Abstract
Objective
NOD-like receptors (NLRs) sense sterile and non-sterile signals and form inflammasomes which trigger an inflammatory response through the activation of caspase-1 and release of IL-1β. Recently we have shown the presence of several NLRs in the bladder urothelia and demonstrated the importance of NLRP3 in bladder outlet obstruction and cyclophosphamide-induced cystitis, both models of sterile inflammation. In this study we explore a role for NLRP3 in mediating the response to LPS, a key antigen of uropathogenic bacteria.Method
In order to bypass the protective glycosaminoglycan layer lining the urothelium, LPS was directly injected into the bladder wall of Sprague-Dawley rats. Glyburide (a NLRP3 inhibitor) or vehicle was administered orally prior to and after injection. Rats were analyzed 24 h later. Inflammasome activity (caspase-1 activity, IL-1β release) and inflammation (Evan's Blue extravasation, bladder weight) were assessed, as was physiological bladder function (urodynamics).Results
Injection of LPS stimulated inflammasome activation (caspase-1 activity) and the release of IL-1β into the urine which was prevented by glyburide. Likewise, LPS increased inflammation, (bladder weight and the extravasation of Evan's blue dye), and this was reversed by glyburide. Functionally, animals injected with saline alone demonstrated decreased voiding volume as measured by urodynamics. In the presence of LPS, additional urinary dysfunction was evident with decreased voiding pressures and threshold pressures. The decrease in voiding pressure was blocked by glyburide but the decrease in threshold pressure was not, suggesting that LPS has significant effects mediated by inflammasome-dependent and -independent mechanisms.Conclusion
Overall, the results demonstrate the potential importance of inflammasomes in bacterial cystitis as well as the ability of the bladder wall injection technique to isolate the in vivo effects of specific inflammasome ligands to the physiological changes associated with cystitis.Free full text

The NACHT, LRR and PYD Domains-Containing Protein 3 (NLRP3) Inflammasome Mediates Inflammation and Voiding Dysfunction in a Lipopolysaccharide-Induced Rat Model of Cystitis
Abstract
Objective
NOD-like receptors (NLRs) sense sterile and non-sterile signals and form inflammasomes which trigger an inflammatory response through the activation of caspase-1 and release of IL-1β. Recently we have shown the presence of several NLRs in the bladder urothelia and demonstrated the importance of NLRP3 in bladder outlet obstruction and cyclophosphamide-induced cystitis, both models of sterile inflammation. In this study we explore a role for NLRP3 in mediating the response to LPS, a key antigen of uropathogenic bacteria.
Method
In order to bypass the protective glycosaminoglycan layer lining the urothelium, LPS was directly injected into the bladder wall of Sprague-Dawley rats. Glyburide (a NLRP3 inhibitor) or vehicle was administered orally prior to and after injection. Rats were analyzed 24 h later. Inflammasome activity (caspase-1 activity, IL-1β release) and inflammation (Evan’s Blue extravasation, bladder weight) were assessed, as was physiological bladder function (urodynamics).
Results
Injection of LPS stimulated inflammasome activation (caspase-1 activity) and the release of IL-1β into the urine which was prevented by glyburide. Likewise, LPS increased inflammation, (bladder weight and the extravasation of Evan’s blue dye), and this was reversed by glyburide. Functionally, animals injected with saline alone demonstrated decreased voiding volume as measured by urodynamics. In the presence of LPS, additional urinary dysfunction was evident with decreased voiding pressures and threshold pressures. The decrease in voiding pressure was blocked by glyburide but the decrease in threshold pressure was not, suggesting that LPS has significant effects mediated by inflammasome-dependent and -independent mechanisms.
Conclusion
Overall, the results demonstrate the potential importance of inflammasomes in bacterial cystitis as well as the ability of the bladder wall injection technique to isolate the in vivo effects of specific inflammasome ligands to the physiological changes associated with cystitis.
Introduction
Urinary Tract Infections (UTIs) are the second most common form of infection following those of the respiratory tract (i.e., the common cold) and annually account for 7 million hospital visits at a cost of over $3.5 billion [1,2]. Acute UTI may progress to chronic cystitis through the formation of intracellular bacterial communities within urothelial cells that help protect the uropathogens from the antibiotic [3]. Using the bodies’ immune system as a therapeutic or prophylactic strategy against acute and chronic UTIs is attractive but still in its infancy. A clearer understanding of the host recognition and response to uropathogens would significantly advance that goal.
Inflammation is a complex cascade that occurs in response to both sterile and infectious insults. Pattern-recognition receptors of the innate immune system; specifically Toll-like receptors (TLRs) and Nod-like receptors (NLRs), recognize molecular patterns present in antigens produced by pathogens (pathogen-associated molecular patterns; PAMPs) or in molecules released from damaged cells (damage-associate molecular patterns; DAMPs). TLRS are cell surface molecules that sample PAMPs and DAMPs in the extracellular and endosomal spaces and trigger transcriptional upregulation of pro-IL-1β. NLRs located in the cytoplasm [4-6] also recognize DAMPs and PAMPs [6,7] and form a multimeric structure known as an inflammasome which activates caspase-1. Caspase-1 ultimately cleaves the pro-IL-1β (that was upregulated by the TLRs) to its mature form which is released to act as a potent pro-inflammatory cytokine.
In this manuscript we begin to explore the importance of the NLR/ inflammasome, NLRP3 (NACHT, LRR and PYD domains-containing protein 3) in the response of the bladder to PAMPs that are important mediators of UTIs. NLRP3 is by far the best studied inflammasome and has been shown to respond to numerous DAMPs as well as PAMPs. Our lab has recently documented the presence of this NLR/ inflammasome (and several others) in the bladder urothelia [8] and its central role in two critical models of sterile inflammation (cyclophosphamide-induced hemmoragic cystitis and bladder outlet obstruction) [9,10]. Among the list of PAMPS that activate this inflammasome (directly or indirectly), lipopolysaccharide (LPS) is one of the most potent virulence factors of uropathogens [11]. Consequently, we examine a role for NLRP3 in the induction of inflammation by LPS and the resulting bladder dysfunction in vivo by directly injecting LPS into the bladder wall, which is necessary to bypass the glycosaminoglycan (GAG) layer that forms a protective barrier lining the lumen of the bladder. While uropathogens have evolved mechanisms to overcome this obstacle, our understanding has not progressed to the point where we can duplicate them experimentally with individual PAMPs. Other investigators have utilized pre-treatment with protamine sulfate to disrupt the GAG layer and allow better access of PAMPs to the underlying urothelial layer [12], but this step itself causes significant damage to the urothelium and elicits inflammation independent of the PAMPs [13]. Therefore, our injection technique allows us to experimentally expose the urothelial cells in vivo to specific antigens while minimizing damage to the urothelium.
Methods
Animals and pharmacological treatments
Sprague Dawley rats (adult, female, ≈200 g, Harlan, Prattville AL) were used in all studies. All experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at the Medical University of South Carolina (AR#2960) and performed in accordance with the guidelines set forth in the NIH Guide for the Care and Use of Laboratory Animals, published by the Public Health Service. Rats were housed in an AAALAC-approved colony room maintained at a constant temperature and humidity on a 12-h light:dark cycle and given ad libitum access to food and water. All surgery was performed under ketamine/xylazine anesthesia.
The dosing regimen is illustrated in Figure 1. Animals were randomly assigned to a given group. The dose of glyburide (10 mg/kg) is common in the literature [14,15]. Glyburide was prepared (5 mg/ml; 100% ethanol) by heating at 56°C followed by dilution with PBS (2:3). Animals were gavaged with 1 ml of glyburide (10 mg/kg) or vehicle (40% ethanol) at −16, −4, +4 and +16 h relative to the LPS injection. LPS was injected at 0 h and all analyses performed 24 h later.
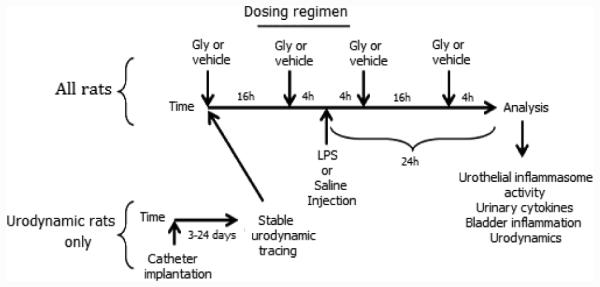
Dosing regimen used in these studies. For rats used in urodynamic studies superpubic catheters were inserted in the bladder and initial tracings taken after 3 days and again at least weekly until tracings normalized. Upon entering the regimen rats were given 10 mg/kg glyburide (Gly, p.o.) the evening before and the morning of injection (−16 h and−4 h, respectively). LPS or saline was injected (200 μl) into the bladder wall and Gly administered again after 4 h and 16 h. 24 h after injection rats were analyzed according to the various methodologies described.
Bladder wall injections
LPS (Esherichia coli 055:B5; Calbiochem-EMD Millipore, Billerica, MA, catalog # 437625), was prepared in saline containing 125 μg/ml Evans blue dye and filter sterilized (0.22 μm). Rats were anesthetized (90 mg/kg ketamine hydrochloride, 10 mg/kg xylazine, i.p.) and the bladder exposed. 200 μl of LPS in Evan’s blue/saline or Evan’s blue/ saline alone (referred to simply as saline) was injected into the bladder wall (typically 50 μl/injection, two injections in the dome and two in the neck) using a 26 g needle. Evans blue dye allows for visual inspection of delivery. Animals with spillage outside the bladder or into the urine were excluded. The muscle and skin layers were then sutured closed.
Caspase-1 assay
To isolate urothelial cells, rats were anesthetized and urine removed by needle puncture and stored at −80°C. Bladders were dissected out, weighed, opened longitudinally and scraped with a scalpel. The cells were transferred to PBS, pelleted (800 × g, 10 min) and resuspended in 25 µl of 10 mM MgCl2, 0.25% Igepal CA-630. Lysates were combined with 25 µl of 40 mM Hepes (pH 7.4), 20 mM NaCl, 2 mM EDTA, 20% Glycerol and stored at −80°C.
For caspase-1 activity, protein concentrations were assessed by Bradford assay [16] and 20 μl of extract combined with 50 μl assay buffer (25 mm HEPES, 5% sucrose, 0.05% CHAPS (pH 7.5), 10 μl 100 mM dithiothreitol and 20 μl 1 mM N-Acetyl-Tyr-Val-Ala-Asp-7-amino-4-trifluoromethylcoumarin (Ac-YVAD-AFC) in blacked-walled 96 well plates. Fluorescence (Excitation 400 nm, Emission 505 nm) was then measured every 30 sec for 15 min and the slope determined. A standard curve of fluorescence versus free AFC was then used to calculate the specific activity of caspase-1 [9].
IL-1β Analysis
Urine levels of mature IL-1β were assayed on the BioPlex platform (BioRad, Luminex Technology, Hercules, CA). Creatinine was assayed using the Urinary Creatinine Kit from Cayman Chemical (Ann Arbor, MI). Cytokine levels were adjusted for creatinine and reported as pg cytokine/mg creatinine.
Evan’s Blue Extravasation
Rats were injected (i.v.) with 25 mg/kg of the dye from a 25 mg/ml stock). Animals were sacrificed 1 h later and bladders removed, weighed and placed in formamide (1 ml) overnight in the dark (56°C). Absorbance (620 nM) was measured and the results calculated from a standard curve [9,17].
Urodynamics
Catheter implantation
Rats were anesthetized and the bladder exposed. A hole was created in the dome with a scalpel and a polyethylene catheter (PE-50 tubing) with a flared end inserted and secured in place with a purse string suture (6-0 polypropylene). The other end was tunneled subcutaneously, externalized at the back of the neck and secured with a suture into the interscapular tissue. The rat was fitted with a SAI Quick Connect Harness from Strategic Applications Incorporated (PHM-119-1, SAI Infusion Technologies, Lake Villa, IL) and given >72 hours to recover.
Cystometry
Initial cystometric tracing were performed after 3 days. If profiles had not stabilized (i.e., formed regular and reproducible micturition cycles with voiding volumes and pressures consistent with published control values [9,17,18]) urodynamics was repeated once per week for up to three weeks. If profiles never stabilized, animals were excluded. Following acquisition of an initial normal tracing, animals were placed into the dosing regimen (Figure 1).
For cystometry [9,18], the harness was removed and the rat placed into a modified restrainer inside a Small Animal Cystometry Lab Station (Catamount, St. Albans, VT). The catheter was attached to an infusion pump and sterile saline infused (80 µl/min). An in-line pressure transducer measured intravesical pressure while an analytical balance measured the voided volume. Both were continuously measured using software provided by Catamount. Voiding pressure is the peak intravesical pressure during voiding, threshold pressure is the intravesical pressure just before initiation of micturition, void volume is the amount of urine voided and the intercontraction interval (ICI) is the time between successive micturitions.
Statistical analysis
Caspase-1 activity, IL-1β levels, bladder weights and Evan’s blue extravasation was assessed by a one-way analysis of variance followed by a Tukey's post-hoc analysis using GraphPad InStat software (La Jolla, CA). Urodynamic parameters were assessed with a general linear model and Tukey's post-hoc analysis and were calculated using SAS v9.3 (Cary, NC).
Results
NLRP3 inflammasome activation/IL-1β release
To assess the effects of LPS injection into the bladder wall and the role of NLRP3, animals were subjected to the dosing regimen in Figure 1. Urothelial cells were isolated and caspase-1 activity was measured to determine inflammasome activation. As shown in Figure 2A, LPS activated caspase-1 activity in a dose-dependent manner compared to injection of saline alone (0 μg/kg), although a statistically significant increase was only detected at 200 μg/kg. Glyburide reduced caspase-1 activity to levels not significantly different from the saline control. There was a concomitant increase in urinary IL-1β (Figure 2B) which was blocked by glyburide.
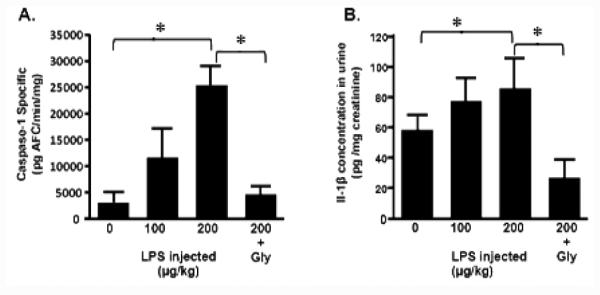
Caspase-1 activity (urothelia) and IL-1β levels (urine) at the end of the dosing regimen. Rats injected with 0, 100 or 200 μg/kg LPS were given vehicle (p.o.) at the times outlined in Figure 1 while the animals in the 200 + Gly group were given 10 mg/kg glyburide. 0 μg/kg LPS is the equivalent to the vehicle-treated, saline-injected rats presented in other figures. 24 h after bladder wall injection rats were sacrificed, urothelia isolated and caspase-1 activity assessed as described. A. Caspase-1 activity in the urothelia. Bars represent the mean ± SEM; n=3 for 0, 100, and 200+gly samples, n=5 for the 200 μg/kg sample. Asterisks indicated significant differences (P<0.05) between the indicated bars. B. IL-1β levels in the urine. Bars represent the mean ± SEM; n=3 for all groups. Asterisks indicated significant differences (P<0.05) between the indicated bars.
Activation of inflammation
To assess bladder inflammation, we examined two well-documented indices; bladder weight and Evan’s blue extravasation [9]. Injection of saline had little effect on bladder weight while LPS injection significantly increased this endpoint (Figure 3A). This increase was blocked by glyburide. Likewise, LPS stimulated a large increase in Evan’s blue extravasation (Figure 3B) compared to saline-injected or untreated controls whereas glyburide attenuated this increase to saline-injected levels (which were not significantly different from untreated controls).
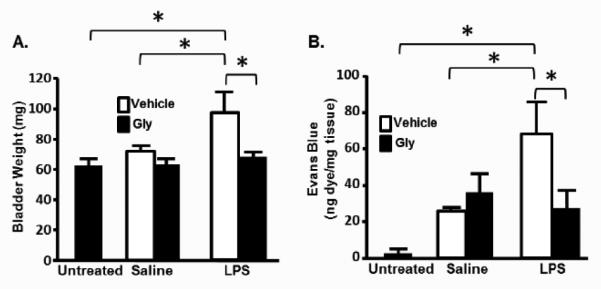
Bladder weight and Evan’s Blue extravasation in rats at the end of the dosing regimen. A. Bladder weights at the end of treatment. Untreated rats were not entered into the dosing regimen and sacrificed with no treatments. At the end of the experiment bladders were harvested, cleaned of all fat and weighed. Bars represent the mean ± SEM. n=4 for untreated, vehicle-treated LPS-injected and Gly-treated LPS-injected groups. n=5 for vehicle-treated saline-injected and glyburide-treated saline-injected groups. Asterisks demark significant differences (P<0.05) between the indicated bars. B. Evan’s blue extravasation into the bladder. Untreated rats were not entered into the dosing regimen and sacrificed with no treatments other than Evan’s Blue injection. At the end of the experiment bladders were harvested and dye extracted and quantitated as described in the Methods section. Bars represent the mean ± SEM. n=8 for untreated, 5 for vehicle-treated saline-injected and glyburide-treated saline-injected groups. n=4 for vehicle-treated LPS-injected and Gly-treated LPS-injected groups. Asterisks demark significant differences (P<0.05) between the indicated bars.
Effects on bladder function (Urodynamics)
Implantation of suprapubic catheters for rodent urodynamics is invasive and likely to elicit an inflammatory response. To insure that such inflammation had been resolved, newly implanted rats were periodically subjected to urodynamics and the tracing assessed for normality. A well-defined filling and voiding cycle was the most critical marker but other indices were assessed such as baseline pressure, voiding volumes (≥ 650 μl) and clear threshold pressures. Animals not displaying normal urodynamics after three weeks were excluded. Importantly, obtaining baseline tracings prior to entering the dosing regimen allows each rat to serve as their own control, providing a direct comparison between that rat’s urodynamic profile before and after bladder wall injection. Thus, each parameter is reported as % of initial value.
Figure 4A shows representative tracings from the 4 groups depicted in Figure 1, before and 24 h after injection. Figures 4B--EE are quantitative analyses of various endpoints. As shown in Figure 4B, injection of saline reduced voiding volume to 69 ± 14% of initial. This change was not affected by inclusion of LPS. A similar change was seen in the intercontraction interval (ICI) (Figure 4C). Interestingly, glyburide not only prevented these changes but increased both parameters to levels significantly above the initial values (i.e., >100%). In contrast, LPS injection reduced voiding pressure to 61 ± 9% initial while saline alone had no real effect (Figure 4D). Glyburide treatment prevented this change in voiding pressure. Finally, LPS significantly reduced the threshold pressure (Figure 4E) with no observed effect of Glyburide.
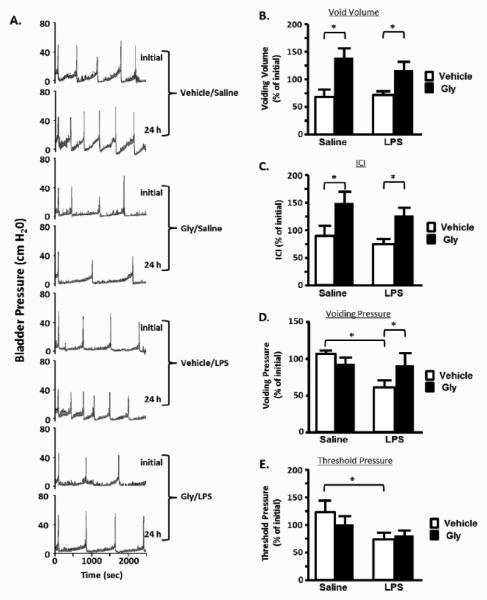
Effects of the treatment regimen on bladder function (urodynamics); A. Representative bladder pressure tracings from a single rat from each of the 4 experimental groups indicated. Included for each are the initial tracing taken just before the rat was entered into the experimental regimen and one taken at the end, 24 h after bladder wall injection. Not shown is the scale tracing which indicate the voided amounts each time there is a major peak in bladder pressure (voiding pressure); B-E. Quantitation of various urodynamics parameters recorded from rats in each of the indicated experimental groups. Initial tracings were made and each of the parameters quantitated as described in the Methods section. Rats were then subjected to the dosing regimen with a final urodynamics tracing made at the end and quantitated. Results for any given parameter were then calculated as the % of the initial value for that individual rat. The percentages of all individual rats in a given group were then averaged and the bars in the figure represent the mean ± SEM of those percentages; B. Void Volume; C. Intercontraction Interval (time between two voiding contractions). D. Voiding Pressure (maximum pressure associated with a void); E. Threshold Pressure (pressure just before the initiation of a voiding contraction). n=4 for vehicle-treated saline-injected, 5 for Gly-treated LPS-injected, 6 for Gly-treated saline-injected and 11 for vehicle-treated LPS-injected. Asterisks indicated significant differences (P<0.05) between the indicated bars.
Discussion
Once uropathogens breach the barriers to the urothelia they trigger a response from the innate immune system through one or more PAMPs. While the number of known PAMPs is long, LPS is the best studied and is one of the antigens most frequently encountered during gram negative UTIs [11]. Numerous studies have shown that LPS is involved in triggering the NLRP3 inflammasome, release of IL-1β and subsequent inflammation. Our discovery of NLRP3 in the urothelia [9] led us to explore a role for NLRP3 in mediating the response to LPS in the bladder.
Intravesicular instillation of LPS has been used to initiate biochemical and morphological cystitis in the mouse [19] and rat [12,20,21] and some degree of inflammasome activation has been reported, by us, using this technique [9].
However, our attempts to measure changes in bladder function via urodynamics were unsuccessful (data not shown), suggesting the level of activation was insufficient to exert physiological effects. Those findings are consistent with [22], who found no change in void volume or urinary frequency in rats that had undergone intravesical instillation of LPS. The results from both labs are likely due to limited access of purified LPS to the urothelia. To overcome this we injected LPS directly into the bladder wall, bypassing the GAG and uroplakin layers. The result was activation of caspase-1 in the urothelia and release of IL-1β into the urine. While we measured caspase-1 activity in urothelia, it is reasonable to expect that injection of LPS or saline into the bladder wall would recruit mobile cellular elements of the innate immune system (e.g. macrophage) into the smooth muscle layer. Activation of inflammasomes in these cells would contribute, at least to some degree, to the IL-1β measured in the urine. Both caspase-1 activity in the urothelia and IL-1β in the urine was prevented or attenuated by glyburide. Injection of LPS also produced full-blown inflammation in only 24 h resulting in increases in bladder weight and Evans blue extravasation. Both of these indices were reversed or attenuated by glyburide. These two observations suggest a role for NLRP3 in initiating the bladder’s inflammatory response to LPS. The mechanisms by which LPS is exerting this effect is not entirely clear from these experiments. In the canonical pathway of NLRP3 activation, LPS works through Toll-like receptors (TLRs) to “prime’ the response by upregulating transcription/translation of pro-IL-1β [7,23]. A second signal, provided by other DAMPS such as ATP, is required to activate the inflammasome. In the present model, second signals could easily be provided from cells damaged by the injection or by molecules crossing into the urothelia from the urine. Conversely, NLRP3 can be activated by a noncanonical pathway in which LPS directly activates caspase-11 which then triggers NLRP3 inflammasome formation and activation of caspase-1 [24,25]. A contribution of this pathway certainly cannot be ruled out and future studies are envisioned to assess the contribution of the various pathways to the results seen in this study.
Although not statistically different, the Evans blue assay did detect a trend with saline injection alone, suggesting that our protocol is an invasive technique whose effects must be carefully controlled for. Likewise, glyburide reduced urinary IL-1β levels below that seen with vehicle (0 µg/kg LPS) alone. Although this reduction was also not significantly different, it is suggestive of an inflammatory effect of injection alone.
Using our protocol this study yielded demonstrable changes in voiding physiology. To begin with, a decrease in voiding volume was noted after injection of saline whereas addition of LPS to the inoculum did not change this parameter. Not only was this effect blocked by glyburide (suggesting dependency on NLRP3) but void volume increased beyond initial values (>100%). This result suggests basal activation of NLRP3 which contributes to normal bladder physiology. Although further experiments are needed to confirm this, similar effects have been reported [9].
Voiding pressure is determined by the strength of contraction and outlet resistance. Since there is no mechanism by which the latter should be altered in this model, the pressure should be wholly dependent on the contractility of the detrusor. The decrease in voiding pressure caused by LPS injection demonstrates that this PAMP had a negative effect on the bladder’s ability to generate a contraction. The ability of glyburide to block this change indicates that it is mediated by NLRP3. While the mechanism of this effect is unknown, inflamed muscles are well known to be weakened and to fatigue easily [26].
Threshold pressure is the set point where micturition is initiated and our data suggests that LPS lowers that threshold. That was not affected by glyburide, indicating that this is not mediated by the NLRP3 inflammasome. It could be dependent on other pathways of the innate immune system or even due to neurological effects. Nevertheless, it does indicate the presence of NLRP3-independent mechanisms activated by LPS.
One potential limitation of or study is that glyburide is exerting effects other than inhibition of NLRP3. For example, this drug also inhibits KATP channels which have been implicated in overactive bladder. However, glyburide is a KATP channel inhibitor and openers of the channel cause detrusor relaxation which is antagonized by glyburide [27]. Thus, one might expect that glyburide, as a KATP channel inhibitor, would increase bladder activity, which was not seen in our study. Glyburide is also an inhibitor of the cholesterol transporter ABCA1. In the bladder the ABCA1 transporter is expressed in detrusor muscles, and to a lesser extent in the urothelium, but there is no known function of these in bladder physiology. Glyburide also exerts mild diuretic effects due to its increase in renal free water clearance. If this is significant in our model, then one would expect increased urinary frequency (or decreased intercontraction interval) and this is the opposite of what we saw in the BOO animals. Thus, direct inhibiton of NLRP3 is the most likely explanation of the effect of glyburide in our study.
Most evidence points to the urothelia as the cellular location of NLRP3 activation by LPS in this study. We have recently shown that this receptor is highly expressed in this epithelial layer [8,9] which is logically the first to encounter this antigen expressed on the surface of invading bacteria. Moreover, as described in the introduction, LPS placed in the lumen of the bladder can activate NLRP3 to some degree [9] although in follow up studies we did not find the level used to be sufficient to change bladder function. We also showed in an in vitro study using a well-established urothelial cell line that the NLRP3 is activated by ATP [8], the quintessential NLRP3 activator, which removes any possible contribution of other cell types and definitively demonstrates the urothelia NLRP3 can be activated. Thus, the urothelia is the most likely center of NLRP3 activation in the current study. The detrusor is another possible location of inflammasome activation. However, we did not detect expression of NLRP3 in the detrusor muscle in two separate studies [8,9] (although other NLRs were present in that tissue) making it unlikely that the detrusor contributes significantly to the response. We cannot, however, rule out a contribution of resident and recruited immune cells, particularly macrophages, in this in vivo study. Once bacteria penetrate the urothelia they inevitably encounter these cells which would be expected to activate their inflammasomes and further contribute to the stimulation of inflammation. Indeed, as the number of immune cells in the tissue accumulates, and the bacteria invade beyond the epithelia, the relative contribution of each cell type to the overall inflammasome activity is likely to shift from the urothelia towards the migrating immune cells. Analysis of the possible changing contributions of these cell types to the inflammation and bladder dysfunction associated with the time course of a UTI will providing fascinating fodder for future studies.
A percentage of UTI patients develop chronic cystitis characterized by intracellular bacterial communities in the urothelium [3,28-31]. NLRs are cytosolic sensors of bacterial PAMPs, putting them in an ideal location to respond to these communities. Not surprisingly, bacteria possess many mechanisms to combat host recognition and inflammasome activation [32] and it is the interplay between the various arsenals that ultimately results in bacterial proliferation or clearance. Now that we have implicated urothelial NLRP3 in the response to LPS, further studies will be necessary to understand how uropathogens counteract this mechanism. Ultimately the goal is to prevent or control bacterial cystitis through the modulation of the innate immune system. Along those lines, Hedl and Abraham [33] demonstrated in human macrophages that chronic exposure to LPS results in a down regulation of pro-inflammatory cytokines but an increase in the bacterial killing capacity of the cell. This effect was mediated by NLRP3/NLRP1, demonstrating the importance of better understanding this molecule(s) to develop these types of strategies.
Our results show that direct injection of LPS into the bladder wall is an effective method of inducing many of the parameters associated with cystitis. It is important to be mindful that results with LPS may not always be consistent with actual infections caused by uropathogens because LPS is only one of many PAMPs involved in clinical UTIs. Invading uropathogens therefore may stimulate the innate immune system in multiple ways depending on contact with specific cells and the repertoire of receptors the responding cell may possess, which can be considerable. Indeed, we have recently shown the presence of 7 different NLRs in the uninfected rat urothelia, with many also expressed in the detrusor [8]. Different NLRs for the most part respond to different PAMPs. Thus, the innate response to an invading bacteria will be the summarized and integrated response of all the PAMPs interacting with all the NLRs (and TLRs as well). One major benefit of our bladder wall injection technique is that it allows a single PAMP to be studied at a time and this will allow us to incrementally develop a more complete model for understanding host recognition of actual pathogens.
Overall, the important finding of this study is that LPS activates NLRP3 inflammasomes in the urothelia which stimulate an inflammatory response and these molecules effectuate some, if not all of the negative changes to physiological bladder function. This discovery strengthens the developing concept that the urothelium is not merely a barrier, but also an important front line sensor in the lumen of the bladder and suggests the NLRs may constitute a major molecular sensor for this tissue.
Acknowledgement
Research reported in this publication was supported by the National Institute Of Diabetes And Digestive And Kidney Diseases of the National Institutes of Health under Award Number R01DK103534. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was also supported in part by intradepartmental funds from the Medical University of South Carolina Department of Urology.
Financial support
NIDDK grant R01DK103534 and intramural funds from the Medical University of South Carolina Department of Urology.
References
Full text links
Read article at publisher's site: https://doi.org/10.4172/2155-9899.1000396
Read article for free, from open access legal sources, via Unpaywall:
https://www.omicsonline.org/open-access/the-nacht-lrr-and-pyd-domainscontaining-protein-3-nlrp3-inflammasome-mediates-inflammation-and-voiding-dysfunction-in-a-lipopolysa-2155-9899-1000396.pdf
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.4172/2155-9899.1000396
Article citations
Male Akita mice develop signs of bladder underactivity independent of NLRP3 as a result of a decrease in neurotransmitter release from efferent neurons.
Am J Physiol Renal Physiol, 325(1):F61-F72, 11 May 2023
Cited by: 4 articles | PMID: 37167271
Inflammation triggered by the NLRP3 inflammasome is a critical driver of diabetic bladder dysfunction.
Front Physiol, 13:920487, 25 Nov 2022
Cited by: 10 articles | PMID: 36505062 | PMCID: PMC9733912
Review Free full text in Europe PMC
The NLRP3 Inflammasome Inhibitor Dapansutrile Attenuates Cyclophosphamide-Induced Interstitial Cystitis.
Front Immunol, 13:903834, 03 Jun 2022
Cited by: 5 articles | PMID: 35720309 | PMCID: PMC9205468
Integrated Analysis of lncRNA and circRNA Mediated ceRNA Regulatory Networks in Skin Reveals Innate Immunity Differences Between Wild-Type and Yellow Mutant Rainbow Trout (Oncorhynchus mykiss).
Front Immunol, 13:802731, 17 May 2022
Cited by: 6 articles | PMID: 35655786 | PMCID: PMC9152293
Specialized pro-resolution mediators in the bladder: Receptor expression and recovery of bladder function from cystitis.
Exp Biol Med (Maywood), 247(8):700-711, 19 Jan 2022
Cited by: 7 articles | PMID: 35044873 | PMCID: PMC9039492
Go to all (22) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The NLRP3 Inflammasome Mediates Inflammation Produced by Bladder Outlet Obstruction.
J Urol, 195(5):1598-1605, 18 Dec 2015
Cited by: 54 articles | PMID: 26707508 | PMCID: PMC4870136
Inflammasomes are important mediators of cyclophosphamide-induced bladder inflammation.
Am J Physiol Renal Physiol, 306(3):F299-308, 27 Nov 2013
Cited by: 52 articles | PMID: 24285499 | PMCID: PMC4073918
Bladder decompensation and reduction in nerve density in a rat model of chronic bladder outlet obstruction are attenuated with the NLRP3 inhibitor glyburide.
Am J Physiol Renal Physiol, 316(1):F113-F120, 24 Oct 2018
Cited by: 15 articles | PMID: 30353742 | PMCID: PMC6383202
NLRP3 Inflammasome and Inflammatory Bowel Disease.
Front Immunol, 10:276, 28 Feb 2019
Cited by: 255 articles | PMID: 30873162 | PMCID: PMC6403142
Review Free full text in Europe PMC




