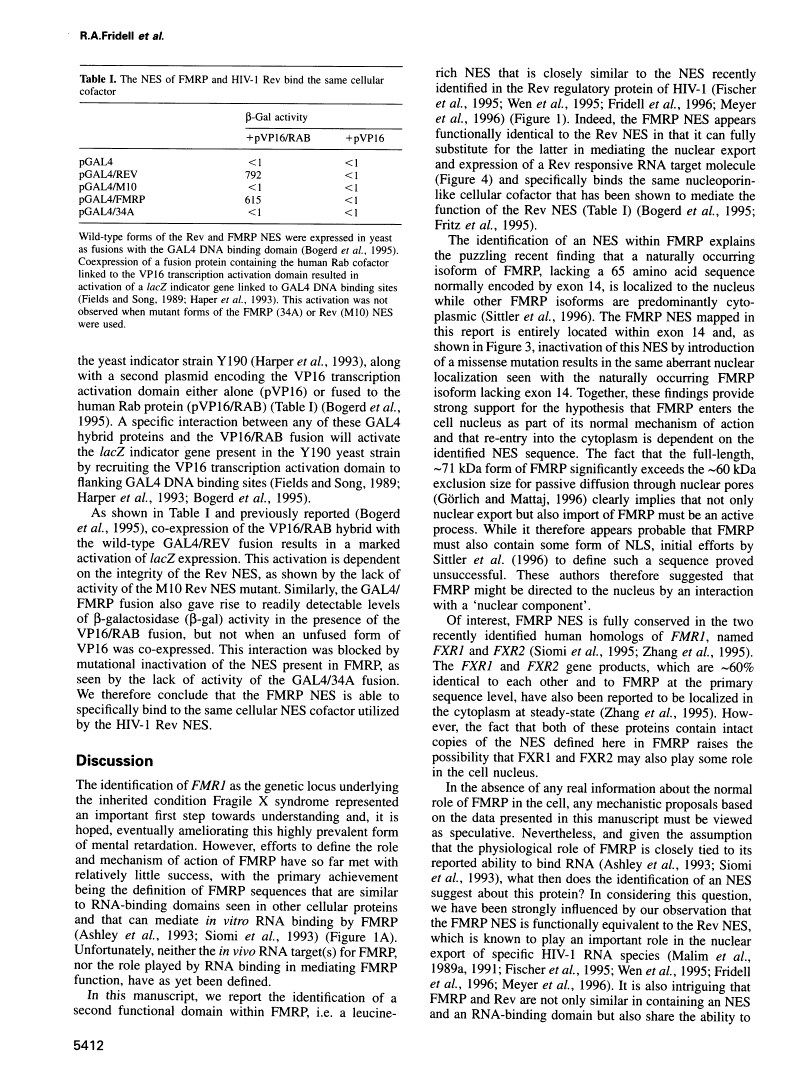
Abstract
Free full text

A nuclear role for the Fragile X mental retardation protein.
Abstract
Fragile X syndrome results from lack of expression of a functional form of Fragile X mental retardation protein (FMRP), a cytoplasmic RNA-binding protein of uncertain function. Here, we report that FMRP contains a nuclear export signal (NES) that is similar to the NES recently identified in the Rev regulatory protein of human immunodeficiency virus type 1 (HIV-1). Mutation of this FMRP NES results in mis-localization of FMRP to the cell nucleus. The FMRP NES is encoded within exon 14 of the FMR1 gene, thus explaining the aberrant nuclear localization of a natural isoform of FMRP that lacks this exon. The NES of FMRP can substitute fully for the Rev NES in mediating Rev-dependent nuclear RNA export and specifically binds a nucleoporin-like cellular cofactor that has been shown to mediate Rev NES function. Together, these findings demonstrate that the normal function of FMRP involves entry into the nucleus followed by export via a pathway that is identical to the one utilized by HIV-1 Rev. In addition, these data raise the possibility that FMRP could play a role in mediating the nuclear export of its currently undefined cellular RNA target(s).
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.5M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashley CT, Sutcliffe JS, Kunst CB, Leiner HA, Eichler EE, Nelson DL, Warren ST. Human and murine FMR-1: alternative splicing and translational initiation downstream of the CGG-repeat. Nat Genet. 1993 Jul;4(3):244–251. [Abstract] [Google Scholar]
- Ashley CT, Jr, Wilkinson KD, Reines D, Warren ST. FMR1 protein: conserved RNP family domains and selective RNA binding. Science. 1993 Oct 22;262(5133):563–566. [Abstract] [Google Scholar]
- Bogerd HP, Fridell RA, Madore S, Cullen BR. Identification of a novel cellular cofactor for the Rev/Rex class of retroviral regulatory proteins. Cell. 1995 Aug 11;82(3):485–494. [Abstract] [Google Scholar]
- Bogerd HP, Fridell RA, Benson RE, Hua J, Cullen BR. Protein sequence requirements for function of the human T-cell leukemia virus type 1 Rex nuclear export signal delineated by a novel in vivo randomization-selection assay. Mol Cell Biol. 1996 Aug;16(8):4207–4214. [Europe PMC free article] [Abstract] [Google Scholar]
- De Boulle K, Verkerk AJ, Reyniers E, Vits L, Hendrickx J, Van Roy B, Van den Bos F, de Graaff E, Oostra BA, Willems PJ. A point mutation in the FMR-1 gene associated with fragile X mental retardation. Nat Genet. 1993 Jan;3(1):31–35. [Abstract] [Google Scholar]
- Devys D, Lutz Y, Rouyer N, Bellocq JP, Mandel JL. The FMR-1 protein is cytoplasmic, most abundant in neurons and appears normal in carriers of a fragile X premutation. Nat Genet. 1993 Aug;4(4):335–340. [Abstract] [Google Scholar]
- Felber BK, Hadzopoulou-Cladaras M, Cladaras C, Copeland T, Pavlakis GN. rev protein of human immunodeficiency virus type 1 affects the stability and transport of the viral mRNA. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1495–1499. [Europe PMC free article] [Abstract] [Google Scholar]
- Fields S, Song O. A novel genetic system to detect protein-protein interactions. Nature. 1989 Jul 20;340(6230):245–246. [Abstract] [Google Scholar]
- Fischer U, Meyer S, Teufel M, Heckel C, Lührmann R, Rautmann G. Evidence that HIV-1 Rev directly promotes the nuclear export of unspliced RNA. EMBO J. 1994 Sep 1;13(17):4105–4112. [Europe PMC free article] [Abstract] [Google Scholar]
- Fischer U, Huber J, Boelens WC, Mattaj IW, Lührmann R. The HIV-1 Rev activation domain is a nuclear export signal that accesses an export pathway used by specific cellular RNAs. Cell. 1995 Aug 11;82(3):475–483. [Abstract] [Google Scholar]
- Fridell RA, Fischer U, Lührmann R, Meyer BE, Meinkoth JL, Malim MH, Cullen BR. Amphibian transcription factor IIIA proteins contain a sequence element functionally equivalent to the nuclear export signal of human immunodeficiency virus type 1 Rev. Proc Natl Acad Sci U S A. 1996 Apr 2;93(7):2936–2940. [Europe PMC free article] [Abstract] [Google Scholar]
- Fritz CC, Zapp ML, Green MR. A human nucleoporin-like protein that specifically interacts with HIV Rev. Nature. 1995 Aug 10;376(6540):530–533. [Abstract] [Google Scholar]
- Gedeon AK, Baker E, Robinson H, Partington MW, Gross B, Manca A, Korn B, Poustka A, Yu S, Sutherland GR, et al. Fragile X syndrome without CCG amplification has an FMR1 deletion. Nat Genet. 1992 Aug;1(5):341–344. [Abstract] [Google Scholar]
- Görlich D, Mattaj IW. Nucleocytoplasmic transport. Science. 1996 Mar 15;271(5255):1513–1518. [Abstract] [Google Scholar]
- Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993 Nov 19;75(4):805–816. [Abstract] [Google Scholar]
- Hinds HL, Ashley CT, Sutcliffe JS, Nelson DL, Warren ST, Housman DE, Schalling M. Tissue specific expression of FMR-1 provides evidence for a functional role in fragile X syndrome. Nat Genet. 1993 Jan;3(1):36–43. [Abstract] [Google Scholar]
- Hope TJ, Bond BL, McDonald D, Klein NP, Parslow TG. Effector domains of human immunodeficiency virus type 1 Rev and human T-cell leukemia virus type I Rex are functionally interchangeable and share an essential peptide motif. J Virol. 1991 Nov;65(11):6001–6007. [Europe PMC free article] [Abstract] [Google Scholar]
- Khandjian EW, Corbin F, Woerly S, Rousseau F. The fragile X mental retardation protein is associated with ribosomes. Nat Genet. 1996 Jan;12(1):91–93. [Abstract] [Google Scholar]
- Malim MH, Cullen BR. HIV-1 structural gene expression requires the binding of multiple Rev monomers to the viral RRE: implications for HIV-1 latency. Cell. 1991 Apr 19;65(2):241–248. [Abstract] [Google Scholar]
- Malim MH, Böhnlein S, Hauber J, Cullen BR. Functional dissection of the HIV-1 Rev trans-activator--derivation of a trans-dominant repressor of Rev function. Cell. 1989 Jul 14;58(1):205–214. [Abstract] [Google Scholar]
- Malim MH, Hauber J, Le SY, Maizel JV, Cullen BR. The HIV-1 rev trans-activator acts through a structured target sequence to activate nuclear export of unspliced viral mRNA. Nature. 1989 Mar 16;338(6212):254–257. [Abstract] [Google Scholar]
- Malim MH, McCarn DF, Tiley LS, Cullen BR. Mutational definition of the human immunodeficiency virus type 1 Rev activation domain. J Virol. 1991 Aug;65(8):4248–4254. [Europe PMC free article] [Abstract] [Google Scholar]
- Meyer BE, Malim MH. The HIV-1 Rev trans-activator shuttles between the nucleus and the cytoplasm. Genes Dev. 1994 Jul 1;8(13):1538–1547. [Abstract] [Google Scholar]
- Meyer BE, Meinkoth JL, Malim MH. Nuclear transport of human immunodeficiency virus type 1, visna virus, and equine infectious anemia virus Rev proteins: identification of a family of transferable nuclear export signals. J Virol. 1996 Apr;70(4):2350–2359. [Europe PMC free article] [Abstract] [Google Scholar]
- Musco G, Stier G, Joseph C, Castiglione Morelli MA, Nilges M, Gibson TJ, Pastore A. Three-dimensional structure and stability of the KH domain: molecular insights into the fragile X syndrome. Cell. 1996 Apr 19;85(2):237–245. [Abstract] [Google Scholar]
- Oostra BA, Willems PJ. A fragile gene. Bioessays. 1995 Nov;17(11):941–947. [Abstract] [Google Scholar]
- Siomi H, Siomi MC, Nussbaum RL, Dreyfuss G. The protein product of the fragile X gene, FMR1, has characteristics of an RNA-binding protein. Cell. 1993 Jul 30;74(2):291–298. [Abstract] [Google Scholar]
- Siomi H, Choi M, Siomi MC, Nussbaum RL, Dreyfuss G. Essential role for KH domains in RNA binding: impaired RNA binding by a mutation in the KH domain of FMR1 that causes fragile X syndrome. Cell. 1994 Apr 8;77(1):33–39. [Abstract] [Google Scholar]
- Siomi MC, Siomi H, Sauer WH, Srinivasan S, Nussbaum RL, Dreyfuss G. FXR1, an autosomal homolog of the fragile X mental retardation gene. EMBO J. 1995 Jun 1;14(11):2401–2408. [Europe PMC free article] [Abstract] [Google Scholar]
- Sittler A, Devys D, Weber C, Mandel JL. Alternative splicing of exon 14 determines nuclear or cytoplasmic localisation of fmr1 protein isoforms. Hum Mol Genet. 1996 Jan;5(1):95–102. [Abstract] [Google Scholar]
- Stutz F, Neville M, Rosbash M. Identification of a novel nuclear pore-associated protein as a functional target of the HIV-1 Rev protein in yeast. Cell. 1995 Aug 11;82(3):495–506. [Abstract] [Google Scholar]
- Verheij C, Bakker CE, de Graaff E, Keulemans J, Willemsen R, Verkerk AJ, Galjaard H, Reuser AJ, Hoogeveen AT, Oostra BA. Characterization and localization of the FMR-1 gene product associated with fragile X syndrome. Nature. 1993 Jun 24;363(6431):722–724. [Abstract] [Google Scholar]
- Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, Pizzuti A, Reiner O, Richards S, Victoria MF, Zhang FP, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991 May 31;65(5):905–914. [Abstract] [Google Scholar]
- Verkerk AJ, de Graaff E, De Boulle K, Eichler EE, Konecki DS, Reyniers E, Manca A, Poustka A, Willems PJ, Nelson DL, et al. Alternative splicing in the fragile X gene FMR1. Hum Mol Genet. 1993 Apr;2(4):399–404. [Abstract] [Google Scholar]
- Wen W, Meinkoth JL, Tsien RY, Taylor SS. Identification of a signal for rapid export of proteins from the nucleus. Cell. 1995 Aug 11;82(3):463–473. [Abstract] [Google Scholar]
- Wöhrle D, Kotzot D, Hirst MC, Manca A, Korn B, Schmidt A, Barbi G, Rott HD, Poustka A, Davies KE, et al. A microdeletion of less than 250 kb, including the proximal part of the FMR-I gene and the fragile-X site, in a male with the clinical phenotype of fragile-X syndrome. Am J Hum Genet. 1992 Aug;51(2):299–306. [Europe PMC free article] [Abstract] [Google Scholar]
- Yu S, Pritchard M, Kremer E, Lynch M, Nancarrow J, Baker E, Holman K, Mulley JC, Warren ST, Schlessinger D, et al. Fragile X genotype characterized by an unstable region of DNA. Science. 1991 May 24;252(5009):1179–1181. [Abstract] [Google Scholar]
- Zhang Y, O'Connor JP, Siomi MC, Srinivasan S, Dutra A, Nussbaum RL, Dreyfuss G. The fragile X mental retardation syndrome protein interacts with novel homologs FXR1 and FXR2. EMBO J. 1995 Nov 1;14(21):5358–5366. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from The EMBO Journal are provided here courtesy of Nature Publishing Group
Full text links
Read article at publisher's site: https://doi.org/10.1002/j.1460-2075.1996.tb00924.x
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc452283?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Article citations
A Double Jeopardy: Loss of FMRP Results in DSB and Down-regulated DNA Repair.
21 Century Pathol, 2(5):125, 17 Oct 2022
Cited by: 0 articles | PMID: 36688938 | PMCID: PMC9850805
Combining affinity purification and mass spectrometry to define the network of the nuclear proteins interacting with the N-terminal region of FMRP.
Front Mol Biosci, 9:954087, 27 Sep 2022
Cited by: 3 articles | PMID: 36237573 | PMCID: PMC9553004
The RGG motif proteins: Interactions, functions, and regulations.
Wiley Interdiscip Rev RNA, 14(1):e1748, 03 Jun 2022
Cited by: 16 articles | PMID: 35661420 | PMCID: PMC9718894
Review Free full text in Europe PMC
Fmr1 exon 14 skipping in late embryonic development of the rat forebrain.
BMC Neurosci, 23(1):32, 31 May 2022
Cited by: 0 articles | PMID: 35641906 | PMCID: PMC9158170
Fragile X-related protein family: a double-edged sword in neurodevelopmental disorders and cancer.
Crit Rev Biochem Mol Biol, 55(5):409-424, 02 Sep 2020
Cited by: 25 articles | PMID: 32878499 | PMCID: PMC7695039
Review Free full text in Europe PMC
Go to all (80) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Interactions between HIV Rev and nuclear import and export factors: the Rev nuclear localisation signal mediates specific binding to human importin-beta.
J Mol Biol, 274(5):693-707, 01 Dec 1997
Cited by: 166 articles | PMID: 9405152
Chimeras containing influenza NS1 and HIV-1 Rev protein sequences: mechanism of their inhibition of nuclear export of Rev protein-RNA complexes.
Virology, 241(2):234-250, 01 Feb 1998
Cited by: 3 articles | PMID: 9499798
The fragile X mental retardation protein is a ribonucleoprotein containing both nuclear localization and nuclear export signals.
Hum Mol Genet, 5(8):1083-1091, 01 Aug 1996
Cited by: 239 articles | PMID: 8842725
The HIV-1 Rev protein.
Annu Rev Microbiol, 52:491-532, 01 Jan 1998
Cited by: 449 articles | PMID: 9891806
Review