Abstract
Free full text

A cellular protein mediates association of p53 with the E6 oncoprotein of human papillomavirus types 16 or 18.
Abstract
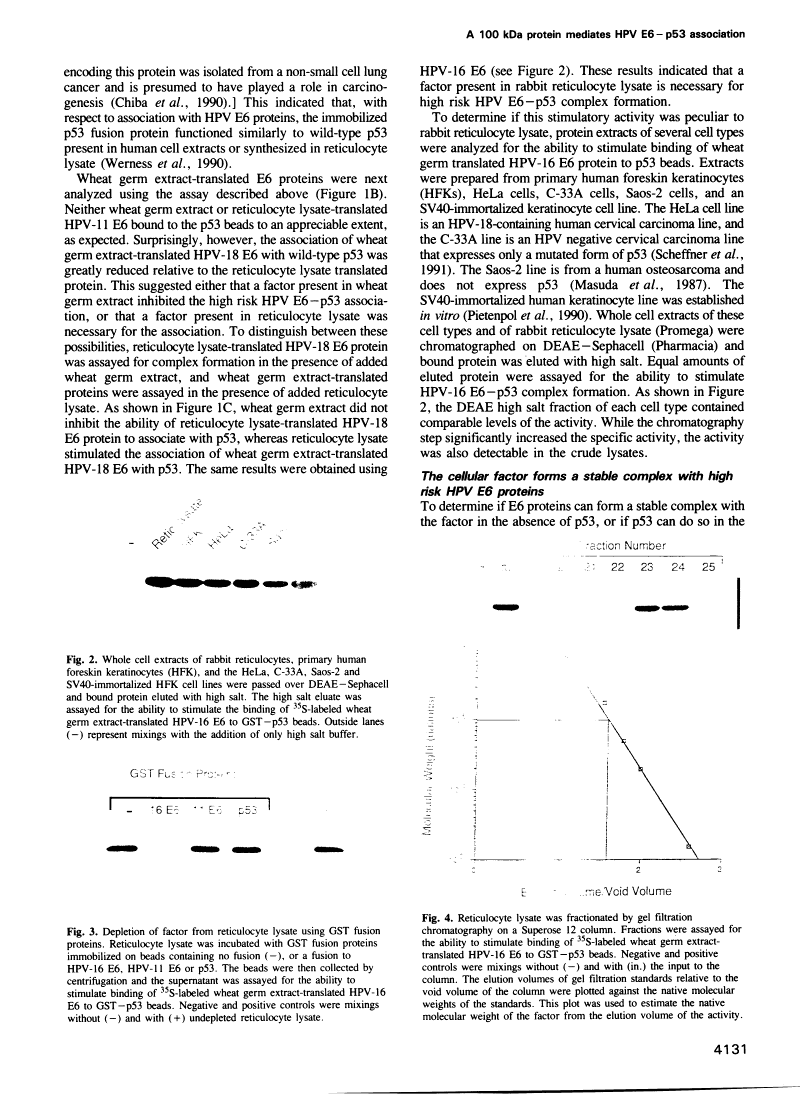
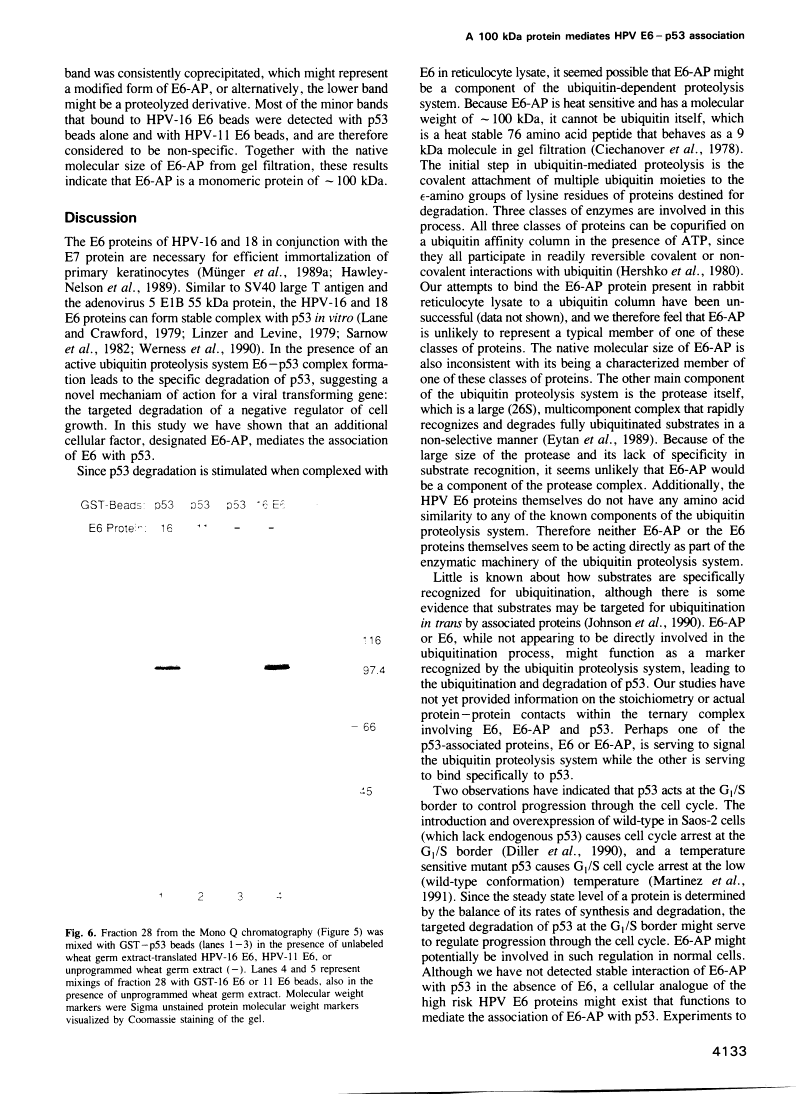
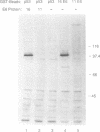
The E6 protein of human papillomavirus types 16 and 18 (HPV-16 and HPV-18) can stably associate with the p53 protein in vitro. In the presence of rabbit reticulocyte lysate, this association leads to the specific degradation of p53 through the ubiquitin-dependent proteolysis system. We have examined the E6-p53 complex in more detail and have found that association of E6 with p53 is mediated by an additional cellular factor. This factor is present in rabbit reticulocyte lysate, primary human keratinocytes and in each of five human cell lines examined. The factor is designated E6-AP, for E6-associated protein, based on the observation that the E6 proteins of HPV-16 and 18 can form a stable complex with the factor in the absence of p53, whereas p53 association with the factor can be detected only in the presence of E6. Gel filtration and coprecipitation experiments indicate that E6-AP is a monomeric protein of approximately 100 kDa.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.7M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker CC, Phelps WC, Lindgren V, Braun MJ, Gonda MA, Howley PM. Structural and transcriptional analysis of human papillomavirus type 16 sequences in cervical carcinoma cell lines. J Virol. 1987 Apr;61(4):962–971. [Europe PMC free article] [Abstract] [Google Scholar]
- Bargonetti J, Friedman PN, Kern SE, Vogelstein B, Prives C. Wild-type but not mutant p53 immunopurified proteins bind to sequences adjacent to the SV40 origin of replication. Cell. 1991 Jun 14;65(6):1083–1091. [Abstract] [Google Scholar]
- Braithwaite AW, Sturzbecher HW, Addison C, Palmer C, Rudge K, Jenkins JR. Mouse p53 inhibits SV40 origin-dependent DNA replication. Nature. 1987 Oct 1;329(6138):458–460. [Abstract] [Google Scholar]
- Chiba I, Takahashi T, Nau MM, D'Amico D, Curiel DT, Mitsudomi T, Buchhagen DL, Carbone D, Piantadosi S, Koga H, et al. Mutations in the p53 gene are frequent in primary, resected non-small cell lung cancer. Lung Cancer Study Group. Oncogene. 1990 Oct;5(10):1603–1610. [Abstract] [Google Scholar]
- Ciechanover A, Schwartz AL. How are substrates recognized by the ubiquitin-mediated proteolytic system? Trends Biochem Sci. 1989 Dec;14(12):483–488. [Abstract] [Google Scholar]
- Ciehanover A, Hod Y, Hershko A. A heat-stable polypeptide component of an ATP-dependent proteolytic system from reticulocytes. Biochem Biophys Res Commun. 1978 Apr 28;81(4):1100–1105. [Abstract] [Google Scholar]
- Ciechanover A, DiGiuseppe JA, Bercovich B, Orian A, Richter JD, Schwartz AL, Brodeur GM. Degradation of nuclear oncoproteins by the ubiquitin system in vitro. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):139–143. [Europe PMC free article] [Abstract] [Google Scholar]
- Crook T, Wrede D, Vousden KH. p53 point mutation in HPV negative human cervical carcinoma cell lines. Oncogene. 1991 May;6(5):873–875. [Abstract] [Google Scholar]
- DeCaprio JA, Ludlow JW, Figge J, Shew JY, Huang CM, Lee WH, Marsilio E, Paucha E, Livingston DM. SV40 large tumor antigen forms a specific complex with the product of the retinoblastoma susceptibility gene. Cell. 1988 Jul 15;54(2):275–283. [Abstract] [Google Scholar]
- Diller L, Kassel J, Nelson CE, Gryka MA, Litwak G, Gebhardt M, Bressac B, Ozturk M, Baker SJ, Vogelstein B, et al. p53 functions as a cell cycle control protein in osteosarcomas. Mol Cell Biol. 1990 Nov;10(11):5772–5781. [Europe PMC free article] [Abstract] [Google Scholar]
- Dürst M, Dzarlieva-Petrusevska RT, Boukamp P, Fusenig NE, Gissmann L. Molecular and cytogenetic analysis of immortalized human primary keratinocytes obtained after transfection with human papillomavirus type 16 DNA. Oncogene. 1987;1(3):251–256. [Abstract] [Google Scholar]
- Dyson N, Howley PM, Münger K, Harlow E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science. 1989 Feb 17;243(4893):934–937. [Abstract] [Google Scholar]
- Eytan E, Ganoth D, Armon T, Hershko A. ATP-dependent incorporation of 20S protease into the 26S complex that degrades proteins conjugated to ubiquitin. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7751–7755. [Europe PMC free article] [Abstract] [Google Scholar]
- Fields S, Jang SK. Presence of a potent transcription activating sequence in the p53 protein. Science. 1990 Aug 31;249(4972):1046–1049. [Abstract] [Google Scholar]
- Gannon JV, Lane DP. p53 and DNA polymerase alpha compete for binding to SV40 T antigen. Nature. 1987 Oct 1;329(6138):456–458. [Abstract] [Google Scholar]
- Glotzer M, Murray AW, Kirschner MW. Cyclin is degraded by the ubiquitin pathway. Nature. 1991 Jan 10;349(6305):132–138. [Abstract] [Google Scholar]
- Goebl MG, Yochem J, Jentsch S, McGrath JP, Varshavsky A, Byers B. The yeast cell cycle gene CDC34 encodes a ubiquitin-conjugating enzyme. Science. 1988 Sep 9;241(4871):1331–1335. [Abstract] [Google Scholar]
- Hawley-Nelson P, Vousden KH, Hubbert NL, Lowy DR, Schiller JT. HPV16 E6 and E7 proteins cooperate to immortalize human foreskin keratinocytes. EMBO J. 1989 Dec 1;8(12):3905–3910. [Europe PMC free article] [Abstract] [Google Scholar]
- Hershko A. Ubiquitin-mediated protein degradation. J Biol Chem. 1988 Oct 25;263(30):15237–15240. [Abstract] [Google Scholar]
- Hershko A, Ciechanover A, Heller H, Haas AL, Rose IA. Proposed role of ATP in protein breakdown: conjugation of protein with multiple chains of the polypeptide of ATP-dependent proteolysis. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1783–1786. [Europe PMC free article] [Abstract] [Google Scholar]
- Johnson ES, Gonda DK, Varshavsky A. cis-trans recognition and subunit-specific degradation of short-lived proteins. Nature. 1990 Jul 19;346(6281):287–291. [Abstract] [Google Scholar]
- Kaur P, McDougall JK. Characterization of primary human keratinocytes transformed by human papillomavirus type 18. J Virol. 1988 Jun;62(6):1917–1924. [Europe PMC free article] [Abstract] [Google Scholar]
- Kern SE, Kinzler KW, Bruskin A, Jarosz D, Friedman P, Prives C, Vogelstein B. Identification of p53 as a sequence-specific DNA-binding protein. Science. 1991 Jun 21;252(5013):1708–1711. [Abstract] [Google Scholar]
- Lane DP, Crawford LV. T antigen is bound to a host protein in SV40-transformed cells. Nature. 1979 Mar 15;278(5701):261–263. [Abstract] [Google Scholar]
- Linzer DI, Levine AJ. Characterization of a 54K dalton cellular SV40 tumor antigen present in SV40-transformed cells and uninfected embryonal carcinoma cells. Cell. 1979 May;17(1):43–52. [Abstract] [Google Scholar]
- Martinez J, Georgoff I, Martinez J, Levine AJ. Cellular localization and cell cycle regulation by a temperature-sensitive p53 protein. Genes Dev. 1991 Feb;5(2):151–159. [Abstract] [Google Scholar]
- Masuda H, Miller C, Koeffler HP, Battifora H, Cline MJ. Rearrangement of the p53 gene in human osteogenic sarcomas. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7716–7719. [Europe PMC free article] [Abstract] [Google Scholar]
- Münger K, Phelps WC, Bubb V, Howley PM, Schlegel R. The E6 and E7 genes of the human papillomavirus type 16 together are necessary and sufficient for transformation of primary human keratinocytes. J Virol. 1989 Oct;63(10):4417–4421. [Europe PMC free article] [Abstract] [Google Scholar]
- Münger K, Werness BA, Dyson N, Phelps WC, Harlow E, Howley PM. Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumor suppressor gene product. EMBO J. 1989 Dec 20;8(13):4099–4105. [Europe PMC free article] [Abstract] [Google Scholar]
- Oren M, Maltzman W, Levine AJ. Post-translational regulation of the 54K cellular tumor antigen in normal and transformed cells. Mol Cell Biol. 1981 Feb;1(2):101–110. [Europe PMC free article] [Abstract] [Google Scholar]
- Pietenpol JA, Stein RW, Moran E, Yaciuk P, Schlegel R, Lyons RM, Pittelkow MR, Münger K, Howley PM, Moses HL. TGF-beta 1 inhibition of c-myc transcription and growth in keratinocytes is abrogated by viral transforming proteins with pRB binding domains. Cell. 1990 Jun 1;61(5):777–785. [Abstract] [Google Scholar]
- Pirisi L, Yasumoto S, Feller M, Doniger J, DiPaolo JA. Transformation of human fibroblasts and keratinocytes with human papillomavirus type 16 DNA. J Virol. 1987 Apr;61(4):1061–1066. [Europe PMC free article] [Abstract] [Google Scholar]
- Raycroft L, Wu HY, Lozano G. Transcriptional activation by wild-type but not transforming mutants of the p53 anti-oncogene. Science. 1990 Aug 31;249(4972):1049–1051. [Europe PMC free article] [Abstract] [Google Scholar]
- Reich NC, Oren M, Levine AJ. Two distinct mechanisms regulate the levels of a cellular tumor antigen, p53. Mol Cell Biol. 1983 Dec;3(12):2143–2150. [Europe PMC free article] [Abstract] [Google Scholar]
- Riou G, Favre M, Jeannel D, Bourhis J, Le Doussal V, Orth G. Association between poor prognosis in early-stage invasive cervical carcinomas and non-detection of HPV DNA. Lancet. 1990 May 19;335(8699):1171–1174. [Abstract] [Google Scholar]
- Sarnow P, Ho YS, Williams J, Levine AJ. Adenovirus E1b-58kd tumor antigen and SV40 large tumor antigen are physically associated with the same 54 kd cellular protein in transformed cells. Cell. 1982 Feb;28(2):387–394. [Abstract] [Google Scholar]
- Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990 Dec 21;63(6):1129–1136. [Abstract] [Google Scholar]
- Scheffner M, Münger K, Byrne JC, Howley PM. The state of the p53 and retinoblastoma genes in human cervical carcinoma cell lines. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5523–5527. [Europe PMC free article] [Abstract] [Google Scholar]
- Schlegel R, Phelps WC, Zhang YL, Barbosa M. Quantitative keratinocyte assay detects two biological activities of human papillomavirus DNA and identifies viral types associated with cervical carcinoma. EMBO J. 1988 Oct;7(10):3181–3187. [Europe PMC free article] [Abstract] [Google Scholar]
- Schwarz E, Freese UK, Gissmann L, Mayer W, Roggenbuck B, Stremlau A, zur Hausen H. Structure and transcription of human papillomavirus sequences in cervical carcinoma cells. Nature. 1985 Mar 7;314(6006):111–114. [Abstract] [Google Scholar]
- Smith DB, Johnson KS. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. [Abstract] [Google Scholar]
- Smotkin D, Wettstein FO. Transcription of human papillomavirus type 16 early genes in a cervical cancer and a cancer-derived cell line and identification of the E7 protein. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4680–4684. [Europe PMC free article] [Abstract] [Google Scholar]
- Wrede D, Tidy JA, Crook T, Lane D, Vousden KH. Expression of RB and p53 proteins in HPV-positive and HPV-negative cervical carcinoma cell lines. Mol Carcinog. 1991;4(3):171–175. [Abstract] [Google Scholar]
- Wang EH, Friedman PN, Prives C. The murine p53 protein blocks replication of SV40 DNA in vitro by inhibiting the initiation functions of SV40 large T antigen. Cell. 1989 May 5;57(3):379–392. [Abstract] [Google Scholar]
- Werness BA, Levine AJ, Howley PM. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990 Apr 6;248(4951):76–79. [Abstract] [Google Scholar]
- Whyte P, Buchkovich KJ, Horowitz JM, Friend SH, Raybuck M, Weinberg RA, Harlow E. Association between an oncogene and an anti-oncogene: the adenovirus E1A proteins bind to the retinoblastoma gene product. Nature. 1988 Jul 14;334(6178):124–129. [Abstract] [Google Scholar]
Associated Data
Articles from The EMBO Journal are provided here courtesy of Nature Publishing Group
Full text links
Read article at publisher's site: https://doi.org/10.1002/j.1460-2075.1991.tb04990.x
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc453163?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1002/j.1460-2075.1991.tb04990.x
Article citations
Fibroblast stromal support model for predicting human papillomavirus-associated cancer drug responses.
J Virol, 98(10):e0102424, 13 Sep 2024
Cited by: 1 article | PMID: 39269177 | PMCID: PMC11494926
Transoral Robotic Surgery and Human Papillomavirus Infection: Impact on Oropharyngeal Cancer Prognosis.
J Clin Med, 13(15):4455, 30 Jul 2024
Cited by: 0 articles | PMID: 39124727 | PMCID: PMC11313069
Ceramide-induced cleavage of GPR64 intracellular domain drives Ewing sarcoma.
Cell Rep, 43(8):114497, 17 Jul 2024
Cited by: 0 articles | PMID: 39024100 | PMCID: PMC11416865
Structural insights into the functional mechanism of the ubiquitin ligase E6AP.
Nat Commun, 15(1):3531, 26 Apr 2024
Cited by: 1 article | PMID: 38670961 | PMCID: PMC11053172
Structure of the p53 degradation complex from HPV16.
Nat Commun, 15(1):1842, 28 Feb 2024
Cited by: 2 articles | PMID: 38418456 | PMCID: PMC10902388
Go to all (480) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Targeted degradation of the retinoblastoma protein by human papillomavirus E7-E6 fusion proteins.
EMBO J, 11(7):2425-2431, 01 Jul 1992
Cited by: 63 articles | PMID: 1321031 | PMCID: PMC556717
Interaction of the human papillomavirus type 16 E6 oncoprotein with wild-type and mutant human p53 proteins.
J Virol, 66(8):5100-5105, 01 Aug 1992
Cited by: 78 articles | PMID: 1321290 | PMCID: PMC241378
Cloning and expression of the cDNA for E6-AP, a protein that mediates the interaction of the human papillomavirus E6 oncoprotein with p53.
Mol Cell Biol, 13(2):775-784, 01 Feb 1993
Cited by: 328 articles | PMID: 8380895 | PMCID: PMC358960
The role of TP53 in Cervical carcinogenesis.
Hum Mutat, 21(3):307-312, 01 Mar 2003
Cited by: 97 articles | PMID: 12619117
Review