Abstract
Free full text

Molecular Mechanisms of Preeclampsia
Abstract
Preeclampsia is a pregnancy-specific disease characterized by new onset hypertension and proteinuria after 20 wk of gestation. It is a leading cause of maternal and fetal morbidity and mortality worldwide. Exciting discoveries in the last decade have contributed to a better understanding of the molecular basis of this disease. Epidemiological, experimental, and therapeutic studies from several laboratories have provided compelling evidence that an antiangiogenic state owing to alterations in circulating angiogenic factors leads to preeclampsia. In this review, we highlight the role of key circulating antiangiogenic factors as pathogenic biomarkers and in the development of novel therapies for preeclampsia.
Preeclampsia is a leading cause of maternal morbidity and mortality worldwide, complicating 2%–8% of pregnancies (Duley 2009). It is a multisystemic disorder with both maternal and fetal manifestations. In the mother, alongside with hypertension and proteinuria, the disease can progress to widespread microangiopathy that mainly affects the kidney, liver, and brain. Thrombocytopenia, liver dysfunction, microangiopathic hemolytic anemia, acute renal failure, placental abruption, visual disturbances, stroke, seizures, and maternal death are serious consequences of preeclampsia. In the fetus, preeclampsia is associated with intrauterine growth restriction and prematurity. Besides adverse pregnancy outcomes, women with preeclampsia have an increased risk of future health complications (Irgens et al. 2001; Bellamy et al. 2007; Vikse et al. 2008; Wang et al. 2013b; Chen et al. 2014).
Recent data suggest that alterations in circulating angiogenic factors play a pathogenic role in preeclampsia. Angiogenesis, the process of new blood vessel formation from preexisting ones, is tightly regulated by angiogenic factors. Importantly, angiogenic factors are also essential for maintenance of normal vessel health; they provide important cues for organ development. Increased levels of the antiangiogenic factors, soluble fms-like tyrosine kinase 1 (sFlt-1) and soluble Endoglin (sEng) trap circulating vascular endothelial growth factor (VEGF), placental growth factor (PlGF) and transforming growth factor β (TGFβ) respectively, decreasing their free levels, leading to endothelial dysfunction and the clinical manifestations of the disease (Maynard et al. 2003; Levine et al. 2004, 2006b; Venkatesha et al. 2006; Romero and Chaiworapongsa 2013). Interestingly, the preeclampsia-like signs and symptoms are also seen in cancer patients receiving antiangiogenic chemotherapy (Hurwitz et al. 2004; Patel et al. 2008; Vigneau et al. 2014).
The search for the ability to better diagnose, predict, and prevent preeclampsia as well as the mechanisms of its pathogenesis to develop a therapy that safely prolongs gestation has been extensive. Exciting data on angiogenic factors as central contributors to the pathogenesis of preeclampsia, candidate biomarkers, and therapeutic targets open clinically meaningful perspectives for the near future.
PATHOGENESIS
Angiogenic Factors as Mediators of the Maternal Syndrome
Taylor and Roberts posited that a dysfunctional placenta releases “toxic” factors into the maternal circulation that trigger the clinical syndrome of preeclampsia (Roberts et al. 1989). The placenta is both necessary and sufficient to cause the disease, and delivery of the placenta is the only treatment (Powe et al. 2011). Over the last decade, preeclampsia has been recognized as an antiangiogenic state (Romero and Chaiworapongsa 2013). Excessive placental production of antiangiogenic factors sFlt-1 and sEng are liberated into the maternal circulation inducing the clinical syndrome. Preeclamptic placentas show overexpression of sFlt-1 and sEng. Their levels are increased in the serum of preeclamptic women weeks before the appearance of overt clinical manifestations of the disease and they correlate with disease severity (Levine et al. 2006b)
Importantly, sFlt-1 overexpression in pregnant rodents is sufficient to induce the hallmarks of the disease: hypertension, proteinuria, and the characteristic kidney lesion, glomerular endotheliosis (Maynard et al. 2003). In addition, we showed that alterations in circulating sFlt-1 may explain a number of risk factors for preeclampsia such as multiple gestation, trisomy 13, nulliparity, and molar pregnancies (Powe et al. 2011). Addition of sEng to this model induces a more severe phenotype including thrombocytopenia, cerebral edema, and fetal growth restriction (Venkatesha et al. 2006; Maharaj et al. 2008).
Fms-like tyrosine kinase 1 (Flt-1), and soluble Flt-1 (sFlt-1) are the products of FLT-1 generated by differential mRNA processing. Flt-1, also known as vascular endothelial growth factor receptor 1 (VEGFR-1), is a membrane spanning receptor for VEGF and PlGF. It comprises an extracellular domain with seven immunoglobulin-like domains (ligand-binding), a membrane domain, and a cytoplasmic tyrosine kinase domain. Soluble Flt-1 is a shorter isoform that retains the ligand-binding domain but lacks the cytoplasmic and transmembrane domains and is therefore secreted into the circulation (Kendall and Thomas 1993; Huckle and Roche 2004; Thomas et al. 2007). sFlt-1 can also be generated by cleavage of the membrane receptor by proteases, although the physiologic significance of this process is not known (Rahimi et al. 2009; Zhao et al. 2010). Soluble Flt-1 binds VEGF and PlGF in circulation and locally in tissues acting as a scavenger that prevents them from interacting with their membrane receptors on the endothelium (Fig. 1). Therefore, an increase in the concentration of sFlt-1 decreases free, and thus bioactive, PlGF and VEGF. Another splicing variant of VEGFR-1, sFlt1-14, generated primarily in nonendothelial cells, functions similarly to sFlt-1 as a potent VEGF inhibitor. Interestingly, conversion of VEGFR-1 mRNA to sFlt1-14 prevents VEGFR-1-mediated signal transmission, in contrast to sFlt-1, whose production in endothelial cells is accompanied by a large excess of the transmembrane receptor. Expression of sFlt1-14 has been shown to be significantly increased in preeclampsia. Specifically, syncytial knots were identified as the major source of local and circulating sFlt1-14 (Sela et al. 2008). The exact role of the different splicing variants of VEGFR-1 in antiangiogenic state remains to be elucidated.
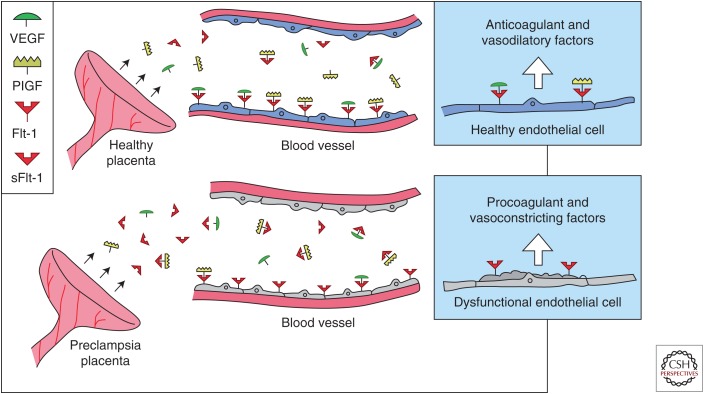
VEGF signaling and role of sFlt-1 in maternal endothelial dysfunction. There is mounting evidence that VEGF, and possibly PlGF, are required to maintain endothelial health in several tissues including the kidney and perhaps the placenta. In normal pregnancy, the placenta produces modest concentrations of VEGF, PlGF, and sFlt-1. In preeclampsia, excess placental sFlt-1 binds circulating VEGF and PlGF and prevents their interaction with endothelial cell-surface receptors leading to endothelial dysfunction. (From Karumanchi et al. 2005; reproduced, with permission, from the author.)
The physiologic function of sFlt-1 is not clearly understood. sFlt-1 is crucial for maintenance of cornea avascularity (Ambati et al. 2006) and provides important cues for vessel sprouting (Chappell et al. 2009). Flt-1 signaling appears to be nonessential for normal placental development (Hiratsuka et al. 1998), and therefore has been debated as a viable therapeutic target in preeclampsia. Evolutionary biologists have argued that one important function of sFlt-1 may be to improve placental vascular perfusion for the benefit of the fetus (Yuan et al. 2005).
Plasma sFlt-1 levels are increased before the onset of clinical signs and symptoms and they are correlated with disease severity (Chaiworapongsa et al. 2004, 2005). This is mirrored by a decrease of free VEGF and PlGF (Maynard et al. 2003; Polliotti et al. 2003; Levine et al. 2004). The main source of sFlt-1 in pregnancy is the placenta (Maynard et al. 2003; Gu et al. 2008). sFlt-1 is highly overexpressed in preeclamptic placentas, in particular in syncytial knots. Additional sources for sFlt-1 production, such as peripheral blood mononuclear cells (Rajakumar et al. 2005), have been described, but the clinical significance is unknown.
sFlt-1 contains heparin-binding domains in the third and fourth immunoglobulin loops that account for its strong avidity to the heparan sulfate on cell surfaces or on the extracellular matrix (Kendall and Thomas 1993; Park and Lee 1999; Sela et al. 2011). This chemical property raised questions about the mechanism by which sFlt-1 gains access to the maternal circulation. Heparanase releases sFlt-1 retained in these storages (Sela et al. 2011). However, expression of heparanases in the placenta is only modestly altered in preeclampsia (Ginath et al. 2014).
Release of trophoblast-derived material/fragments into the maternal circulation has long been recognized (Redman and Sargent 2000). It is an intriguing phenomenon in which the physiological function remains to be elucidated. Trophoblast fragments are a significant source of sFlt-1 in the maternal circulation in preeclampsia. The first report of trophoblast deportation comes from Schmorl who showed trophoblast material in lungs of patients that died from eclampsia (Lapaire et al. 2007). It is now recognized that the detachment of syncytial knots from the placenta results in free, multinucleated trophoblast microparticles that are metabolically active and capable of de novo gene translation, including the ability to synthesize sFlt-1 protein from endogenous stores of mRNA (Rajakumar et al. 2012). This phenomenon is enhanced in preeclampsia, suggesting that an accelerated shedding of syncytial aggregates is a proximal event in the pathogenesis of preeclampsia (Buurma et al. 2013).
VEGF signaling is not only important for angiogenesis but also for maintaining endothelial health in certain specialized vascular beds such as liver, kidney, brain as well as endocrine organs such as thyroid tissue, where it is constitutively expressed to maintain endothelial fenestrations (Kamba et al. 2006). There is compelling evidence that the growth of some tumors is dependent on VEGF-induced angiogenesis (Sitohy et al. 2011). VEGF inhibitor therapy caused early striking vascular changes in islet cell tumors. Within 24 hours, endothelial fenestrations and sprouts disappeared, patency was lost, and flow ceased in some vessels. Tumors vessels lacking fenestrations were less responsive to the inhibitors, suggesting that vessel phenotype may be predictive of responsiveness to VEGF inhibitors (Inai et al. 2004). Cessation of anti-VEGF therapy led to complete revascularization within the first week after the treatment stopped which fully regressed during a second treatment (Mancuso et al. 2006). VEGF, which is strongly expressed by podocytes, although its binding sites are localized on glomerular endothelial cells, plays a paramount role in maintaining glomerular integrity under normal physiologic conditions, such that when intraglomerular VEGF levels decrease capillary endothelial cells swell, capillary loops collapse, glomerular filtration is impaired, and proteinuria develops (Mattot et al. 2002; Eremina and Quaggin 2004). Moreover, after renal injury, there is a transient increase in VEGF expression followed by a decrease in its expression resulting in glomerular and peritubular capillary loss, which correlates with the severity of glomerulosclerosis, interstitial fibrosis, and tubular atrophy. This shows that lack of persistence of VEGF expression may be a primary factor in the progression of renal insufficiency (Kang et al. 2001a,b).
VEGF treatment promoted glomerulogenesis and vascularization of implanted organoids and enabled the generation of vascularized nephrons from single cell suspensions (Xinaris et al. 2012). Genetic studies in mice have provided compelling evidence that VEGF is involved in the induction and maintenance of the fenestrae in glomerular capillary endothelial cells (Eremina et al. 2003). Moreover, slit-diaphragm proteins such as nephrin are significantly reduced in podocytes in animals receiving VEGF inhibitors (Sugimoto et al. 2003) as well as in renal biopsy studies in preeclamptic subjects (Garovic et al. 2007). Podocyte VEGF knockdown disrupts integrin activity via decreased VEGFR2 signaling, thereby damaging the glomerular filtration barrier, causing proteinuria and acute renal failure (Veron et al. 2012). VEGF also mediates endothelium-dependent vasodilatation and is thus important for blood pressure regulation (He et al. 1999; Facemire et al. 2009). Hypertension and proteinuria with glomerular damage resembling preeclampsia are adverse effects of VEGF inhibitors therapy given to nonpregnant cancer patients (Eremina et al. 2008; Patel et al. 2008; Vigneau et al. 2014). Furthermore, the treatment of pregnant rats or mice with sFlt-1 results in hypertension, proteinuria, and glomerular endotheliosis, the classic lesions of preeclampsia (Maynard et al. 2003; Lu et al. 2007). Taken together, these studies and others suggest that VEGF inhibition prompts endothelial dysfunction, hypertension, and proteinuria.
Other factors released by the placenta act synergistically with sFlt-1 to induce an antiangiogenic environment. Placental endoglin is up-regulated in preeclampsia, releasing sEng to the maternal circulation. sEng interferes with TGF-β signaling and eNOS activation and thereby causes endothelial dysfunction (Venkatesha et al. 2006). Overexpression of sEng and sFlt-1 in mice also induces cerebral edema that resembles eclampsia and severe preeclampsia (Maharaj et al. 2008). sEng is increased in the sera of preeclamptic women 2–3 mo before clinical signs of preeclampsia develop, and its blood levels correlate with disease severity (Levine et al. 2006a; Noori et al. 2010). Semaphorin 3B, a novel trophoblastic secreted antiangiogenic protein may also synergize with sFlt1 by interfering with VEGF signaling and contributing to the pathogenesis of preeclampsia (Zhou et al. 2013).
Recently, a pathophysiological explanation was provided for the epidemiological association of peripartum cardiomyopathy (PPCM) with preeclampsia (Bello et al. 2013). Exogenous sFlt-1 alone caused diastolic dysfunction in wild type mice, and profound systolic dysfunction in mice lacking cardiac PGC-1α, a powerful regulator of angiogenesis. Furthermore, plasma samples from women with PPCM contained abnormally high levels of sFlt-1. These data indicate that PPCM is a two-hit combination of, first, systemic antiangiogenesis during late pregnancy which is substantially worsened in preeclampsia and/or in multiple gestation and second, a host susceptibility attributed to insufficient local proangiogenic defenses in the heart (Patten et al. 2012).
Mechanisms of Placentation Defects in Preeclampsia
Although high levels of sFlt-1 and sEng are widely recognized as contributors to the pathogenesis of the disease, the upstream mechanisms of placental damage remain to be elucidated. Several mechanisms have been proposed such as placental hypoxia, oxidative stress, endoplasmic stress, inflammation, altered NK cell signaling, autoantibodies against the angiotensin receptor, and others (Chaiworapongsa et al. 2014).
During normal placental development, the maternal uterine spiral arteries undergo an extensive remodeling, transforming them into low resistance, high bore blood vessels adequate to provide nutrients to the placenta and fetus (Brosens et al. 1967). In preeclampsia, cytotrophoblasts (CTBs) fail to invade the myometrium and the physiological changes of the spiral arteries are restricted to the decidua (Fig. 2). Invading CTBs up-regulate expression of molecules that are central to uterine invasion and pseudovasculogenesis (the process by which CTB switch their adhesion molecules to mimic that of vascular cells) (Damsky and Fisher 1998). Expression of molecules from the VEGF family changes as invasion of these cells through the uterine wall takes place. Invasive CTBs in early gestation express VEGF-A, VEGF-C, PlGF, VEGFR-1 and VEGFR-3 and at term, VEGF-A, PlGF, and VEGFR-1. The interactions between these molecules are critical for invasion and pseudovasculogenesis because interference with ligand binding altered integrin α1, an adhesion molecule highly expressed by endovascular CTB, resulting in increased apoptosis of these cells. Interestingly, in severe preeclampsia, expression of VEGF-A and VEGFR-1 in the cytotrophoblasts is down-regulated but sFlt-1 release is increased (Zhou et al. 2002). These findings suggest that dysregulation of angiogenic factors in the maternal–fetal interface is associated with failed pseudovasculogenesis and impaired placentation (Zhou et al. 2002; Fisher 2004).
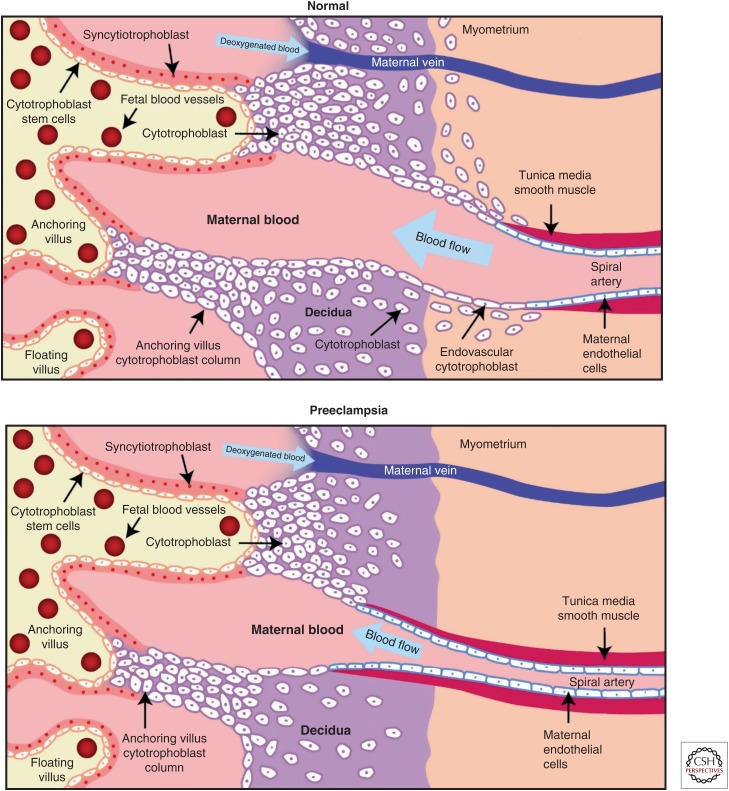
Spiral artery defects in preeclampsia. Exchange of oxygen, nutrients, and waste products between the fetus and the mother depends on adequate placental perfusion by maternal vessels. In normal placental development, invasive cytotrophoblasts of fetal origin invade the maternal spiral arteries, transforming them from small-caliber resistance vessels to high-caliber capacitance vessels capable of providing placental perfusion adequate to sustain the growing fetus. During the process of vascular invasion, the cytotrophoblasts differentiate from an epithelial phenotype to an endothelial phenotype, a process referred to as “pseudovasculogenesis” (upper panel). In preeclampsia, cytotrophoblasts fail to adopt an invasive endothelial phenotype. Instead, invasion of the spiral arteries is shallow and they remain small caliber, resistance vessels leading to placental ischemia (lower panel). (From Lam et al. 2005; reproduced, with permission, from the author.)
Epidemiological data suggest that the immune system may play a role in the pathogenesis of preeclampsia (Robillard et al. 2011). Uterine natural killer (uNK) cells, which are abundant in decidualized endometrium, surround spiral arteries and secrete a range of angiogenic growth factors play that a major role in immunology-related placentation, vascular remodeling of uterine arteries, and angiogenesis (Croy et al. 2000). When chorionic plate arteries and spiral arteries are cultured with conditioned medium from uNK cells, disruption of vascular smooth muscle cells and breakdown of extracellular matrix components is induced. These results show that uNK cells contribute to the early stages of spiral artery remodeling (Robson et al. 2012). Genetic studies have associated natural killer (NK) cells with preeclampsia. Specific combinations of killer immunoglobulin-like receptors (NK cell receptors for receptors for MHC class I ligands) haplotypes in the mother and trophoblast HLA-C types in the fetus are strongly associated with preeclampsia (Hiby et al. 2004). Moreover, uNK cells have a distinct phenotype, as well as a differential gene expression, compared with circulating NK cells (Koopman et al. 2003). Recently it has been shown that exposure to a combination of hypoxia, TGF-β1, and a demethylating agent results in transformation of peripheral NK cells to decidual NK-like cells, including expression of decidual NK cell markers, the ability to secrete VEGF, reduced cytotoxicity, and promotion of invasion of human trophoblast cell lines. These striking findings have potential therapeutic applications for placental disorders associated with altered NK cell biology (Cerdeira et al. 2013).
Impaired Corin expression or function in the pregnant uterus has been suggested as another mechanism for failed spiral artery remodeling. Local atrial natriuretic peptide (ANP) production by Corin, a cardiac protease that activates ANP, promoted trophoblast invasion and spiral artery remodeling to prevent hypertension in pregnancy (Cui et al. 2012). Pregnant Corin- or ANP-deficient mice develop hypertension and proteinuria. Moreover, in preeclamptic women, uterine, Corin mRNA and protein levels are significantly lower than in normal pregnancies. Of note, Corin levels in plasma, probably derived from the heart, are higher in preeclamptic women and did not reflect the levels in tissues (Cui et al. 2012).
Inadequate placentation, owing to deficient trophoblast invasion of uterine spiral arteries, a characteristic of preeclampsia, can lead to placental hypoxia that can lead to abnormal expression of angiogenic factors. Persistent placental hypoxia promotes hypoxia-inducible factor-1 alpha (HIF-1α) release, which fosters a proliferative noninvasive trophoblast phenotype (Rajakumar et al. 2005), further aggravating hypoxia. HIF-1α promotes transforming growth factor-β3 (TGF-β3) expression, an inhibitor of trophoblast differentiation (Caniggia et al. 2000). Endoglin (Eng), related to a decrease in invasive functions, is a coreceptor for TGF-β, which is expressed on cytotrophoblasts during the first trimester and is promoted by TGF-β1 and TGF-β3. TGF-β3 also promotes the production of sEng (Mano et al. 2011). In addition, VEGF and PlGF are induced under hypoxic conditions (Tissot van Patot et al. 2004; Nishimoto et al. 2009) and are inhibited by sFlt-1. Interestingly, sEng and sFlt-1 expression are both stimulated under ischemic conditions, thus contributing to the systemic endothelial dysfunction (Gilbert et al. 2007, 2009).
The renin–angiotensin system (RAS) may be involved in the pathogenesis of preeclampsia. Decidual expression of AT1 receptor, the gene encoding the angiotensin (Ang II) type 1 receptor is higher in preeclampsia than in normal pregnancies (Herse et al. 2007). Ang concentration, which is also increased in the chorionic villi in preeclampsia, may promote uteroplacental dysfunction via vasoconstriction (Anton et al. 2008). Levels of stimulatory Ang type 1 receptor autoantibodies (AT1-AAs) are elevated in ~70%–95% of preeclamptic women (Siddiqui et al. 2010). Injection of AT1-AAs from pregnant women to pregnant mice induced hypertension, proteinuria, placental abnormalities, and glomerular endotheliosis, which are the key features of preeclampsia (Zhou et al. 2008). The binding of AT1-AAs to AT1-R induces sFlt-1 and sEng production by human villous explants through tumor necrosis factor (TNF)-α pathways (Irani et al. 2010). TNF-α is also increased in preeclampsia owing to placental ischemia and it stimulates placental production of sEng and sFlt-1 (Parrish et al. 2010). Interestingly, high levels of AT1-AAs correlate with the disease severity because of their association with the presence of hypertension, proteinuria, and sFlt-1 (Siddiqui et al. 2010).
A pathogenic role of heme-oxygenase-1 (HO-1) in preeclampsia has been identified (Ahmed and Cudmore 2009). Heme breakdown metabolites generated by the enzymatic activity of HO-1 account for its angiogenic and vasodilatory properties. Placental HO-1 expression and exhaled CO levels were both found to be reduced in severe preeclamptic women. Interestingly, in vitro experiments showed that HO-1 induction increased CO production and down-regulated secretion of sFlt-1 (Levytska et al. 2013). More recently, endometrial VEGF up-regulation has also been shown in a mouse model to induce placental sFlt1 production and contribute to preeclampsia (Fan et al. 2014).
Placental underperfusion, secondary to deficient conversion of the spiral arteries was recently linked to endoplasmic reticulum (ER) stress, which can lead to activation of proinflammatory pathways, contributing to maternal endothelial cell activation (Burton and Yung 2011). The ER is a hub for proper folding and export of peptides, guided by ER-specific chaperones. ER stress can dysregulate the function of chaperones, resulting in export of misfolded proteins into the circulation (Redman 2008). Administration of aberrant transthyretin, immunoprecipitated from PE serum to pregnant mice induces a full spectrum of preeclampsia-like features, whereas immunodepletion of the transthyretin from preeclamptic serum ameliorates most of the disease features and reduces sFlt-1 and sEng. Moreover, aggregation of transthyretin protein is found in serum and in placental tissue from preeclamptic pregnancies. It is possible that hypoxia, which has been shown to control transthyretin expression and uptake, and ER stress destabilize transthyretin into a misfolded conformation and aggregation acquiring the potential to induce antiangiogenic factors (Kalkunte et al. 2013). The presence of β-amyloid aggregates in placentas of women with PE and fetal growth restriction (Buhimschi et al. 2014) may further support the pathogenic role of ER stress and misfolded proteins in preeclampsia.
Placental pathological lesions similar to preeclampsia also occur in cases of intrauterine growth restriction (IUGR), but without maternal disease (Khong et al. 1986). Increased syncytiotrophoblast microparticles are found in women with early onset preeclampsia, but not in cases of normotensive IUGR suggesting that syncytial pathology may be unique to preeclampsia (Goswami et al. 2006). These biologically active syncytial l microparticles can transport toxic proteins, such as sFlt1 and sEng into the maternal circulation, where they mediate the major manifestations of preeclampsia (Fig. 3) (Rajakumar et al. 2012). We and others have hypothesized that the absence of a second insult of syncytial debris shedding, crucial for the appearance of the systemic disease in PE, protects women with fetal IUGR from the full blown maternal syndrome observed in preeclamptic women. Changes not only in quantity, but also in the size of the circulating microparticles, might be important in the pathogenesis of preeclampsia (Redman et al. 2012). Moreover, differences in physical and antigenic characteristics of normal and preeclamptic placental micro- and nanovesicles produced by placental perfusion or mechanical disruption have been observed (Tannetta et al. 2013). It is therefore possible that specific differences in microparticles of preeclampsia versus normal or IUGR pregnancies are the basis for their distinct pathology.
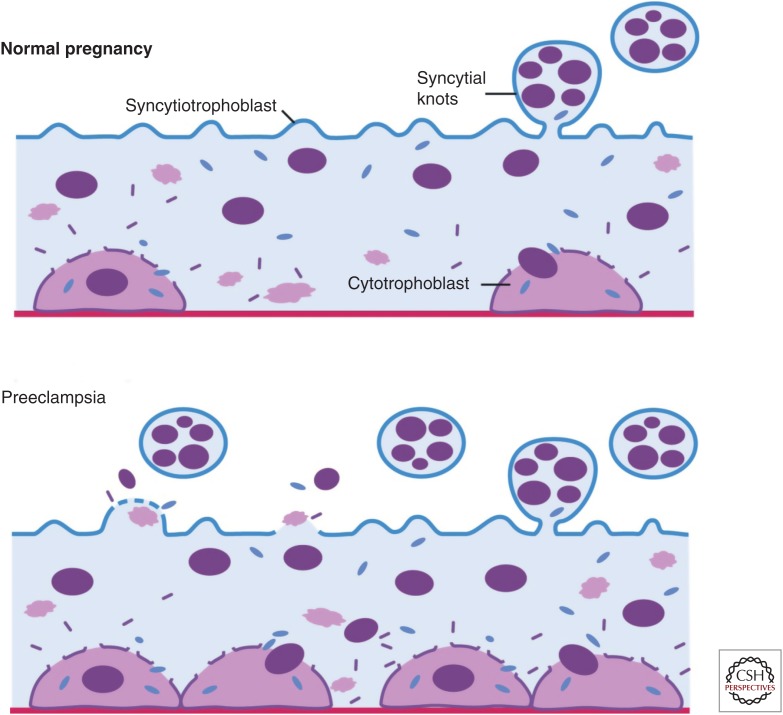
Schematic of syncytiotrophoblast pathology in preeclampsia. Syncytialization, knot formation, and subsequent microparticle shedding appear to be normal events of pregnancy. Preeclampsia rises from an augmentation of this sequence. Syncytial fragments shed into the maternal circulation are a significant source of circulating sFlt-1 in preeclampsia. (Created from data in Huppertz and Herrler 2005.)
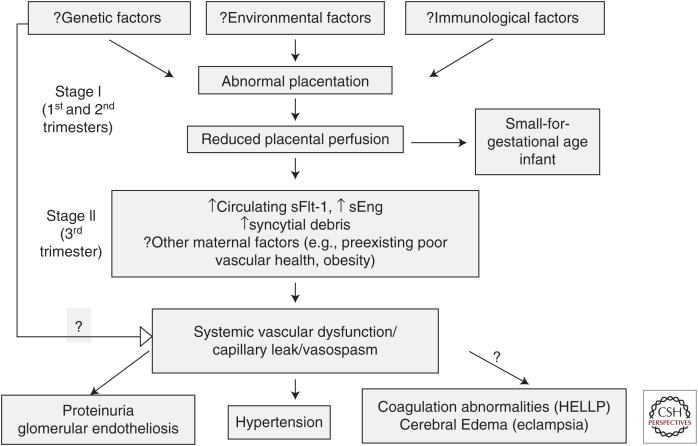
The mechanisms by which angiogenic factor expression in the placenta contribute to the pathophysiology of preeclampsia are yet to be defined. However, most models described above and others show increased sFlt-1 and sEng secretion, which further strengthens its role in the stage II or the effector pathway that leads to the clinical syndrome of preeclampsia (Fig. 4).
ANGIOGENIC FACTORS AS BIOMARKERS
Measurement of angiogenic factors using automated assays (sFlt-1 and PlGF) has proven to be clinically useful for routine diagnosis of preeclampsia (Ohkuchi et al. 2010; Schiettecatte et al. 2010; Sunderji et al. 2010; Verlohren et al. 2010; Benton et al. 2011; Knudsen et al. 2012). Several groups have shown that sFlt-1 and free PlGF levels can be used to differentiate preeclampsia from diseases that mimic preeclampsia, such as chronic hypertension, gestational hypertension, kidney disease, and gestational thrombocytopenia (Salahuddin et al. 2007; Sunderji et al. 2010; Perni et al. 2012; Rolfo et al. 2012; Verdonk et al. 2012).
Similar to the field of cardiovascular medicine with the introduction of troponins, risk stratification of women presenting at obstetrical triage with suspected preeclampsia may be an important advance in obstetrical clinical practice. Rana et al. tested the performance of angiogenic factors as predictors of adverse outcomes in patients presenting at obstetrical triage with suspected preeclampsia (Rana et al. 2012). In women presenting at <34 wk, plasma sFlt-1/PlGF ratio predicted adverse maternal and perinatal outcomes better than current clinical assessment (AUC 0.93) and importantly correlated strongly with duration of pregnancy (Rana et al. 2012). An extension of this study showed that plasma sEng, for which levels correlated with sFlt-1, also performed better than clinical current standards and had a performance similar to sFlt-1/PlGF ratio (Rana et al. 2012). Similarly, Chaiworapongsa et al. showed a good performance of plasma angiogenic factors assessed in obstetric triage as prognostic predictors of risk for developing preeclampsia requiring preterm delivery (Chaiworapongsa et al. 2011). In patients presenting at <34 wk gestation, plasma PlGF/sFlt-1 ratio of <0.033 MoM identified patients who delivered within 2 wk because of preeclampsia with a sensitivity of 93% and a specificity of 78%, likelihood ratio for a positive test of 4.23, AUC 88. Likewise, Moore et al. also showed a robust association of serum angiogenic biomarkers with maternal and neonatal complications in women presenting to triage with suspected preeclampsia (Moore et al. 2012). Verlohren et al. (2012) also assessed the value of serum sFlt-1/PlGF as prognostic markers, showing an increased risk of imminent delivery in preeclamptic women with high sFlt-1/PLGF ratios. In addition, Rana et al. (2013) showed that women diagnosed with preeclampsia, but with a normal plasma angiogenic profile, are not at risk for adverse maternal or fetal outcomes. Chappell et al. (2013) studied the performance of plasma PlGF alone for prediction of delivery for preeclampsia within 14 d in women at <35 wk with suspected preeclampsia. They showed a high sensitivity and negative predictive value for PlGF levels <5th centile (Chappell et al. 2013). Taken together, these studies suggest that angiogenic factors have a significantly better prognostic accuracy than the current clinical standard and may be useful for risk stratification and management. Automated assays are already available for sFlt-1 and PlGF measurements in various biological fluids (serum plasma or urine), with results available in <20 min (Schiettecatte et al. 2010), which makes these factors easily translatable to clinical decision making in labor and delivery.
Numerous studies have evaluated the performance of angiogenic factors in prediction of preeclampsia alone or in combination with other markers (for a detailed review, see Hagmann et al. 2012). In particular, first trimester prediction has been developed using models that incorporate angiogenic factors, maternal characteristics, biophysical, and other biochemical markers. A large prospective study of 7797 women with singleton pregnancies at 11–13 wk of gestational age evaluated the performance of an algorithm that combined PlGF, uterine pulsatility index, pregnancy-associated plasma protein A, mean arterial pressure, body mass index, and presence of nulliparity or previous preeclampsia, for prediction of preeclampsia. At a 5% false positive rate, the detection rate for early preeclampsia was 93.1% (Poon et al. 2009). The calculated positive likelihood ratio was 16.5, and negative likelihood ratio was 0.06 (Levine and Lindheimer 2009). The same group subsequently developed variations of this algorithm (including PlGF and sEng) that also showed a good performance (Akolekar et al. 2011, 2013). Other groups have studied the performance of angiogenic factors in the second trimester. Kusanovic et al. (2009) conducted a longitudinal study of 1622 consecutive pregnancies, analyzing the performance of angiogenic factors sEng, PlGF and sFlt-1 alone or combined in an angiogenic index. PlGF/sEng ratios in the mid trimester (20–25 wk) showed a sensitivity of 100% and a specificity of 98.3%, a positive likelihood ratio of 57.6 (95%CI:37.6–57.6) and a negative likelihood ratio of 0.0 (95%CI:0.0–0.3) for prediction of early onset preeclampsia (Kusanovic et al. 2009), suggesting that angiogenic factors could be reliable tools for second trimester prediction of preeclampsia. However, there is no evidence that interventions or close follow up may improve maternal and/or fetal outcome even if one were to robustly predict early onset preeclampsia. Recent guidelines of the American College of Obstetrics and Gynecology Task Force also do not support the use of biomarkers to predict preeclampsia (ACOG Task Force on Hypertension and Pregnancy 2013). Studies evaluating the utility of low-dose aspirin to reduce the incidence of preeclampsia in those at high risk based on abnormal first trimester biochemical profile are currently in progress.
ANGIOGENIC FACTORS AS THERAPEUTIC TARGETS
To date, delivery of the placenta is the only treatment, which in the case of preterm delivery may adversely affect neonatal outcomes. The development of a therapy that safely prolongs gestation would constitute a major advance in this field. Several strategies have been developed to target angiogenic factors using both in vitro and in vivo models to reestablish the angiogenic balance. Depletion of sFlt-1 in preeclamptic villous condition media by immunoprecipitation reverses the antiangiogenic properties in vitro (Ahmad and Ahmed 2004). Administration of VEGFA-121 into a rodent model of preeclampsia improved hypertension, proteinuria, glomerular endotheliosis, and reverted sFlt-1 induced changes in gene expression (Li et al. 2007). Coadministration of adenovirus expressing VEGF in a similar model, resulted in a significant reduction of free sFlt-1, rescued the renal damage and normalized blood pressure (Bergmann et al. 2010). VEGF-121 infusion has also been reported to lower blood pressure and improve renal function in rats with placental ischemia-induced hypertension (Gilbert et al. 2010). Furthermore, increased expression of placental growth factor induced by pravastatin decreased the levels of circulating sFlt-1 and ameliorated preeclamptic symptoms in a preeclampsia mouse model of placental-specific sFlt-1 overexpression (Kumasawa et al. 2011).
In humans, resolution of signs of preeclampsia were observed in cases of parvovirus-induced hydrops (Stepan and Faber 2006), mirror syndrome (Llurba et al. 2012), fetal demise in a twin pregnancies (Hladunewich et al. 2009), all of which correlated with fall in sFlt1 levels. These experiments of nature have strengthened the notion that reduction of sFlt-1 during pregnancy may be safe without generating significant adverse effects to the fetus.
Thadhani et al. (2011) recently translated some fundamental discoveries to the bedside. Taking advantage of the positive charge of sFlt-1, they used a negatively charged dextran sulfate cellulose column for extracorporeal removal of sFlt-1. In a pilot study of eight women with preterm preeclampsia, dextran sulfate apheresis lead to a reduction in sFlt-1 levels and improvement in proteinuria and blood pressure, without evident adverse effects to the mother and fetus. Five women were treated once, two women twice, and a third patient treated four times. Of the two women undergoing two apheresis treatments, one remained pregnant for 15 d and the other for 19 d. A third woman, treated four times, remained pregnant for 23 d. In all of these cases, there was evidence of continued fetal growth. In untreated women, average prolongation of pregnancy was 3.6 d (Thadhani et al. 2011). This is an exciting study that opens the way for a safe prolongation of gestation in severe preterm preeclamptic patients. Other modalities for targeting the angiogenic imbalance are administration of agents that scavenge sFlt-1, such as sFlt-1 antibodies, PlGF, VEGF, or decrease sFlt-1 production by siRNA strategies or small molecules.
Relaxin, a peptide hormone that is increased in pregnancy, is evolving as a potential target for preeclampsia, given its vasodilatory and vascular remodeling properties (Conrad and Shroff 2011). A phase I safety study in women with severe preeclampsia is currently ongoing (Unemori et al. 2009). Interestingly, its effects could be partially mediated by VEGF and PlGF (McGuane et al. 2011). Thus, relaxin could offset the increased sFlt-1 levels in preeclampsia by potentiating local VEGF and PlGF signaling in the vasculature.
During pregnancy, heme oxygenase-1 (HO-1) negatively regulates sFlt-1 and the loss of HO-1 activity early in pregnancy could be partly responsible for the cascade of events observed subsequently in preeclamptic pregnancies, such as the elevation in sFlt-1 as well as the increase in oxidative stress or inflammation (Ahmed, 2011). Carbon monoxide (CO), one of the metabolites of the HO system, has also been implicated in PE, as exposure to CO reduced endothelial and placental sFlt-1 and sEng release (Cudmore et al. 2007). Statins were found to increase HO-1 expression, which in turn leads to the subsequent production of CO and bilirubin, which inhibit sFlt-1 and sEng (Ramma and Ahmed 2014). Pravastatin treatment in injected adenovirus carrying sFlt-1 mice reduced sFlt-1 and sEng concentrations to levels similar to control group whereas placental PlGF and VEGF expression were up-regulated (Saad et al. 2014). Cardiac glycosides such as ouabain have also been reported to be potent inhibitors of sFlt-1 protein and mRNA synthesis through the HIF-1α pathway. Furthermore, in an animal model of pregnancy-induced hypertension, ouabain therapy improved hypertension without any adverse consequences to the pups (Rana et al. 2014). Additional studies are needed to evaluate the role of HO-1 and HIF-1 as potential therapeutic targets in preeclampsia.
Finally, complement activation downstream from the endothelial dysfunction has also been recently reported to be critical to the maternal phenotype in preeclampsia (Burwick et al. 2013). Eculizumab, a monoclonal antibody that targets C5, has been used in one patient with severe HELLP syndrome (Burwick and Feinberg 2013). More studies are needed to evaluate whether specific subgroups of preeclampsia may benefit from complement inhibition.
LONG TERM COMPLICATIONS OF PREECLAMPSIA
Endothelial dysfunction, which has been linked to atherosclerosis, persists in formerly preeclamptic women many years after an affected pregnancy (Chambers et al. 2001; Sattar et al. 2003; Saxena et al. 2010). Large retrospective epidemiologic studies have shown an elevated risk for many types of cardiovascular disease in women with a history of preeclampsia (Smith et al. 2001; Wilson et al. 2003; Ray et al. 2005; Lykke et al. 2009). The prevalence of hypertension in women with previous preeclampsia is >50% an average of 14 yr after pregnancy. This is three to four times the risk found in women without preeclampsia (Bellamy et al. 2007). Similarly, the risk of death from cardiovascular disease and cerebrovascular disease is about twofold greater in women with a history of preeclampsia. Women who have had preeclampsia before 34 wk or preeclampsia complicated by fetal growth restriction, have even higher risks of death from cardiovascular disease (Ray et al. 2005; Bellamy et al. 2007; Lykke et al. 2009). Studies have shown that levels of sFlt-1 remain modestly higher in women with a history of preeclampsia compared with those without preeclampsia in the postpartum period, which may explain the elevated cardiovascular risk in women with a history of preeclampsia (Wolf et al. 2004; Saxena et al. 2010). Although persistent alterations in antiangiogenic proteins levels leading to endothelial dysfunction can be a potential explanatory mechanism, it is possible that shared risk factors may jointly predispose to preeclampsia, endothelial dysfunction, and cardiovascular disease, and that pregnancy as a stress test reveals subclinical cardiovascular disease phenotypes long before manifestation of overt disease. Further investigation is necessary to determine whether preeclampsia itself may cause permanent vascular damage from inflammatory stress, coagulation dysregulation and endothelial damage, contributing directly to cardiovascular disease pathogenesis.
Preeclampsia is also linked to endocrine and metabolic disorders, specifically subsequent development of hypothyroidism, hyperlipidemia, and diabetes mellitus (Hubel et al. 2000; Levine et al. 2009; Lykke et al. 2009). A significantly increased risk of hypothyroidism was observed among nulliparous women with late-onset preeclampsia in a cohort of 15,935 women with 20–40 yr of follow-up (HR: 1.82, 95%CI:1.04–3.19) (Mannisto et al. 2013). Women with preeclampsia have higher thyrotropin (TSH) levels in late pregnancy and women with history of recurrent preeclampsia have been shown to have a higher risk of developing subsequently reduced thyroid function many years after preeclampsia. The association was stronger if the high concentration of TSH was combined with absence of thyroid peroxidase antibodies, and particularly strong if preeclampsia had occurred in two pregnancies, suggesting that the hypothyroid function in preeclampsia may occur independent of the autoimmune mechanisms accepted as the most likely cause of hypothyroidism in iodine replete women (Levine et al. 2009). The increased risk for thyroid dysfunction among women with preeclampsia is thought to be mediated through antiangiogenic proteins, which act as antagonists to VEGF and PlGF, causing endothelial dysfunction and capillary regression in several tissues, including the thyroid (Maynard et al. 2005; Levine et al. 2006b). This theory is further strengthened by studies in mice using VEGF inhibitors such as sFlt-1, which have shown substantial thyroid capillary regression and increased TSH concentrations (Kamba et al. 2006; Kamba and McDonald 2007).
Hypertensive diseases are also associated with worsened renal outcomes, mainly increased albuminuria and an increased risk of future chronic kidney disease (CKD) and end-stage renal disease (ESRD). A meta-analysis confirms the association between preeclampsia and albuminuria in the long term. Thirty-one percent of women who had preeclampsia developed microalbuminuria at an average of 7.1 yr postpartum, in contrast to only 7% of women who had uncomplicated pregnancies (McDonald et al. 2010). An increased risk of renal disease was shown in a large retrospective cohort study of 570,433 women and an average follow-up of 17 yr after initial pregnancy. The relative risk of ESRD in women who had preeclampsia in the first pregnancy was close to five. Women with two or three episodes of preeclampsia had a 15-fold increase in the risk of developing ESRD (Vikse et al. 2008). Moreover, the severity of hypertensive disease during pregnancy correlated with progression to ESRD (Wang et al. 2013a).
In addition to the maternal complications of preeclampsia detailed above, preeclampsia significantly increases perinatal and neonatal morbidities. Maternal preeclampsia is a frequent cause of preterm birth (Basso et al. 2006) and serum levels of sFlt-1 in the mother are inversely related to gestational age and birth weight of the newborn (Veas et al. 2011). sFlt-1, which is markedly increased in amniotic fluid during the second and third trimesters of pregnancies complicated by preeclampsia (Vuorela et al. 2000; Wang et al. 2010), may directly reach the fetal lung via fetal breathing or through an intramembranous pathway and fetal swallowing to enter the fetal lung. Studies have shown that VEGF inhibition during early neonatal period results in persistent abnormalities of alveolar and pulmonary vascular structures into and beyond infancy, which are characteristic of pathological changes in human bronchopulmonary dysplasia (BPD) (Le Cras et al. 2002; Tang et al. 2004). Furthermore, excess intra-amniotic sFlt-1, causing transient impairment of VEGF signaling in the fetus, was sufficient to cause sustained abnormalities of lung structure during infancy as well as biventricular hypertrophy most probably secondary to pulmonary and systemic hypertension in 14-d-old rats (Tang et al. 2012). These findings are interesting because offspring of mothers with preeclampsia are at risk for pulmonary vascular dysfunction later in life (Jayet et al. 2010). Preeclampsia-induced vascular dysfunction may have other clinical consequences in offspring, such as an increased risk of hypertension (Seidman et al. 1991; Vatten et al. 2003) and stroke (Kajantie et al. 2009). Interestingly, the antiangiogenic state of gestational hypertension/preeclampsia protects the infant from retinopathy of prematurity (Yu et al. 2012), a disorder characterized by the overproduction of VEGF (Pierce et al. 1995; Robbins et al. 1997).
Taken together, all of the maternal and fetal complications mentioned above suggest a central role of antiangiogenic factors, not only in the pathogenesis of preeclampsia but also as leading contributing factors to unfavorable maternal as well as fetal long term consequences.
CONCLUSION
Preeclampsia is a pregnancy-specific disease characterized by an antiangiogenic state (see Fig. 4, for summary). Many questions remain to be answered, namely the upstream mechanisms of the deregulation of angiogenic factors. The angiogenic imbalance can be quantified in plasma or serum by automated assays and used for clinical decision making and therapeutic monitoring in clinical trials. Administration of proangiogenic factors or removal of antiangiogenic factors is a promising approach for preeclampsia treatment. Preeclampsia is also associated with long-term health risks to both mother and child. More studies are needed to evaluate the underlying mechanisms leading to long-term cardiovascular disease in women exposed to preeclampsia.
COMPETING INTEREST STATEMENT
S.A.K has financial interest in Aggamin Therapeutics, a consultant to Siemens and has Grant funding from Thermofisher. S.A.K is a co-inventor on patents related to angiogenic biomarkers in preeclampsia that are held by the Beth Israel Deaconess Medical Center.
Footnotes
Editors: Diana W. Bianchi and Errol R. Norwitz
Additional Perspectives on Molecular Approaches to Reproductive and Newborn Medicine available at www.perspectivesinmedicine.org
REFERENCES
- Ahmed A. 2011. New insights into the etiology of preeclampsia: Identification of key elusive factors for the vascular complications. Thromb Res 127: S72–S75. [Abstract] [Google Scholar]
- Ahmad S, Ahmed A. 2004. Elevated placental soluble vascular endothelial growth factor receptor-1 inhibits angiogenesis in preeclampsia. Circ Res 95: 884–891. [Abstract] [Google Scholar]
- Ahmed A, Cudmore MJ. 2009. Can the biology of VEGF and haem oxygenases help solve pre-eclampsia? Biochem Soc Trans 37: 1237–1242. [Abstract] [Google Scholar]
- Akolekar R, Syngelaki A, Sarquis R, Zvanca M, Nicolaides KH. 2011. Prediction of early, intermediate and late pre-eclampsia from maternal factors, biophysical and biochemical markers at 11–13 weeks. Prenat Diagn 31: 66–74. [Abstract] [Google Scholar]
- Akolekar R, Syngelaki A, Poon L, Wright D, Nicolaides KH. 2013. Competing risks model in early screening for preeclampsia by biophysical and biochemical markers. Fetal Diagn Ther 33: 8–15. [Abstract] [Google Scholar]
- Ambati BK, Nozaki M, Singh N, Takeda A, Jani PD, Suthar T, Albuquerque RJ, Richter E, Sakurai E, Newcomb MT, et al. 2006. Corneal avascularity is due to soluble VEGF receptor-1. Nature 443: 993–997. [Europe PMC free article] [Abstract] [Google Scholar]
- American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy. 2013. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol 122: 1122–1131. [Abstract] [Google Scholar]
- Anton L, Merrill DC, Neves LA, Stovall K, Gallagher PE, Diz DI, Moorefield C, Gruver C, Ferrario CM, Brosnihan KB. 2008. Activation of local chorionic villi angiotensin II levels but not angiotensin (1–7) in preeclampsia. Hypertension 51: 1066–1072. [Europe PMC free article] [Abstract] [Google Scholar]
- Basso O, Rasmussen S, Weinberg CR, Wilcox AJ, Irgens LM, Skjaerven R. 2006. Trends in fetal and infant survival following preeclampsia. JAMA 296: 1357–1362. [Abstract] [Google Scholar]
- Bellamy L, Casas JP, Hingorani AD, Williams DJ. 2007. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: Systematic review and meta-analysis. BMJ 335: 974. [Europe PMC free article] [Abstract] [Google Scholar]
- Bello N, Rendon IS, Arany Z. 2013. The relationship between pre-eclampsia and peripartum cardiomyopathy: A systematic review and meta-analysis. J Am Coll Cardiol 62: 1715–1723. [Europe PMC free article] [Abstract] [Google Scholar]
- Benton SJ, Hu Y, Xie F, Kupfer K, Lee SW, Magee LA, von Dadelszen P. 2011. Angiogenic factors as diagnostic tests for preeclampsia: A performance comparison between two commercial immunoassays. Am J Obstet Gynecol 205: 469 e461–e468. [Abstract] [Google Scholar]
- Bergmann A, Ahmad S, Cudmore M, Gruber AD, Wittschen P, Lindenmaier W, Christofori G, Gross V, Gonzalves A, Grone HJ, et al. 2010. Reduction of circulating soluble Flt-1 alleviates preeclampsia-like symptoms in a mouse model. J Cell Mol Med 14: 1857–1867. [Europe PMC free article] [Abstract] [Google Scholar]
- Brosens I, Robertson WB, Dixon HG. 1967. The physiological response of the vessels of the placental bed to normal pregnancy. J Pathol Bacteriol 93: 569–579. [Abstract] [Google Scholar]
- Buhimschi IA, Nayeri UA, Zhao G, Shook LL, Pensalfini A, Funai EF, Bernstein IM, Glabe CG, Buhimschi CS. 2014. Protein misfolding, congophilia, oligomerization, and defective amyloid processing in preeclampsia. Sci Transl Med 6: 245ra292. [Abstract] [Google Scholar]
- Burton GJ, Yung HW. 2011. Endoplasmic reticulum stress in the pathogenesis of early-onset pre-eclampsia. Pregnancy Hypertens 1: 72–78. [Europe PMC free article] [Abstract] [Google Scholar]
- Burwick RM, Feinberg BB. 2013. Eculizumab for the treatment of preeclampsia/HELLP syndrome. Placenta 34: 201–203. [Abstract] [Google Scholar]
- Burwick RM, Fichorova RN, Dawood HY, Yamamoto HS, Feinberg BB. 2013. Urinary excretion of C5b-9 in severe preeclampsia: Tipping the balance of complement activation in pregnancy. Hypertension 62: 1040–1045. [Abstract] [Google Scholar]
- Buurma AJ, Penning ME, Prins F, Schutte JM, Bruijn JA, Wilhelmus S, Rajakumar A, Bloemenkamp KW, Karumanchi SA, Baelde HJ. 2013. Preeclampsia is associated with the presence of transcriptionally active placental fragments in the maternal lung. Hypertension 62: 608–613. [Abstract] [Google Scholar]
- Caniggia I, Mostachfi H, Winter J, Gassmann M, Lye SJ, Kuliszewski M, Post M. 2000. Hypoxia-inducible factor-1 mediates the biological effects of oxygen on human trophoblast differentiation through TGFβ3. J Clin Invest 105: 577–587. [Europe PMC free article] [Abstract] [Google Scholar]
- Cerdeira AS, Rajakumar A, Royle CM, Lo A, Husain Z, Thadhani RI, Sukhatme VP, Karumanchi SA, Kopcow HD. 2013. Conversion of peripheral blood NK cells to a decidual NK-like phenotype by a cocktail of defined factors. J Immunol 190: 3939–3948. [Europe PMC free article] [Abstract] [Google Scholar]
- Chaiworapongsa T, Romero R, Espinoza J, Bujold E, Mee Kim Y, Goncalves LF, Gomez R, Edwin S. 2004. Evidence supporting a role for blockade of the vascular endothelial growth factor system in the pathophysiology of preeclampsia. Am J Obstet Gynecol 190: 1541–1547; discussion 1547–1550. [Abstract] [Google Scholar]
- Chaiworapongsa T, Romero R, Kim YM, Kim GJ, Kim MR, Espinoza J, Bujold E, Goncalves L, Gomez R, Edwin S, et al. 2005. Plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated prior to the clinical diagnosis of pre-eclampsia. J Matern Fetal Neonatal Med 17: 3–18. [Abstract] [Google Scholar]
- Chaiworapongsa T, Romero R, Savasan ZA, Kusanovic JP, Ogge G, Soto E, Dong Z, Tarca A, Gaurav B, Hassan SS. 2011. Maternal plasma concentrations of angiogenic/anti-angiogenic factors are of prognostic value in patients presenting to the obstetrical triage area with the suspicion of preeclampsia. J Matern Fetal Neona 24: 1187–1207. [Europe PMC free article] [Abstract] [Google Scholar]
- Chaiworapongsa T, Chaemsaithong P, Yeo L, Romero R. 2014. Pre-eclampsia part 1: Current understanding of its pathophysiology. Nat Rev Nephrol 10: 466–480. [Abstract] [Google Scholar]
- Chambers JC, Fusi L, Malik IS, Haskard DO, De Swiet M, Kooner JS. 2001. Association of maternal endothelial dysfunction with preeclampsia. JAMA 285: 1607–1612. [Abstract] [Google Scholar]
- Chappell JC, Taylor SM, Ferrara N, Bautch VL. 2009. Local guidance of emerging vessel sprouts requires soluble Flt-1. Dev Cell 17: 377–386. [Europe PMC free article] [Abstract] [Google Scholar]
- Chappell LC, Duckworth S, Seed PT, Griffin M, Myers J, Mackillop L, Simpson N, Waugh J, Anumba D, Kenny LC, et al. 2013. Diagnostic accuracy of placental growth factor in women with suspected preeclampsia: A prospective multicenter study. Circulation 128: 2121–2131. [Abstract] [Google Scholar]
- Chen CW, Jaffe IZ, Karumanchi SA. 2014. Pre-eclampsia and cardiovascular disease. Cardiovasc Res 101: 579–586. [Europe PMC free article] [Abstract] [Google Scholar]
- Conrad KP, Shroff SG. 2011. Effects of relaxin on arterial dilation, remodeling, and mechanical properties. Curr Hypertens Rep 13: 409–420. [Abstract] [Google Scholar]
- Croy BA, Ashkar AA, Minhas K, Greenwood JD. 2000. Can murine uterine natural killer cells give insights into the pathogenesis of preeclampsia? J Soc Gynecol Investig 7: 12–20. [Abstract] [Google Scholar]
- Cudmore M, Ahmad S, Al-Ani B, Fujisawa T, Coxall H, Chudasama K, Devey LR, Wigmore SJ, Abbas A, Hewett PW, et al. 2007. Negative regulation of soluble Flt-1 and soluble endoglin release by heme oxygenase-1. Circulation 115: 1789–1797. [Abstract] [Google Scholar]
- Cui Y, Wang W, Dong N, Lou J, Srinivasan DK, Cheng W, Huang X, Liu M, Fang C, Peng J, et al. 2012. Role of corin in trophoblast invasion and uterine spiral artery remodelling in pregnancy. Nature 484: 246–250. [Europe PMC free article] [Abstract] [Google Scholar]
- Damsky CH, Fisher SJ. 1998. Trophoblast pseudo-vasculogenesis: Faking it with endothelial adhesion receptors. Curr Opin Cell Biol 10: 660–666. [Abstract] [Google Scholar]
- Duley L. 2009. The global impact of pre-eclampsia and eclampsia. Semin Perinatol 33: 130–137. [Abstract] [Google Scholar]
- Eremina V, Quaggin SE. 2004. The role of VEGF-A in glomerular development and function. Curr Opin Nephrol Hypertens 13: 9–15. [Abstract] [Google Scholar]
- Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, Ferrara N, Gerber HP, Kikkawa Y, Miner JH, Quaggin SE. 2003. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest 111: 707–716. [Europe PMC free article] [Abstract] [Google Scholar]
- Eremina V, Jefferson JA, Kowalewska J, Hochster H, Haas M, Weisstuch J, Richardson C, Kopp JB, Kabir MG, Backx PH, et al. 2008. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med 358: 1129–1136. [Europe PMC free article] [Abstract] [Google Scholar]
- Facemire CS, Nixon AB, Griffiths R, Hurwitz H, Coffman TM. 2009. Vascular endothelial growth factor receptor 2 controls blood pressure by regulating nitric oxide synthase expression. Hypertension 54: 652–658. [Europe PMC free article] [Abstract] [Google Scholar]
- Fan X, Rai A, Kambham N, Sung JF, Singh N, Petitt M, Dhal S, Agrawal R, Sutton RE, Druzin ML, et al. 2014. Endometrial VEGF induces placental sFLT1 and leads to pregnancy complications. J Clin Invest 124: 4941–4952. [Europe PMC free article] [Abstract] [Google Scholar]
- Fisher SJ. 2004. The placental problem: Linking abnormal cytotrophoblast differentiation to the maternal symptoms of preeclampsia. Reprod Biol Endocrinol 2: 53. [Europe PMC free article] [Abstract] [Google Scholar]
- Garovic VD, Wagner SJ, Petrovic LM, Gray CE, Hall P, Sugimoto H, Kalluri R, Grande JP. 2007. Glomerular expression of nephrin and synaptopodin, but not podocin, is decreased in kidney sections from women with preeclampsia. Nephrol Dial Transplant 22: 1136–1143. [Abstract] [Google Scholar]
- Gilbert JS, Babcock SA, Granger JP. 2007. Hypertension produced by reduced uterine perfusion in pregnant rats is associated with increased soluble fms-like tyrosine kinase-1 expression. Hypertension 50: 1142–1147. [Abstract] [Google Scholar]
- Gilbert JS, Gilbert SA, Arany M, Granger JP. 2009. Hypertension produced by placental ischemia in pregnant rats is associated with increased soluble endoglin expression. Hypertension 53: 399–403. [Europe PMC free article] [Abstract] [Google Scholar]
- Gilbert JS, Verzwyvelt J, Colson D, Arany M, Karumanchi SA, Granger JP. 2010. Recombinant vascular endothelial growth factor 121 infusion lowers blood pressure and improves renal function in rats with placentalischemia-induced hypertension. Hypertension 55: 380–385. [Europe PMC free article] [Abstract] [Google Scholar]
- Ginath S, Lurie S, Golan A, Amsterdam A, Sandbank J, Sadan O, Kovo M. 2014. The expression of heparanase in normal and preeclamptic placentas. J Matern Fetal Neonatal Med: 1–5. 10.3109/14767058.2014.962506. [Abstract] [CrossRef] [Google Scholar]
- Goswami D, Tannetta DS, Magee LA, Fuchisawa A, Redman CW, Sargent IL, von Dadelszen P. 2006. Excess syncytiotrophoblast microparticle shedding is a feature of early-onset pre-eclampsia, but not normotensive intrauterine growth restriction. Placenta 27: 56–61. [Abstract] [Google Scholar]
- Gu Y, Lewis DF, Wang Y. 2008. Placental productions and expressions of soluble endoglin, soluble fms-like tyrosine kinase receptor-1, and placental growth factor in normal and preeclamptic pregnancies. J Clin Endocrinol Metab 93: 260–266. [Europe PMC free article] [Abstract] [Google Scholar]
- Hagmann H, Thadhani R, Benzing T, Karumanchi SA, Stepan H. 2012. The promise of angiogenic markers for the early diagnosis and prediction of preeclampsia. Clin Chem 58: 837–845. [Abstract] [Google Scholar]
- He H, Venema VJ, Gu X, Venema RC, Marrero MB, Caldwell RB. 1999. Vascular endothelial growth factor signals endothelial cell production of nitric oxide and prostacyclin through flk-1/KDR activation of c-Src. J Biol Chem 274: 25130–25135. [Abstract] [Google Scholar]
- Herse F, Dechend R, Harsem NK, Wallukat G, Janke J, Qadri F, Hering L, Muller DN, Luft FC, Staff AC. 2007. Dysregulation of the circulating and tissue-based renin-angiotensin system in preeclampsia. Hypertension 49: 604–611. [Abstract] [Google Scholar]
- Hiby SE, Walker JJ, O’Shaughnessy KM, Redman CW, Carrington M, Trowsdale J, Moffett A. 2004. Combinations of maternal KIR and fetal HLA-C genes influence the risk of preeclampsia and reproductive success. J Exp Med 200: 957–965. [Europe PMC free article] [Abstract] [Google Scholar]
- Hiratsuka S, Minowa O, Kuno J, Noda T, Shibuya M. 1998. Flt-1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. Proc Natl Acad Sci 95: 9349–9354. [Europe PMC free article] [Abstract] [Google Scholar]
- Hladunewich MA, Steinberg G, Karumanchi SA, Levine RJ, Keating S, Kingdom J, Keunen J. 2009. Angiogenic factor abnormalities and fetal demise in a twin pregnancy. Nat Rev Nephrol 5: 658–662. [Abstract] [Google Scholar]
- Hubel CA, Snaedal S, Ness RB, Weissfeld LA, Geirsson RT, Roberts JM, Arngrimsson R. 2000. Dyslipoproteinaemia in postmenopausal women with a history of eclampsia. BJOG 107: 776–784. [Abstract] [Google Scholar]
- Huckle WR, Roche RI. 2004. Post-transcriptional control of expression of sFlt-1, an endogenous inhibitor of vascular endothelial growth factor. J Cell Biochem 93: 120–132. [Abstract] [Google Scholar]
- Huppertz B, Herrler A. 2005. Regulation of proliferation and apoptosis during development of the preimplantation embryo and the placenta. Birth Defects Res C Embryo Today 75: 249–261. [Abstract] [Google Scholar]
- Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, et al. 2004. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350: 2335–2342. [Abstract] [Google Scholar]
- Inai T, Mancuso M, Hashizume H, Baffert F, Haskell A, Baluk P, Hu-Lowe DD, Shalinsky DR, Thurston G, Yancopoulos GD, et al. 2004. Inhibition of vascular endothelial growth factor (VEGF) signaling in cancer causes loss of endothelial fenestrations, regression of tumor vessels, and appearance of basement membrane ghosts. Am J Pathol 165: 35–52. [Europe PMC free article] [Abstract] [Google Scholar]
- Irani RA, Zhang Y, Zhou CC, Blackwell SC, Hicks MJ, Ramin SM, Kellems RE, Xia Y. 2010. Autoantibody-mediated angiotensin receptor activation contributes to preeclampsia through tumor necrosis factor-α signaling. Hypertension 55: 1246–1253. [Europe PMC free article] [Abstract] [Google Scholar]
- Irgens HU, Reisaeter L, Irgens LM, Lie RT. 2001. Long term mortality of mothers and fathers after pre-eclampsia: Population based cohort study. BMJ 323: 1213–1217. [Europe PMC free article] [Abstract] [Google Scholar]
- Jayet PY, Rimoldi SF, Stuber T, Salmon CS, Hutter D, Rexhaj E, Thalmann S, Schwab M, Turini P, Sartori-Cucchia C, et al. 2010. Pulmonary and systemic vascular dysfunction in young offspring of mothers with preeclampsia. Circulation 122: 488–494. [Abstract] [Google Scholar]
- Kajantie E, Eriksson JG, Osmond C, Thornburg K, Barker DJ. 2009. Pre-eclampsia is associated with increased risk of stroke in the adult offspring: The Helsinki birth cohort study. Stroke 40: 1176–1180. [Abstract] [Google Scholar]
- Kalkunte SS, Neubeck S, Norris WE, Cheng SB, Kostadinov S, Vu Hoang D, Ahmed A, von Eggeling F, Shaikh Z, Padbury J, et al. 2013. Transthyretin is dysregulated in preeclampsia, and its native form prevents the onset of disease in a preclinical mouse model. Am J Pathol 183: 1425–1436. [Europe PMC free article] [Abstract] [Google Scholar]
- Kamba T, McDonald DM. 2007. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br J Cancer 96: 1788–1795. [Europe PMC free article] [Abstract] [Google Scholar]
- Kamba T, Tam BY, Hashizume H, Haskell A, Sennino B, Mancuso MR, Norberg SM, O’Brien SM, Davis RB, Gowen LC, et al. 2006. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol Heart Circ Physiol 290: H560–H576. [Abstract] [Google Scholar]
- Kang DH, Anderson S, Kim YG, Mazzalli M, Suga S, Jefferson JA, Gordon KL, Oyama TT, Hughes J, Hugo C, et al. 2001a. Impaired angiogenesis in the aging kidney: Vascular endothelial growth factor and thrombospondin-1 in renal disease. Am J Kidney Dis 7: 601–611. [Abstract] [Google Scholar]
- Kang DH, Hughes J, Mazzali M, Schreiner GF, Johnson RJ. 2001b. Impaired angiogenesis in the remnant kidney model. II: Vascular endothelial growth factor administration reduces renal fibrosis and stabilizes renal function. J Am Soc Nephrol 12: 1448–1457. [Abstract] [Google Scholar]
- Karumanchi SA, Maynard SE, Stillman IE, Epstein FH, Sukhatme VP. 2005. Preeclampsia: A renal perspective. Kidney Int 67: 2101–2113. [Abstract] [Google Scholar]
- Kendall RL, Thomas KA. 1993. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci 90: 10705–10709. [Europe PMC free article] [Abstract] [Google Scholar]
- Khong TY, De Wolf F, Robertson WB, Brosens I. 1986. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol 93: 1049–1059. [Abstract] [Google Scholar]
- Knudsen UB, Kronborg CS, von Dadelszen P, Kupfer K, Lee S-W, Vittinghus E, Allen JG, Redman CW. 2012. A single rapid point-of-care placental growth factor determination as an aid in the diagnosis of preeclampsia. Pregnancy Hypertension: An International Journal of Women’s Cardiovascular Health 2: 8–15. [Abstract] [Google Scholar]
- Koopman LA, Kopcow HD, Rybalov B, Boyson JE, Orange JS, Schatz F, Masch R, Lockwood CJ, Schachter AD, Park PJ, et al. 2003. Human decidual natural killer cells are a unique NK cell subset with immunomodulatory potential. J Exp Med 198: 1201–1212. [Europe PMC free article] [Abstract] [Google Scholar]
- Kumasawa K, Ikawa M, Kidoya H, Hasuwa H, Saito-Fujita T, Morioka Y, Takakura N, Kimura T, Okabe M. 2011. Pravastatin induces placental growth factor (PGF) and ameliorates preeclampsia in a mouse model. Proc Natl Acad Sci 108: 1451–1455. [Europe PMC free article] [Abstract] [Google Scholar]
- Kusanovic JP, Romero R, Chaiworapongsa T, Erez O, Mittal P, Vaisbuch E, Mazaki-Tovi S, Gotsch F, Edwin SS, Gomez R, et al. 2009. A prospective cohort study of the value of maternal plasma concentrations of angiogenic and anti-angiogenic factors in early pregnancy and midtrimester in the identification of patients destined to develop preeclampsia. J Matern Fetal Neona 22: 1021–1038. [Europe PMC free article] [Abstract] [Google Scholar]
- Lam C, Lim KH, Karumanchi SA. 2005. Circulating angiogenic factors in the pathogenesis and prediction of preeclampsia. Hypertension 46: 1077–1085. [Abstract] [Google Scholar]
- Lapaire O, Holzgreve W, Oosterwijk JC, Brinkhaus R, Bianchi DW. 2007. Georg Schmorl on trophoblasts in the maternal circulation. Placenta 28: 1–5. [Abstract] [Google Scholar]
- Le Cras TD, Markham NE, Tuder RM, Voelkel NF, Abman SH. 2002. Treatment of newborn rats with a VEGF receptor inhibitor causes pulmonary hypertension and abnormal lung structure. Am J Physiol Lung Cell Mol Physiol 283: L555–L562. [Abstract] [Google Scholar]
- Levine RJ, Lindheimer MD. 2009. First-trimester prediction of early preeclampsia: A possibility at last! Hypertension 53: 747–748. [Europe PMC free article] [Abstract] [Google Scholar]
- Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, et al. 2004. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 350: 672–683. [Abstract] [Google Scholar]
- Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, Sibai BM, Epstein FH, Romero R, Thadhani R, et al. 2006a. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med 355: 992–1005. [Abstract] [Google Scholar]
- Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, Sibai BM, Epstein FH, Romero R, Thadhani R, et al. 2006b. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med 355: 992–1005. [Abstract] [Google Scholar]
- Levine RJ, Vatten LJ, Horowitz GL, Qian C, Romundstad PR, Yu KF, Hollenberg AN, Hellevik AI, Asvold BO, Karumanchi SA. 2009. Pre-eclampsia, soluble fms-like tyrosine kinase 1, and the risk of reduced thyroid function: Nested case-control and population based study. BMJ 339: b4336. [Abstract] [Google Scholar]
- Levytska K, Kingdom J, Baczyk D, Drewlo S. 2013. Heme oxygenase-1 in placental development and pathology. Placenta 34: 291–298. [Abstract] [Google Scholar]
- Li Z, Zhang Y, Ying Ma J, Kapoun AM, Shao Q, Kerr I, Lam A, O’Young G, Sannajust F, Stathis P, et al. 2007. Recombinant vascular endothelial growth factor 121 attenuates hypertension and improves kidney damage in a rat model of preeclampsia. Hypertension 50: 686–692. [Abstract] [Google Scholar]
- Llurba E, Marsal G, Sanchez O, Dominguez C, Alijotas-Reig J, Carreras E, Cabero L. 2012. Angiogenic and antiangiogenic factors before and after resolution of maternal mirror syndrome. Ultrasound Obstet Gynecol 40: 367–369. [Abstract] [Google Scholar]
- Lu F, Longo M, Tamayo E, Maner W, Al-Hendy A, Anderson GD, Hankins GD, Saade GR. 2007. The effect of over-expression of sFlt-1 on blood pressure and the occurrence of other manifestations of preeclampsia in unrestrained conscious pregnant mice. Am J Obstet Gynecol 196: 396 e391–e397; discussion 396 e397. [Abstract] [Google Scholar]
- Lykke JA, Langhoff-Roos J, Sibai BM, Funai EF, Triche EW, Paidas MJ. 2009. Hypertensive pregnancy disorders and subsequent cardiovascular morbidity and type 2 diabetes mellitus in the mother. Hypertension 53: 944–951. [Abstract] [Google Scholar]
- Maharaj AS, Walshe TE, Saint-Geniez M, Venkatesha S, Maldonado AE, Himes NC, Matharu KS, Karumanchi SA, D’Amore PA. 2008. VEGF and TGF-β are required for the maintenance of the choroid plexus and ependyma. J Exp Med 205: 491–501. [Europe PMC free article] [Abstract] [Google Scholar]
- Mancuso MR, Davis R, Norberg SM, O’Brien S, Sennino B, Nakahara T, Yao VJ, Inai T, Brooks P, Freimark B, et al. 2006. Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J Clin Invest 116: 2610–2621. [Europe PMC free article] [Abstract] [Google Scholar]
- Mannisto T, Karumanchi SA, Pouta A, Vaarasmaki M, Mendola P, Miettola S, Surcel HM, Bloigu A, Ruokonen A, Jarvelin MR, et al. 2013. Preeclampsia, gestational hypertension and subsequent hypothyroidism. Pregnancy Hypertens 3: 21–27. [Europe PMC free article] [Abstract] [Google Scholar]
- Mano Y, Kotani T, Shibata K, Matsumura H, Tsuda H, Sumigama S, Yamamoto E, Iwase A, Senga T, Kikkawa F. 2011. The loss of endoglin promotes the invasion of extravillous trophoblasts. Endocrinology 152: 4386–4394. [Abstract] [Google Scholar]
- Mattot V, Moons L, Lupu F, Chernavvsky D, Gomez RA, Collen D, Carmeliet P. 2002. Loss of the VEGF(164) and VEGF(188) isoforms impairs postnatal glomerular angiogenesis and renal arteriogenesis in mice. J Am Soc Nephrol 13: 1548–1560. [Abstract] [Google Scholar]
- Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, et al. 2003. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 111: 649–658. [Europe PMC free article] [Abstract] [Google Scholar]
- Maynard SE, Venkatesha S, Thadhani R, Karumanchi SA. 2005. Soluble fms-like tyrosine kinase 1 and endothelial dysfunction in the pathogenesis of preeclampsia. Pediatr Res 57: 1R–7R. [Abstract] [Google Scholar]
- McDonald SD, Han Z, Walsh MW, Gerstein HC, Devereaux PJ. 2010. Kidney disease after preeclampsia: A systematic review and meta-analysis. Am J Kidney Dis 55: 1026–1039. [Abstract] [Google Scholar]
- McGuane JT, Danielson LA, Debrah JE, Rubin JP, Novak J, Conrad KP. 2011. Angiogenic growth factors are new and essential players in the sustained relaxin vasodilatory pathway in rodents and humans. Hypertension 57: 1151–1160. [Europe PMC free article] [Abstract] [Google Scholar]
- Moore AG, Young H, Keller JM, Ojo LR, Yan J, Simas TA, Maynard SE. 2012. Angiogenic biomarkers for prediction of maternal and neonatal complications in suspected preeclampsia. J Matern Fetal Neona 25: 2651–2657. [Abstract] [Google Scholar]
- Nishimoto F, Sakata M, Minekawa R, Okamoto Y, Miyake A, Isobe A, Yamamoto T, Takeda T, Ishida E, Sawada K, et al. 2009. Metal transcription factor-1 is involved in hypoxia-dependent regulation of placenta growth factor in trophoblast-derived cells. Endocrinology 150: 1801–1808. [Abstract] [Google Scholar]
- Noori M, Donald AE, Angelakopoulou A, Hingorani AD, Williams DJ. 2010. Prospective study of placental angiogenic factors and maternal vascular function before and after preeclampsia and gestational hypertension. Circulation 122: 478–487. [Abstract] [Google Scholar]
- Ohkuchi A, Hirashima C, Suzuki H, Takahashi K, Yoshida M, Matsubara S, Suzuki M. 2010. Evaluation of a new and automated electrochemiluminescence immunoassay for plasma sFlt-1 and PlGF levels in women with preeclampsia. Hypertens Res 33: 422–427. [Abstract] [Google Scholar]
- Park M, Lee ST. 1999. The fourth immunoglobulin-like loop in the extracellular domain of FLT-1, a VEGF receptor, includes a major heparin-binding site. Biochem Biophys Res Commun 264: 730–734. [Abstract] [Google Scholar]
- Parrish MR, Murphy SR, Rutland S, Wallace K, Wenzel K, Wallukat G, Keiser S, Ray LF, Dechend R, Martin JN, et al. 2010. The effect of immune factors, tumor necrosis factor-α, and agonistic autoantibodies to the angiotensin II type I receptor on soluble fms-like tyrosine-1 and soluble endoglin production in response to hypertension during pregnancy. Am J Hypertens 23: 911–916. [Europe PMC free article] [Abstract] [Google Scholar]
- Patel TV, Morgan JA, Demetri GD, George S, Maki RG, Quigley M, Humphreys BD. 2008. A preeclampsia-like syndrome characterized by reversible hypertension and proteinuria induced by the multitargeted kinase inhibitors sunitinib and sorafenib. J Natl Cancer Inst 100: 282–284. [Abstract] [Google Scholar]
- Patten IS, Rana S, Shahul S, Rowe GC, Jang C, Liu L, Hacker MR, Rhee JS, Mitchell J, Mahmood F, et al. 2012. Cardiac angiogenic imbalance leads to peripartum cardiomyopathy. Nature 485: 333–338. [Europe PMC free article] [Abstract] [Google Scholar]
- Perni U, Sison C, Sharma V, Helseth G, Hawfield A, Suthanthiran M, August P. 2012. Angiogenic factors in superimposed preeclampsia: A longitudinal study of women with chronic hypertension during pregnancy. Hypertension 59: 740–746. [Abstract] [Google Scholar]
- Pierce EA, Avery RL, Foley ED, Aiello LP, Smith LE. 1995. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci 92: 905–909. [Europe PMC free article] [Abstract] [Google Scholar]
- Polliotti BM, Fry AG, Saller DN, Mooney RA, Cox C, Miller RK. 2003. Second-trimester maternal serum placental growth factor and vascular endothelial growth factor for predicting severe, early-onset preeclampsia. Obstet Gynecol 101: 1266–1274. [Abstract] [Google Scholar]
- Poon LC, Kametas NA, Maiz N, Akolekar R, Nicolaides KH. 2009. First-trimester prediction of hypertensive disorders in pregnancy. Hypertension 53: 812–818. [Abstract] [Google Scholar]
- Powe CE, Levine RJ, Karumanchi SA. 2011. Preeclampsia, a disease of the maternal endothelium: The role of antiangiogenic factors and implications for later cardiovascular disease. Circulation 123: 2856–2869. [Europe PMC free article] [Abstract] [Google Scholar]
- Rahimi N, Golde TE, Meyer RD. 2009. Identification of ligand-induced proteolytic cleavage and ectodomain shedding of VEGFR-1/FLT1 in leukemic cancer cells. Cancer Res 69: 2607–2614. [Europe PMC free article] [Abstract] [Google Scholar]
- Rajakumar A, Michael HM, Rajakumar PA, Shibata E, Hubel CA, Karumanchi SA, Thadhani R, Wolf M, Harger G, Markovic N. 2005. Extra-placental expression of vascular endothelial growth factor receptor-1, (Flt-1) and soluble Flt-1 (sFlt-1), by peripheral blood mononuclear cells (PBMCs) in normotensive and preeclamptic pregnant women. Placenta 26: 563–573. [Abstract] [Google Scholar]
- Rajakumar A, Cerdeira AS, Rana S, Zsengeller Z, Edmunds L, Jeyabalan A, Hubel CA, Stillman IE, Parikh SM, Karumanchi SA. 2012. Transcriptionally active syncytial aggregates in the maternal circulation may contribute to circulating soluble fms-like tyrosine kinase 1 in preeclampsia. Hypertension 59: 256–264. [Europe PMC free article] [Abstract] [Google Scholar]
- Ramma W, Ahmed A. 2014. Therapeutic potential of statins and the induction of heme oxygenase-1 in preeclampsia. J Reprod Immunol 101–102: 153–160. [Europe PMC free article] [Abstract] [Google Scholar]
- Rana S, Cerdeira AS, Wenger J, Salahuddin S, Lim KH, Ralston SJ, Thadhani RI, Karumanchi SA. 2012. Plasma concentrations of soluble endoglin versus standard evaluation in patients with suspected preeclampsia. PLoS ONE 7: e48259. [Europe PMC free article] [Abstract] [Google Scholar]
- Rana S, Schnettler WT, Powe C, Wenger J, Salahuddin S, Cerdeira AS, Verlohren S, Perschel FH, Arany Z, Lim KH, et al. 2013. Clinical characterization and outcomes of preeclampsia with normal angiogenic profile. Hypertens Pregnancy 32: 189–201. [Europe PMC free article] [Abstract] [Google Scholar]
- Rana S, Rajakumar A, Geahchan C, Salahuddin S, Cerdeira AS, Burke SD, George EM, Granger JP, Karumanchi SA. 2014. Ouabain inhibits placental sFlt1 production by repressing HSP27-dependent HIF-1α pathway. FASEB J 28: 4324–4334. [Europe PMC free article] [Abstract] [Google Scholar]
- Ray JG, Vermeulen MJ, Schull MJ, Redelmeier DA. 2005. Cardiovascular health after maternal placental syndromes (CHAMPS): Population-based retrospective cohort study. Lancet 366: 1797–1803. [Abstract] [Google Scholar]
- Redman CW. 2008. The endoplasmic reticulum stress of placental impoverishment. Am J Pathol 173: 311–314. [Europe PMC free article] [Abstract] [Google Scholar]
- Redman CW, Sargent IL. 2000. Placental debris, oxidative stress and pre-eclampsia. Placenta 21: 597–602. [Abstract] [Google Scholar]
- Redman CW, Tannetta DS, Dragovic RA, Gardiner C, Southcombe JH, Collett GP, Sargent IL. 2012. Review: Does size matter? Placental debris and the pathophysiology of pre-eclampsia. Placenta 33: S48–S54. [Abstract] [Google Scholar]
- Robbins SG, Conaway JR, Ford BL, Roberto KA, Penn JS. 1997. Detection of vascular endothelial growth factor (VEGF) protein in vascular and non-vascular cells of the normal and oxygen-injured rat retina. Growth Factors 14: 229–241. [Abstract] [Google Scholar]
- Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, McLaughlin MK. 1989. Preeclampsia: An endothelial cell disorder. Am J Obstet Gynecol 161: 1200–1204. [Abstract] [Google Scholar]
- Robillard PY, Dekker G, Chaouat G, Hulsey TC, Saftlas A. 2011. Epidemiological studies on primipaternity and immunology in preeclampsia—A statement after twelve years of workshops. J Reprod Immunol 89: 104–117. [Abstract] [Google Scholar]
- Robson A, Harris LK, Innes BA, Lash GE, Aljunaidy MM, Aplin JD, Baker PN, Robson SC, Bulmer JN. 2012. Uterine natural killer cells initiate spiral artery remodeling in human pregnancy. FASEB J 26: 4876–4885. [Abstract] [Google Scholar]
- Rolfo A, Attini R, Nuzzo AM, Piazzese A, Parisi S, Ferraresi M, Todros T, Piccoli GB. 2012. Chronic kidney disease may be differentially diagnosed from preeclampsia by serum biomarkers. Kidney Int 83: 177–181. [Abstract] [Google Scholar]
- Romero R, Chaiworapongsa T. 2013. Preeclampsia: A link between trophoblast dysregulation and an antiangiogenic state. J Clin Invest 123: 2775–2777. [Europe PMC free article] [Abstract] [Google Scholar]
- Saad AF, Kechichian T, Yin H, Sbrana E, Longo M, Wen M, Tamayo E, Hankins GD, Saade GR, Costantine MM. 2014. Effects of pravastatin on angiogenic and placental hypoxic imbalance in a mouse model of preeclampsia. Reprod Sci 21: 138–145. [Abstract] [Google Scholar]
- Salahuddin S, Lee Y, Vadnais M, Sachs BP, Karumanchi SA, Lim KH. 2007. Diagnostic utility of soluble fms-like tyrosine kinase 1 and soluble endoglin in hypertensive diseases of pregnancy. Am J Obstet Gynecol 197: 28 e21–e26. [Abstract] [Google Scholar]
- Sattar N, Ramsay J, Crawford L, Cheyne H, Greer IA. 2003. Classic and novel risk factor parameters in women with a history of preeclampsia. Hypertension 42: 39–42. [Abstract] [Google Scholar]
- Saxena AR, Karumanchi SA, Brown NJ, Royle CM, McElrath TF, Seely EW. 2010. Increased sensitivity to angiotensin II is present postpartum in women with a history of hypertensive pregnancy. Hypertension 55: 1239–1245. [Europe PMC free article] [Abstract] [Google Scholar]
- Schiettecatte J, Russcher H, Anckaert E, Mees M, Leeser B, Tirelli AS, Fiedler GM, Luthe H, Denk B, Smitz J. 2010. Multicenter evaluation of the first automated Elecsys sFlt-1 and PlGF assays in normal pregnancies and preeclampsia. Clin Biochem 43: 768–770. [Abstract] [Google Scholar]
- Seidman DS, Laor A, Gale R, Stevenson DK, Mashiach S, Danon YL. 1991. Pre-eclampsia and offspring’s blood pressure, cognitive ability and physical development at 17-years-of-age. Br J Obstet Gynaecol 98: 1009–1014. [Abstract] [Google Scholar]
- Sela S, Itin A, Natanson-Yaron S, Greenfield C, Goldman-Wohl D, Yagel S, Keshet E. 2008. A novel human-specific soluble vascular endothelial growth factor receptor 1: Cell-type-specific splicing and implications to vascular endothelial growth factor homeostasis and preeclampsia. Circ Res 102: 1566–1574. [Abstract] [Google Scholar]
- Sela S, Natanson-Yaron S, Zcharia E, Vlodavsky I, Yagel S, Keshet E. 2011. Local retention versus systemic release of soluble VEGF receptor-1 are mediated by heparin-binding and regulated by heparanase. Circ Res 108: 1063–1070. [Abstract] [Google Scholar]
- Siddiqui AH, Irani RA, Blackwell SC, Ramin SM, Kellems RE, Xia Y. 2010. Angiotensin receptor agonistic autoantibody is highly prevalent in preeclampsia: Correlation with disease severity. Hypertension 55: 386–393. [Europe PMC free article] [Abstract] [Google Scholar]
- Sitohy B, Nagy JA, Jaminet SC, Dvorak HF. 2011. Tumor-surrogate blood vessel subtypes exhibit differential susceptibility to anti-VEGF therapy. Cancer Res 71: 7021–7028. [Europe PMC free article] [Abstract] [Google Scholar]
- Smith GC, Pell JP, Walsh D. 2001. Pregnancy complications and maternal risk of ischaemic heart disease: A retrospective cohort study of 129,290 births. Lancet 357: 2002–2006. [Abstract] [Google Scholar]
- Stepan H, Faber R. 2006. Elevated sFlt1 level and preeclampsia with parvovirus-induced hydrops. N Engl J Med 354: 1857–1858. [Abstract] [Google Scholar]
- Sugimoto H, Hamano Y, Charytan D, Cosgrove D, Kieran M, Sudhakar A, Kalluri R. 2003. Neutralization of circulating vascular endothelial growth factor (VEGF) by anti-VEGF antibodies and soluble VEGF receptor 1 (sFlt-1) induces proteinuria. J Biol Chem 278: 12605–12608. [Abstract] [Google Scholar]
- Sunderji S, Gaziano E, Wothe D, Rogers LC, Sibai B, Karumanchi SA, Hodges-Savola C. 2010. Automated assays for sVEGF R1 and PlGF as an aid in the diagnosis of preterm preeclampsia: A prospective clinical study. Am J Obstet Gynecol 202: 40 e41–e47. [Abstract] [Google Scholar]
- Tang JR, Markham NE, Lin YJ, McMurtry IF, Maxey A, Kinsella JP, Abman SH. 2004. Inhaled nitric oxide attenuates pulmonary hypertension and improves lung growth in infant rats after neonatal treatment with a VEGF receptor inhibitor. Am J Physiol Lung Cell Mol Physiol 287: L344–L351. [Abstract] [Google Scholar]
- Tang JR, Karumanchi SA, Seedorf G, Markham N, Abman SH. 2012. Excess soluble vascular endothelial growth factor receptor-1 in amniotic fluid impairs lung growth in rats: Linking preeclampsia with bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 302: L36–L46. [Europe PMC free article] [Abstract] [Google Scholar]
- Tannetta DS, Dragovic RA, Gardiner C, Redman CW, Sargent IL. 2013. Characterisation of syncytiotrophoblast vesicles in normal pregnancy and pre-eclampsia: Expression of Flt-1 and endoglin. PLoS ONE 8: e56754. [Europe PMC free article] [Abstract] [Google Scholar]
- Thadhani R, Kisner T, Hagmann H, Bossung V, Noack S, Schaarschmidt W, Jank A, Kribs A, Cornely OA, Kreyssig C, et al. 2011. Pilot study of extracorporeal removal of soluble fms-like tyrosine kinase 1 in preeclampsia. Circulation 124: 940–950. [Abstract] [Google Scholar]
- Thomas CP, Andrews JI, Liu KZ. 2007. Intronic polyadenylation signal sequences and alternate splicing generate human soluble Flt1 variants and regulate the abundance of soluble Flt1 in the placenta. FASEB J 21: 3885–3895. [Abstract] [Google Scholar]
- Tissot van Patot MC, Bendrick-Peart J, Beckey VE, Serkova N, Zwerdlinger L. 2004. Greater vascularity, lowered HIF-1/DNA binding, and elevated GSH as markers of adaptation to in vivo chronic hypoxia. Am J Physiol Lung Cell Mol Physiol 287: L525–L532. [Abstract] [Google Scholar]
- Unemori E, Sibai B, Teichman SL. 2009. Scientific rationale and design of a phase I safety study of relaxin in women with severe preeclampsia. Ann NY Acad Sci 1160: 381–384. [Abstract] [Google Scholar]
- Vatten LJ, Romundstad PR, Holmen TL, Hsieh CC, Trichopoulos D, Stuver SO. 2003. Intrauterine exposure to preeclampsia and adolescent blood pressure, body size, and age at menarche in female offspring. Obstet Gynecol 101: 529–533. [Abstract] [Google Scholar]
- Veas CJ, Aguilera VC, Munoz IJ, Gallardo VI, Miguel PL, Gonzalez MA, Lamperti LI, Escudero CA, Aguayo CR. 2011. Fetal endothelium dysfunction is associated with circulating maternal levels of sE-selectin, sVCAM1, and sFlt-1 during pre-eclampsia. J Matern Fetal Neonatal Med 24: 1371–1377. [Abstract] [Google Scholar]
- Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, Bdolah Y, Lim KH, Yuan HT, Libermann TA, et al. 2006. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med 12: 642–649. [Abstract] [Google Scholar]
- Verdonk K, Visser W, Russcher H, Danser AH, Steegers EA, van den Meiracker AH. 2012. Differential diagnosis of preeclampsia: Remember the soluble fms-like tyrosine kinase 1/placental growth factor ratio. Hypertension 60: 884–890. [Abstract] [Google Scholar]
- Verlohren S, Galindo A, Schlembach D, Zeisler H, Herraiz I, Moertl MG, Pape J, Dudenhausen JW, Denk B, Stepan H. 2010. An automated method for the determination of the sFlt-1/PlGF ratio in the assessment of preeclampsia. Am J Obstet Gynecol 202: 161 e161–161 e111. [Abstract] [Google Scholar]
- Verlohren S, Herraiz I, Lapaire O, Schlembach D, Moertl M, Zeisler H, Calda P, Holzgreve W, Galindo A, Engels T, et al. 2012. The sFlt-1/PlGF ratio in different types of hypertensive pregnancy disorders and its prognostic potential in preeclamptic patients. Am J Obstet Gynecol 206: 58 e51–e58. [Abstract] [Google Scholar]
- Veron D, Villegas G, Aggarwal PK, Bertuccio C, Jimenez J, Velazquez H, Reidy K, Abrahamson DR, Moeckel G, Kashgarian M, et al. 2012. Acute podocyte vascular endothelial growth factor (VEGF-A) knockdown disrupts αVβ3 integrin signaling in the glomerulus. PLoS ONE 7: e40589. [Europe PMC free article] [Abstract] [Google Scholar]
- Vigneau C, Lorcy N, Dolley-Hitze T, Jouan F, Arlot-Bonnemains Y, Laguerre B, Verhoest G, Goujon JM, Belaud-Rotureau MA, Rioux-Leclercq N. 2014. All anti-vascular endothelial growth factor drugs can induce ‘pre-eclampsia-like syndrome’: A RARe study. Nephrol Dial Transplant 29: 325–332. [Abstract] [Google Scholar]
- Vikse BE, Irgens LM, Leivestad T, Skjaerven R, Iversen BM. 2008. Preeclampsia and the risk of end-stage renal disease. N Engl J Med 359: 800–809. [Abstract] [Google Scholar]
- Vuorela P, Helske S, Hornig C, Alitalo K, Weich H, Halmesmaki E. 2000. Amniotic fluid--soluble vascular endothelial growth factor receptor-1 in preeclampsia. Obstet Gynecol 95: 353–357. [Abstract] [Google Scholar]
- Wang CN, Chang SD, Peng HH, Lee YS, Chang YL, Cheng PJ, Chao AS, Wang TH, Wang HS. 2010. Change in amniotic fluid levels of multiple anti-angiogenic proteins before development of preeclampsia and intrauterine growth restriction. J Clin Endocrinol Metab 95: 1431–1441. [Abstract] [Google Scholar]
- Wang IK, Muo CH, Chang YC, Liang CC, Chang CT, Lin SY, Yen TH, Chuang FR, Chen PC, Huang CC, et al. 2013a. Association between hypertensive disorders during pregnancy and end-stage renal disease: A population-based study. CMAJ 185: 207–213. [Europe PMC free article] [Abstract] [Google Scholar]
- Wang IK, Muo CH, Chang YC, Liang JC, Chang CT, Lin SY, Yen TH, Chuang FR, Chen PC, Huang CC, et al. 2013b. Association between hypertensive disorders during pregnancy and end-stage renal disease: A population-based study. CMAJ 185: 207–213. [Europe PMC free article] [Abstract] [Google Scholar]
- Wilson BJ, Watson MS, Prescott GJ, Sunderland S, Campbell DM, Hannaford P, Smith WC. 2003. Hypertensive diseases of pregnancy and risk of hypertension and stroke in later life: Results from cohort study. BMJ 326: 845. [Europe PMC free article] [Abstract] [Google Scholar]
- Wolf M, Hubel CA, Lam C, Sampson M, Ecker JL, Ness RB, Rajakumar A, Daftary A, Shakir AS, Seely EW, et al. 2004. Preeclampsia and future cardiovascular disease: Potential role of altered angiogenesis and insulin resistance. J Clin Endocrinol Metab 89: 6239–6243. [Abstract] [Google Scholar]
- Xinaris C, Benedetti V, Rizzo P, Abbate M, Corna D, Azzollini N, Conti S, Unbekandt M, Davies JA, Morigi M, et al. 2012. In vivo maturation of functional renal organoids formed from embryonic cell suspensions. J Am Soc Nephrol 23: 1857–1868. [Europe PMC free article] [Abstract] [Google Scholar]
- Yu XD, Branch DW, Karumanchi SA, Zhang J. 2012. Preeclampsia and retinopathy of prematurity in preterm births. Pediatrics 130: e101–e107. [Abstract] [Google Scholar]
- Yuan HT, Haig D, Ananth Karumanchi S. 2005. Angiogenic factors in the pathogenesis of preeclampsia. Curr Top Dev Biol 71: 297–312. [Abstract] [Google Scholar]
- Zhao S, Gu Y, Fan R, Groome LJ, Cooper D, Wang Y. 2010. Proteases and sFlt-1 release in the human placenta. Placenta 31: 512–518. [Europe PMC free article] [Abstract] [Google Scholar]
- Zhou Y, McMaster M, Woo K, Janatpour M, Perry J, Karpanen T, Alitalo K, Damsky C, Fisher SJ. 2002. Vascular endothelial growth factor ligands and receptors that regulate human cytotrophoblast survival are dysregulated in severe preeclampsia and hemolysis, elevated liver enzymes, and low platelets syndrome. Am J Pathol 160: 1405–1423. [Europe PMC free article] [Abstract] [Google Scholar]
- Zhou CC, Zhang Y, Irani RA, Zhang H, Mi T, Popek EJ, Hicks MJ, Ramin SM, Kellems RE, Xia Y. 2008. Angiotensin receptor agonistic autoantibodies induce pre-eclampsia in pregnant mice. Nat Med 14: 855–862. [Europe PMC free article] [Abstract] [Google Scholar]
- Zhou Y, Gormley MJ, Hunkapiller NM, Kapidzic M, Stolyarov Y, Feng V, Nishida M, Drake PM, Bianco K, Wang F, et al. 2013. Reversal of gene dysregulation in cultured cytotrophoblasts reveals possible causes of preeclampsia. J Clin Invest 123: 2862–2872. [Europe PMC free article] [Abstract] [Google Scholar]
Articles from Cold Spring Harbor Perspectives in Medicine are provided here courtesy of Cold Spring Harbor Laboratory Press
Full text links
Read article at publisher's site: https://doi.org/10.1101/cshperspect.a023473
Read article for free, from open access legal sources, via Unpaywall:
http://perspectivesinmedicine.cshlp.org/content/5/10/a023473.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1101/cshperspect.a023473
Article citations
Impacts of low birthweight on kidney development and intergenerational growth of the offspring.
iScience, 27(11):111159, 12 Oct 2024
Cited by: 0 articles | PMID: 39524353
Behind the Curtain of Abnormal Placentation in Pre-Eclampsia: From Molecular Mechanisms to Histological Hallmarks.
Int J Mol Sci, 25(14):7886, 18 Jul 2024
Cited by: 0 articles | PMID: 39063129 | PMCID: PMC11277090
Review Free full text in Europe PMC
MIR193BHG inhibits the proliferation, migration and invasion of trophoblasts by upregulating p53.
Exp Ther Med, 28(2):320, 17 Jun 2024
Cited by: 0 articles | PMID: 38939173
Downregulated PDIA3P1 lncRNA Impairs Trophoblast Phenotype by Regulating Snail and SFRP1 in PE.
Anal Cell Pathol (Amst), 2024:8972022, 27 Apr 2024
Cited by: 0 articles | PMID: 38715918 | PMCID: PMC11074859
Association Between Hypertensive Disorders of Pregnancy and Interval Neurocognitive Decline: An Analysis of the Hispanic Community Health Study/Study of Latinos.
Obstet Gynecol, 143(6):785-793, 05 Apr 2024
Cited by: 0 articles | PMID: 38574370
Go to all (82) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Angiogenic Imbalance as a Contributor of Preeclampsia.
Curr Pharm Biotechnol, 19(10):797-815, 01 Jan 2018
Cited by: 13 articles | PMID: 30255753
Review
Biology of preeclampsia: Combined actions of angiogenic factors, their receptors and placental proteins.
Biochim Biophys Acta Mol Basis Dis, 1866(2):165349, 13 Dec 2018
Cited by: 13 articles | PMID: 30553017
Molecular mechanisms of preeclampsia.
Microvasc Res, 75(1):1-8, 06 May 2007
Cited by: 160 articles | PMID: 17553534 | PMCID: PMC2241748
Review Free full text in Europe PMC
Elevated placental adenosine signaling contributes to the pathogenesis of preeclampsia.
Circulation, 131(8):730-741, 23 Dec 2014
Cited by: 44 articles | PMID: 25538227 | PMCID: PMC4751998
Funding
Funders who supported this work.
National Institute for Health Research (NIHR) (1)
Grant ID: ACF-2012-13-018(1)