Abstract
Background
The nociceptin/orphanin-FQ (or opioid receptor-like [ORL1]) receptor (NOP) is localized in the mesolimbic reward pathway and has been suggested to play a role in feeding, mood, stress, and addiction. Since its deorphanization in 1995, there has been a clear dichotomy in the literature regarding whether an agonist or antagonist would provide therapeutic benefit. Specifically, the literature reports indicate that NOP receptor antagonists produce efficacy in animal models of hyperphagia and antidepressant-like activity, whereas NOP agonists produce anxiolytic-like effects and dampen reward/addiction behaviors including ethanol consumption.Methods
We characterize here the potent, orally bioavailable NOP antagonist, LY2940094, in rodent models of ethanol consumption, including ethanol self-administration, progressive ratio operant self-administration, stress-induced reinstatement of ethanol seeking, and in vivo microdialysis in the nucleus accumbens.Results
LY2940094 dose dependently reduced homecage ethanol self-administration in Indiana alcohol-preferring (P) and Marchigian Sardinian alcohol-preferring (msP) rats, without affecting food/water intake or locomotor activity. Reduced ethanol intake in P rats did not show significant tolerance over 4 days of subchronic dosing. LY2940094 attenuated progressive ratio operant responding and break points for ethanol in P rats. Moreover, stress-induced reinstatement of ethanol seeking in msP rats was completely blocked by LY2940094. Furthermore, LY2940094 blocked ethanol-stimulated dopamine release in response to ethanol challenge (1.1 g/kg, intraperitoneally).Conclusions
Our findings demonstrate for the first time that blockade of NOP receptors attenuates ethanol self-administration and ethanol-motivated behaviors, stress-induced ethanol seeking, and ethanol-induced stimulation of brain reward pathways in lines of rats that exhibit excessive ethanol consumption. Results suggest that LY2940094 may have potential therapeutic utility in treating alcohol addiction.Free full text

A novel, orally-bioavailable nociceptin receptor antagonist, LY2940094, reduces ethanol self-administration and ethanol-seeking in animal models
Abstract
Background
The nociceptin/orphanin-FQ (NOP; or opioid-receptor-like (ORL1)) receptor is localized in the mesolimbic reward pathway and has been suggested to play a role in feeding, mood, stress, and addiction. Since its deorphanization in 1995, there has been a clear dichotomy in the literature regarding whether an agonist or antagonist would provide therapeutic benefit. Specifically, literature reports indicate that NOP receptor antagonists produce efficacy in animal models of hyperphagia and antidepressant-like activity, whereas NOP agonists produce anxiolytic-like effects and dampen reward/addiction behaviors including ethanol consumption.
Methods
We characterize here the potent, orally-bioavailable NOP antagonist, LY2940094, in rodent models of ethanol consumption, including ethanol self-administration, progressive ratio operant self-administration, stress-induced reinstatement of ethanol-seeking, and in vivo microdialysis in the nucleus accumbens.
Results
LY2940094 dose-dependently reduced homecage ethanol self-administration in Indiana Alcohol-Preferring (P) and Marchigian Sardinian Alcohol-Preferring (msP) rats, without affecting food/water intake or locomotor activity. Reduced ethanol intake in P rats did not show significant tolerance over 4 days of subchronic dosing. LY2940094 attenuated progressive ratio operant responding and breakpoints for ethanol in P rats. Moreover, stress-induced reinstatement of ethanol-seeking in msP rats was completely blocked by LY2940094. Furthermore, LY2940094 blocked ethanol-stimulated dopamine release in response to ethanol challenge (1.1 g/kg, IP).
Conclusions
Our findings demonstrate for the first time that blockade of NOP receptors attenuates ethanol self-administration and ethanol-motivated behaviors, stress-induced ethanol-seeking, and ethanol-induced stimulation of brain reward pathways in lines of rats that exhibit excessive ethanol consumption. Results suggest that LY2940094 may have potential therapeutic utility in treating alcohol addiction.
1. Introduction
The peptide neurotransmitter nociceptin/orphanin-FQ (N/OFQ) and the receptor to which it binds (NOP) are localized throughout the mesocorticolimbic reward pathway and related areas (for example, central nucleus of the amygdala, bed nucleus of the stria terminalis, medial prefrontal cortex, ventral tegmental area, lateral hypothalamus, nucleus accumbens) which are critical mediators in motivated behaviors and addiction (Neal et al., 1999a, Neal et al., 1999b, Witkin et al., 2014, Koob et al., 2014). Although classified within the opioid family, N/OFQ does not bind classical mu, kappa, and delta opioid receptor subtypes (MOP, KOP, and DOP, respectively) with appreciable affinity, nor do classical opioid receptor agonists bind to the NOP receptor (Meunier et al., 1995, Reinscheid et al., 1995). Preclinical research supports that selective modulation of NOP receptor signaling may have potential therapeutic benefit in a number of clinical indications including obesity, pain, mood, addiction, cardiovascular control and immunity (Lambert, 2008, Witkin et al., 2014).
Peptide and non-peptide NOP receptor agonists have consistently been reported to regulate drug reward and drug-seeking behaviors associated with opiates, psychostimulants and ethanol (reviewed in (Witkin et al., 2014)). With respect to ethanol-mediated behaviors, NOP agonists attenuate conditioned place preference, ethanol self-administration, and ethanol-seeking behaviors in rat lines genetically-selected for high alcohol preference, including the Marchigian Sardinian Alcohol-Preferring (msP) and Indiana Alcohol-Preferring (P) rats, as well as ethanol-dependent Wistar rats and Naval Medical Research Institute (NMRI) mice (Ciccocioppo et al., 2004, Ciccocioppo et al., 1999, Economidou et al., 2006, Economidou et al., 2008, Kuzmin et al., 2003, Kuzmin et al., 2007, de Guglielmo et al., 2015). The attenuation of ethanol self-administration and ethanol-motivated behaviors in these lines is presumed to be related to suppression of dopamine synthesis and release (Olianas et al., 2008, Murphy et al., 1996).
Since the deorphanization of the NOP receptor in 1995, a clear dichotomy has emerged in the literature regarding whether an agonist or antagonist would provide therapeutic benefit. Specifically, NOP receptor antagonists and NOP receptor knockout mice have been reported to produce efficacy in animal models of obesity and antidepressant-like activity, whereas NOP agonists have been reported to produce efficacy in animal models of reward/addiction and anxiolytic-like efficacy (Gavioli and Calo, 2013, Witkin et al., 2014, Rizzi et al., 2007, Gavioli and Calo, 2006). Given the extant overlap of brain areas involved in the regulation of mood, reward, and food intake, this dichotomy has been difficult to reconcile.
We recently discovered a selective, orally-bioavailable NOP antagonist (LY2940094; compound 36 in (Toledo et al., 2014)) with high affinity (Ki = 0.105 nM) and antagonist potency (Kb = 0.166 nM) at NOP receptors (Statnick et al., 2016). Consistent with its antagonist pharmacological profile, LY2940094 potently inhibits excessive feeding behaviors in rodents (Statnick et al., 2016), demonstrates antidepressant-like efficacy in rodent models, and antidepressant efficacy in patients with major depressive disorder (Witkin et al., under review),(Post et al., 2015). Importantly, hypophagic and antidepressant-like efficacy of LY2940094 in rodent models was lost in mice lacking NOP receptors(Statnick et al., 2016),(Witkin et al., under review). Because literature reports consistently indicated that NOP receptor agonists attenuated ethanol-motivated behaviors, we tested LY2940094 to determine its propensity to facilitate or exacerbate ethanol self-administration and ethanol-seeking behaviors. In contrast to expectations based upon literature findings, we report herein for the first time that NOP receptor antagonism is also sufficient to attenuate ethanol drinking, seeking, and relapse.
2. Materials and Methods
2.1 Animals
Female Alcohol-Preferring (P) rats (generously supplied by the Indiana University School of Medicine and maintained at Taconic Inc. (Germantown, NY; 250–320 g) and male Marchigian Sardinian Alcohol-Preferring (msP) rats obtained from the University of Camerino (Marche, Italy; 400–450 g) were used in the ethanol experiments. Male Sprague-Dawley rats obtained from Taconic Farms (Germantown, NY) weighing 260 – 300 g were used in the microdialysis experiment. For the homecage ethanol self-administration experiments, rats were individually-housed on a 12-hr light:dark cycle with food, water, and ethanol available ad libitum. For the operant ethanol experiments, rats were pair-housed on a 12-hr light:dark cycle with food and water available ad libitum in the homecage. All experiments were conducted in compliance with the NIH Guide for the Care and Use of Laboratory Animals under protocols approved and monitored by a local animal care and use committee.
2.2 Drugs and reagents
[2-[4-[(2-Chloro-4,4-difluorospiro[5H-thieno[2,3-c]pyran-7,4′-piperidine]-1′-yl)methyl]-3-methylpyrazol-1-yl]-3-pyridyl]methanol (LY2940094; (Toledo et al., 2014)) was synthesized at Lilly Research Laboratories (Madrid, Spain). Naltrexone HCl and yohimbine HCl were purchased from Sigma-Aldrich (St. Louis, MO and Milan, Italy, respectively). 95% Ethanol was diluted to 15% (v/v) in tap water for experiments in P rats and 10% (v/v) in distilled water for experiments in msP rats. LY2940094 was dissolved in 20% Captisol in 25 mM phosphate buffer (pH 3, adjusted for L-tartaric acid salt weight), and administered orally in a volume of 2–3 ml/kg. Yohimbine and naltrexone were dissolved in distilled water and injected IP and PO, respectively (1 ml/kg). Solutions were prepared fresh daily.
2.3 Homecage Ethanol Self-Administration
Female P rats were individually-housed with ad libitum access to regular rat chow (Harlan Teklad Diet #2014), water, and 15% (v/v) ethanol in water. Consistent with literature indicating that this genetically-selected line exhibits high alcohol preference (Waller et al., 1982), mean baseline ethanol intake was approximately 4.2 g/kg over the dark phase of the light:dark cycle (lights off at 4:00 pm) prior to initiation of the study. For the first experiment, rats (n = 9) received acute oral administration of vehicle, 10 mg/kg naltrexone, or 3, 10, or 30 mg/kg LY2940094, 5 min before onset of the dark phase, using a within-subjects counterbalanced, Latin-square design (3–4 day washout between subsequent doses). In a separate experiment, rats (n = 19) received 4 daily oral doses of either vehicle or 30 mg/kg LY2940094, 5 min before onset of the dark phase, in a between-subjects design. Voluntary consumption of ethanol, water, and food in both experiments was continuously monitored for the duration of the 12 hr dark phase after each dose, using a force transduction monitoring system (TSE Systems, Bad Homburg, Germany, as previously described (Rorick-Kehn et al., 2014)). At the same time, homecage locomotor activity was monitored using infrared sensors located on top of each cage (TSE Systems).
Male msP rats (n = 10) were individually-housed with ad libitum access to regular chow (Harlan Teklad Diet #2014), water and 10% (v/v) ethanol in water. Mean baseline ethanol intake was approximately 5–6 g/kg/day prior to initiation of the study. Rats received vehicle, 3, or 30 mg/kg LY2940094 (PO) in a within-subjects, counterbalanced design, 60 min before onset of the dark cycle. Voluntary consumption of ethanol and water was measured at 2, 8, or 24 hr after dosing by recording volume of intake from graduated cylinders, as previously described (Economidou et al., 2006). Voluntary consumption of chow was measured at the same time points by weighing the food basket.
2.4 Progressive ratio operant responding for ethanol
Female P rats (n = 13) were pair-housed with chow and water available ad libitum except during the training and test sessions. One subject was excluded after box-plot analysis (JMP; SAS Institute, Inc., Cary, NC) determined this subject to be a significant outlier. Rats were trained to self-administer 15% ethanol (v/v) in 0.1 mL increments in standard rat operant chambers (Med-Associates, St. Albans, VT), starting with a fixed-ratio 1 schedule of reinforcement and slowly transitioning to a progressive ratio schedule. Rats were maintained on an arithmetic progressive ratio operant schedule with counterbalanced active and inactive levers as previously described (Rorick-Kehn et al., 2014). Briefly, response requirements were increased by 2 after every 3 reinforcements received. Because previous analysis demonstrated that P rats in this assay drank most of their alcohol during the first 15–20 min, the rats were removed from the operant chambers after 30 min and returned to their homecages. Breakpoints were measured as the highest fixed-ratio (FR) response requirement reached (breakpoint) during the 30-min session. Ethanol-seeking was defined as the number of responses on the alcohol-associated lever. After stable performance was achieved, rats were acclimated to the dosing procedures and then received oral doses of vehicle, 3, 10, or 30 mg/kg LY2940094 (PO), or 10 mg/kg naltrexone (PO), 60 min prior to the operant session, using a within-subject counterbalanced, Latin-square design, with 3–4 days washout between subsequent doses. Performance was monitored on non-drug days to confirm stable baseline performance.
2.5 Stress-induced reinstatement to ethanol-seeking
Male msP rats (n = 30) were individually-housed with food and water available ad libitum except during the training and test sessions. Experiments were conducted in standard rat operant chambers housed within sound-attenuating cubicles (Med-Associates, St. Albans, VT), as previously described (Ciccocioppo et al., 2004). During daily 30-min operant sessions, rats were trained to self-administer a 10% (v/v) ethanol solution under a fixed-ratio-1 schedule of reinforcement, in which each response resulted in the delivery of 0.1 mL ethanol, followed by a 5-sec time out period, signaled by illumination of a 5W house light. Once stable baseline responding was achieved, rats were subjected to 30-min extinction sessions. During extinction sessions, all procedures were the same, except lever responses were no longer reinforced. After lever responses were extinguished, msP rats were subjected to a stress-induced reinstatement test conducted under the same extinction conditions, except that rats received the pharmacological stressor, yohimbine (2 mg/kg IP), 30 min before the session. Once stable baseline extinction responding was recovered following the previous yohimbine challenge, rats were randomly divided into 3 groups (N=10/group) and pretreated with LY2940094 (0, 3 or 10 mg/kg, PO) 60 min before the reinstatement test (30 min before yohimbine).
2.6 In vivo microdialysis
In vivo microdialysis studies were conducted in male Sprague-Dawley rats with guide cannulae (Bioanalytical Systems Inc, West Lafayette, IN) implanted in the Nucleus Accumbens (NAcc) as previously described (Rorick-Kehn et al., 2014). A 5 μM concentration of nomifensine was perfused via reverse microdialysis for all treatment groups to facilitate detection of extracellular dopamine levels. Reverse microdialysis of nomifensine was previously demonstrated to increase local concentrations of dopamine in the NAcc without altering evoked release of dopamine (Cenci et al., 1992). Importantly, all groups were treated with nomifensine, so any effects on dopamine levels related to the small local concentrations of nomifensine would be the same across all treatment groups. Rats received vehicle or 30 mg/kg LY2940094 (PO) prior to vehicle or ethanol challenge (1.1 g/kg, IP, administered as 15% v/v in 0.9% NaCl in a volume of 2.9 mL per rat). When LY2940094 was administered alone, it was given 10 min into the 4th sample (0-min time point). When administered in combination with ethanol, it was given 10 min into the 1st sample (−60 min time point). Ethanol was administered 10 min into the 4th sample (i.e., at the 0-min time point). All data are presented as the mean + SEM for n = 4–6 rats per group. Probe placements were verified histologically, but histology samples were not archived.
3. Results
3.1 NOP Antagonist LY2940094 Attenuates Ethanol Self-Administration in Indiana Alcohol-Preferring (P) and Marchigian Sardinian Alcohol-Preferring (msP) Rats
The effect of LY2940094 on ethanol self-administration, at doses that fully saturate NOP receptors (Toledo et al., 2014), was assessed in P rats. The mu-preferring opioid receptor antagonist naltrexone was included as a comparator. Following acute dosing, a significant main effect of dose was observed on ethanol self-administration during the first 3 hr of the dark cycle [F(4,32) = 4.43, p < 0.02; Fig 1A]. Post-hoc analyses revealed that ethanol self-administration was significantly attenuated in both naltrexone- (10 mg/kg) and LY2940094-treated (30 mg/kg) groups (p < 0.05 vs. vehicle; Dunnett’s). No significant effects were observed on food or water intake or locomotor activity during the first 3 hr of the dark cycle (all p’s > 0.05; data not shown). Consistent with the pharmacokinetic and receptor occupancy profiles, the significant reduction of ethanol intake was maintained over the entire 12-hr dark cycle in the LY2940094-treated group [F(4,32) = 4.94, p < 0.001], but not the naltrexone-treated group [p > 0.05; Fig 1B]. Post-hoc analyses revealed a significant effect of the 30 mg/kg dose of LY2940094 (p < 0.05 vs. vehicle; Dunnett’s). Neither LY2940094 nor naltrexone significantly altered food or water intake (p’s > 0.05; Fig 1C). Neither LY2940094 nor naltrexone affected homecage locomotor activity (p’s > 0.05; data not shown).
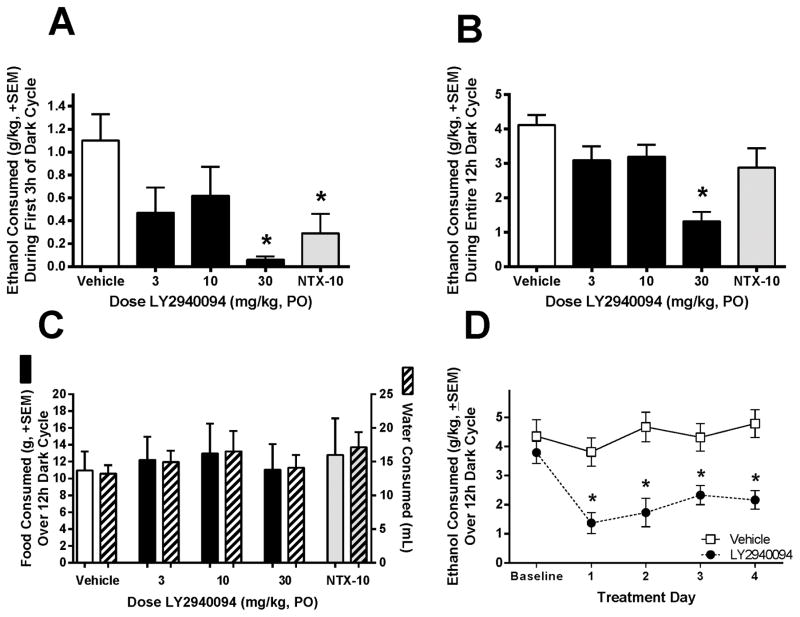
LY2940094 (30 mg/kg, PO) significantly reduced continuous homecage ethanol (15% v/v) self-administration in Indiana Alcohol-Preferring (P) rats with a chronic history of high ethanol consumption at 3 (A) and 12 hr (B) post-treatment, without affecting food or water intake (C). The positive control, naltrexone (10 mg/kg, PO), significantly reduced ethanol self-administration at the 3 hr time point (A, gray bar), but failed to maintain efficacy after 12 hr (B). The suppression of ethanol intake by LY2940094 did not show significant tolerance over 4 days of subchronic dosing (30 mg/kg, D). *, p < 0.05 vs. vehicle-treated rats.
In a subsequent study in Indiana P rats, LY2940094 (30 mg/kg) significantly decreased ethanol intake relative to vehicle control over 4 consecutive days of daily administration [Interaction: F(4,60) = 3.82, p < 0.01; Main Effect of Treatment: F(1,15) = 17.98, p < 0.001; Fig 1D], demonstrating lack of tolerance. Posthoc t-tests tests confirmed a significant effect of LY2940094 on each of the four active treatment days (all p’s < 0.002). Water and food intake were unaffected by LY2940094 in this experiment (p’s > 0.05; data not shown). Locomotor activity was also unaffected by subchronic administration of LY2940094 (p > 0.05; data not shown).
LY2940094 dose-dependently reduced homecage ethanol self-administration in msP rats [F(2,18) = 27.78, p < 0.001; Fig 2A]. Post-hoc analysis revealed a significant reduction in voluntary ethanol consumption in the 30 mg/kg LY2940094 group at 2, 8 and 24 hr (all ps < 0.01). As shown in Fig 2, the lower dose (3 mg/kg), significantly reduced ethanol drinking only at the 24-hr time point (p < 0.05), whereas no effect at the 3 mg/kg dose was observed at 2 and 8 hr post-dose. Consumption of chow diet was not significantly affected by drug treatment [p > 0.05; Fig 2B]. Results showed a slight trend toward an increase in water intake (Fig 2C) after drug treatment, but the effect was not statistically significant [p > 0.05].
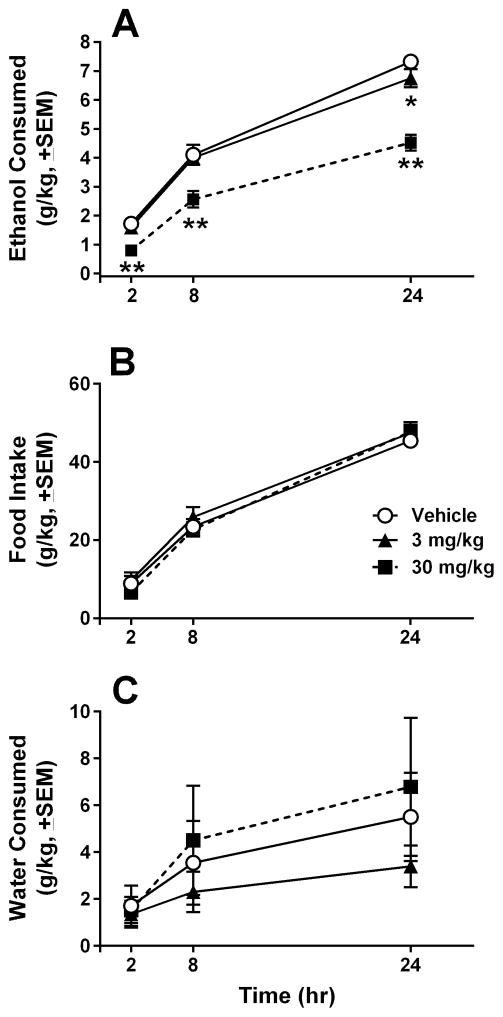
LY2940094 (30 mg/kg, PO) significantly and dose-dependently attenuated continuous ethanol (10% v/v) self-administration in Marchigian Sardinian Alcohol-Preferring (msP) rats at 2, 8, and 24 hr after dosing (A), without affecting food or water intake at any time point (B, C). *, p < 0.05 vs. vehicle-treated rats at the same time point; **, p < 0.01 vs. vehicle-treated rats at the same time point.
3.2 LY2940094 Reduces the Motivation to Consume Ethanol and Ethanol-Seeking in P Rats
Oral administration of LY2940094 attenuated operant ethanol self-administration behavior maintained on a progressive ratio schedule in P rats. LY2940094 dose-dependently reduced the motivation to consume ethanol, as indicated by reduced breakpoints in drug-treated rats [F(4,55) = 7.5, p < 0.001; Fig 3A]. Posthoc tests indicated significant effects at both the 30 mg/kg LY2940094 and 10 mg/kg naltrexone doses (p < 0.05). Reduced motivation for ethanol was also reflected by a significant reduction in the total number of lever presses on the active lever [F(4,55) = 5.2, p = 0.001; Fig 3B], which was reduced by about 50% during the 30-min operant session. Posthoc tests indicated that both LY2940094 (30 mg/kg) and naltrexone (10 mg/kg) reduced active lever presses (p < 0.05). LY2940094 did not alter responding on the inactive lever (lever not producing ethanol) [p=0.68; mean ±SEM inactive lever presses: vehicle = 3.58 ± 1.15; 3 mg/kg = 2.92 ± 0.70; 10 mg/kg = 6 ± 2.25; 30 mg/kg = 6.17 ± 2.82; NTX = 5.17 ± 1.38].
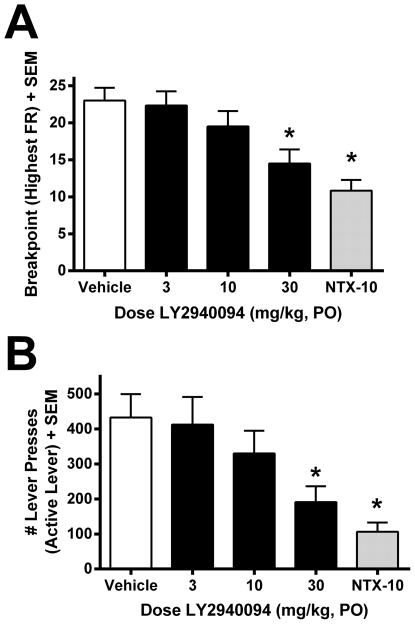
LY2940094 (30 mg/kg, PO) reduced the motivation to consume ethanol (as indicated by reduced breakpoints, A) and ethanol-seeking (B) in P rats maintained on a progressive ratio operant schedule. The positive control, naltrexone (10 mg/kg, PO), also significantly reduced ethanol motivation and ethanol-seeking (A,B). Values in A represent the highest fixed-ratio (FR) response requirement reached (breakpoint) during the 30-min session. Values in B represent the mean (+SEM) number of responses on the alcohol-associated lever. *, p < 0.05 vs. vehicle-treated rats.
3.3 LY2940094 Robustly Blocks Stress-Induced Reinstatement to Ethanol-Seeking in msP Rats
During the extinction phase, rats that were previously responding for ethanol at asymptotic levels (mean ±SEM lever presses: active lever, 49.6 ±1.9; inactive lever, 1.3 ±0.2) reduced their responding on the alcohol-associated lever to a mean of less than 10 responses per session (Fig 4; “Ext”). Administration of the pharmacological stressor and anxiety-provoking agent, yohimbine (2 mg/kg, IP), significantly reinstated responding on the lever that previously delivered ethanol [Fig 4; F(1,18) = 10.36, p < 0.01]. Pre-treatment with LY2940094 significantly attenuated yohimbine-induced reinstatement [F(2,27) = 18.2, p < 0.0001; Fig 4]. Post-hoc analysis revealed a significant inhibition of reinstatement by both 3 and 30 mg/kg doses of LY2940094 (***p < 0.001). Responses on the inactive lever were unaffected by LY2940094 [p > 0.05; data not shown], indicating a behaviorally-specific effect of LY2940094 on yohimbine-elicited reinstatement of alcohol-seeking.
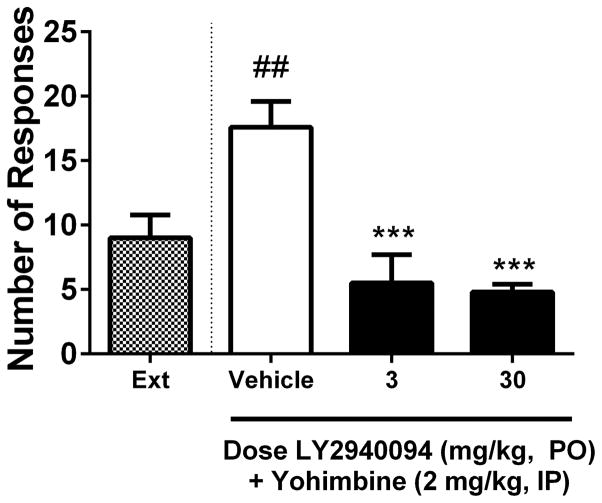
LY2940094 robustly blocked stress-induced reinstatement to ethanol-seeking in msP rats. Compared to extinction levels of responding, the anxiogenic agent and pharmacological stressor, yohimbine (2 mg/kg, IP), elicited a significant reinstatement to ethanol-seeking (white bar), and this effect was significantly reduced in rats receiving LY2940094 (3 or 30 mg/kg; black bars). Values represent the mean (+SEM) number of responses on the alcohol-associated lever. ##, p < 0.01 vs. responding during extinction; ***, p < 0.001 vs. vehicle-treated rats receiving yohimbine (2 mg/kg, IP) during the reinstatement session.
3.4 LY2940094 Blocks Ethanol-Stimulated Dopamine Release in the Nucleus Accumbens
Figure 5 shows the time course for changes in extracellular dopamine levels in the NAcc in response to ethanol challenge (1.1 g/kg, IP). The figure inset shows a summary comparison of the effects of the four treatment groups using the average post-injection dopamine response over 120 min. ANOVA revealed an overall significant difference between groups [(F(3,17) = 4.603, p < 0.02]. Subsequent pairwise comparisons demonstrated a significant ethanol-stimulated increase in extracellular dopamine levels (“Vehicle+Vehicle vs Vehicle+EtOH” group; t(10) = 2.42, p < 0.05), that was fully prevented by pretreatment with 30 mg/kg LY2940094 (“Vehicle+EtOH” vs “LY+EtOH”: t(9) = 3.10, p < 0.02; “Vehicle+Vehicle” vs “LY+EtOH”: p > 0.05).
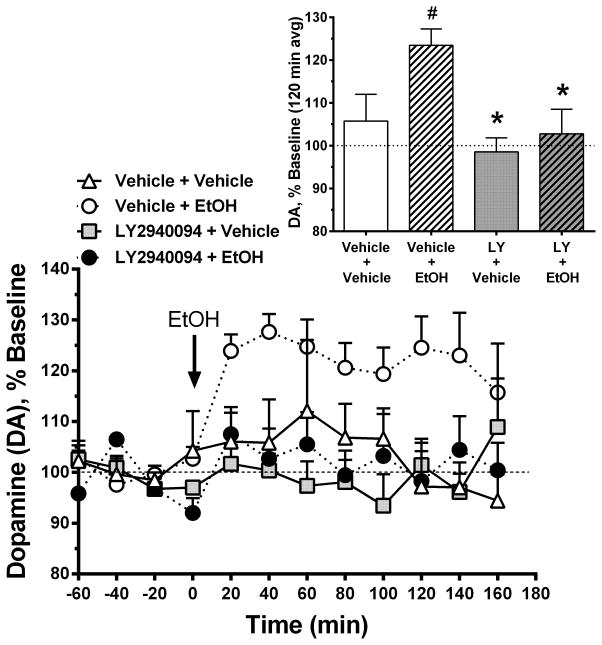
LY2940094 blocked ethanol-stimulated dopamine release in the Nucleus Accumbens. Main panel shows the time course of extracellular dopamine levels in response to ethanol challenge (1.1 g/kg, IP). Figure inset shows a summary of the mean extracellular dopamine levels in the 4 different treatment groups over the first 120 min after ethanol administration. Ethanol increased extracellular dopamine levels in the nucleus accumbens in rats receiving vehicle (“Vehicle + EtOH” group; open circles). Ethanol-stimulated increases in extracellular dopamine levels were prevented by pretreatment with 30 mg/kg LY2940094 (“LY2940094 + EtOH” group; filled circles), which neither increased nor decreased extracellular dopamine levels when administered alone (“LY2940094 + Vehicle” group; gray squares). The blockade of ethanol-stimulated dopamine release in the nucleus accumbens is consistent with decreased ethanol self-administration and decreased motivation to consume ethanol. Values represent the mean (+SEM) concentration of extracellular dopamine in nucleus accumbens dialysate, as a percentage of the baseline concentration (average of three samples taken immediately prior to drug injection).
LY2940094, per se, neither increased nor decreased extracellular dopamine levels when administered alone (“LY2940094+Vehicle” vs “Vehicle+Vehicle”: p > 0.05).
4. Discussion
The current study provides the first in vivo evidence that blockade of endogenous N/OFQ signaling by oral administration of a NOP receptor antagonist attenuates or prevents ethanol intake, motivation to consume ethanol, and stress-induced relapse to ethanol-seeking in animal models. The importance and novelty of these findings can be contextualized by the large literature documenting that NOP receptor agonists also produce these ethanol-attenuating effects and that the effects of agonists can be prevented by NOP receptor antagonists (c.f., overview and discussion in (Witkin et al., 2014); (Zaveri, 2011)).
LY2940094 is a potent and selective NOP receptor antagonist, with a Ki = 0.105 nM and a Kb = 0.166 nM and no agonist efficacy at concentrations up to 10 μM in CHO cells recombinantly expressing human NOP receptors (Statnick et al., 2016). The doses of LY2940094 used in the current study were based on receptor occupancy data; specifically, a 10 mg/kg PO dose of LY2940094 yielded 62% NOP receptor occupancy in the brain (Toledo et al., 2014). In the current study, LY2940094 dose-dependently reduced homecage ethanol self-administration in both P and msP rats, without affecting food or water intake or general locomotor activity, an effect that was maintained without evidence of tolerance for up to 4 days of subchronic dosing (Figures 1 and and22).
We also report here that LY2940094 reduced the motivation to consume ethanol, as indicated by decreased progressive ratio operant responding and breakpoints for ethanol (Arnold and Roberts, 1997) in Indiana P rats. Additionally, LY2940094 completely blocked the ability of the alpha2 adrenoreceptor antagonist, yohimbine (a known anxiogenic agent and pharmacological stressor), to reinstate ethanol-seeking in msP rats, even at the lowest dose tested (3 mg/kg). The current results are paradoxical considering nearly two decades of research indicating that NOP agonists reduce the reinforcing and motivating effects of ethanol and block stress-induced reinstatement to ethanol-seeking (Ciccocioppo et al., 1999), (Ciccocioppo et al., 2004),(Ciccocioppo et al., 2014),(Martin-Fardon et al., 2000). The mechanism by which LY2940094 blocked stress-induced reinstatement and motivation to consume ethanol is unknown, but the data are consistent with recent data showing that LY2940094 produced anxiolytic-like efficacy in preclinical models (Witkin et al., under review). It is interesting to note that, although the level of reinstatement following yohimbine was moderate, LY2940094 showed 10-fold greater potency for blocking this behavior relative to efficacy in other models reported herein that did not involve stress.
The suppression of ethanol self-administration and ethanol-motivated behaviors by LY2940094 reported here are similar to those reported previously using peptide and non-peptide NOP receptor agonists (Ciccocioppo et al., 1999), (Kuzmin et al., 2007), (Ciccocioppo et al., 2004). Therefore, the current results raise the question: how do NOP antagonists produce similar effects as those previously reported for NOP agonists? Our current working hypothesis of the comparable ethanol-attenuating effects of NOP receptor agonists and antagonists is that agonists may produce desensitization and internalization of NOP receptors when administered in vivo, therefore reducing expression of NOP receptors at the plasma membrane which are available to bind N/OFQ agonists, thus resulting in a “functional” receptor blockade. Indeed, multiple labs have demonstrated rapid and robust receptor desensitization and internalization in vitro in response to the endogenous ligand as well as to small molecule synthetic agonists (Corbani et al., 2004, Dautzenberg et al., 2001, Spampinato et al., 2007). For example, the small molecule NOP agonist, Ro 64-6198, produced sustained desensitization and internalization of NOP receptors expressed in recombinant cells, which was prevented by both low temperature and hyperosmolar sucrose conditions that are known to prevent receptor internalization (Dautzenberg et al., 2001). Full recovery of NOP receptor binding sites was observed 24 hr after treatment in that study, consistent with canonical agonist-mediated regulation and trafficking. In another study, when monensin was used to block intracellular protein transport and inhibit receptor recycling, the proportion of membrane-expressed receptors in a desensitized state was increased (Spampinato and Baiula, 2006). Although NOP agonist-induced receptor desensitization and internalization still needs to be demonstrated in vivo, the initial findings reported here lead us to speculate that the efficacy of the agonists reported in the literature may be related to the reduced availability, and attenuated endogenous signaling, of the NOP receptor upon agonist exposure, thus producing a “functional” blockade of NOP receptors after sub-chronic administration. This hypothesis is indirectly supported by published data indicating that acute administration of NOP agonists in vivo was either not effective or even sometimes increased ethanol self-administration (Ciccocioppo et al., 1999), whereas repeated administration of the agonists was required to reliably attenuate ethanol-motivated behaviors (Ciccocioppo et al., 1999, Economidou et al., 2006, Economidou et al., 2008),(Ciccocioppo et al., 2014), suggesting sustained agonist-induced receptor desensitization and internalization in vivo. In contrast, LY2940094 acutely blocks Gi/o-coupled signaling of the NOP receptor (Toledo et al., 2014), (Statnick et al., 2016), presumably without triggering receptor internalization and recycling to the plasma membrane, although this has proven very difficult to measure in native tissue ex vivo preparations. Relatedly, one might also anticipate that chronic administration of a NOP antagonist may lead to compensatory upregulation of NOP receptors, possibly leading to tolerance. Our results provide preliminary evidence for lack of tolerance after subchronic dosing; however, additional work will be required to explore the effects of chronic administration.
An alternative explanation to the receptor desensitization and internalization hypothesis is that agonists and antagonists of NOP receptors may produce different patterns of activation or inhibition in different brain areas relevant to reward and addiction. That is, perhaps the volume of distribution of LY2940094 across relevant reward-related brain areas is different than the volume of brain distribution of the agonists, and the differential modulation of NOP receptors in different brain areas could potentially explain the paradoxical results reported herein. However, distribution patterns of NOP receptor expression (in the unblocked condition) reported in nonhuman primate positron-emission-tomography (PET) studies using the NOP agonist PET ligand 11C-NOP-A (Kimura et al., 2011) and the NOP antagonist PET ligand 11C-(S)-10c (Pike et al., 2011) were remarkably similar to each other in rhesus monkey, and both were strikingly similar to that reported in humans with 11C-NOP-A (Lohith et al., 2012) and 11C-LY2959530 (Post et al., 2014). Therefore, it is unlikely that differential distribution of NOP agonists and antagonists in various reward-related brain regions can explain the anomalous findings reported here. Another alternative hypothesis is that the agonists and antagonists bind different splice variants of the NOP receptor, thus leading to differential pharmacology. Previous studies have reported evidence that the rat NOP receptor appears to be alternatively spliced and that the splice variants displayed differential tissue distribution (Curro et al., 2001). In that study, three different splice variants were detected in rat brain tissue. However, a later study assessing human tissue reported no evidence for expression of alternative splice variants in human brain tissue, the ultimate target of interest (Berthele et al., 2003). Thus, even if splice variants existed in the rodent, yielding differential pharmacology with different ligands, it is unlikely that would translate into observable differences in human pharmacology. Regardless, the possibility that different NOP receptor splice variants exist in the rodent which may contribute to differential pharmacology cannot be completely excluded with the current data. A third alternative hypothesis to explain similar effects produced by NOP agonists and antagonists is ligand-biased signaling (Kenakin, 2011). It is possible that NOP antagonists, or LY2940094 in particular, exhibit biased signaling which leads to functional activation of similar intracellular signaling cascades as produced by NOP agonists. Indeed, a recent report provided the first evidence that NOP agonists MCOPPB and NNC 63-0532 exhibit biased signaling for G-protein versus arrestin recruitment, and that simple mutations of the NOP antagonist J-113,397 resulted in partial agonist activity in vitro (Chang et al., 2015). However, we did not observe agonist activity in vitro with LY2940094 at concentrations up to 10 μM (Statnick et al., 2016). Further work will be required to determine the mechanism by which NOP agonists and antagonists produce similar efficacy in animal models of ethanol-motivated behaviors.
The ethanol-dampening effects of LY2940094 reported here were apparently NOP receptor-mediated and specific to ethanol self-administration rather than reflecting a nonspecific effect on consummatory or locomotor behaviors, as food/water consumption and locomotor activity were not affected in the same rats (Figures 1 and and2).2). Moreover, in a previous study, LY2940094 reduced hyperphagic food intake in several preclinical models, without affecting normal food consumption, and the efficacy was NOP receptor-mediated, as evidenced by the lack of effect in mice lacking NOP receptors (Statnick et al., 2016). Moreover, we previously demonstrated that, over the dose range tested, LY2940094 selectively occupies NOP receptors in the brain and inhibits NOP agonist-mediated decreases in body temperature (Toledo et al., 2014),(Statnick et al., 2016). Therefore, we are fairly confident that the effects produced by LY2940094 reported here were mediated by NOP receptor antagonism.
It has been well-established that drugs abused by humans, including ethanol, stimulate dopamine release, particularly in the nucleus accumbens (Di Chiara and Imperato, 1988). We replicate those findings here, showing that IP-administered ethanol stimulated dopamine release when administered alone. Importantly, LY2940094 completely blocked the IP-ethanol-stimulated dopamine release, without directly affecting dopamine release on its own (Figure 5). Since the ethanol was administered IP, blockade of ethanol-stimulated dopamine release by LY2940094 was independent of changes in ethanol consumption and, therefore, not likely confounded by nonspecific changes in motivation or reward or motor effects. Importantly, LY2940094 did not stimulate dopamine release in the nucleus accumbens when administered alone, suggesting reduced propensity for abuse potential. This neurochemical evidence supports the behavioral results and provides a possible mechanism for the reduction of ethanol drinking by NOP antagonists; specifically, attenuation of the reinforcing effects of ethanol (Weiss and Koob, 2001). As with the behavioral studies, the neurochemical data were not anticipated, considering others have reported that N/OFQ suppressed dopamine release in the nucleus accumbens (Murphy et al., 1996) (Koizumi et al., 2004a) and that NOP antagonists UFP-101 and Compound B (chemical name: 1-[(3R,4R)-1-cyclooctylmethyl-3-hydroxymethyl-4-piperidyl]-3-ethyl-1,3-dihydro-2H-benzimidazol-2-one) blocked the dopamine-suppressing effects of N/OFQ (Koizumi et al., 2004b, Koizumi et al., 2004a). Additional work will have to be conducted to fully clarify the mechanisms through which NOP receptors regulate mesolimbic dopamine transmission.
5. Conclusions
The current study provides the first in vivo evidence that a NOP receptor antagonist, such as the potent and selective NOP antagonist LY2940094, attenuates ethanol self-administration and motivation to consume ethanol in animal models of alcoholism. Moreover, we demonstrate that LY2940094 blocks stress-induced reinstatement to ethanol-seeking and ethanol-stimulated dopamine release in the nucleus accumbens, a critical locus in the brain reward pathway (Koob et al., 2014). The data suggest that NOP receptor antagonists, such as LY2940094, may have therapeutic benefit in the treatment of alcohol dependence and alcohol use disorders, as evidenced by efficacy in multiple behavioral and neurochemical preclinical models of alcoholism.
Footnotes
Funding and Disclosure: Studies were sponsored and funded by Eli Lilly and Co., Indianapolis, IN, USA. LMRK, CJW, JMW, MAMG, BLA, JSK, KWP, MAT, ND, CL, AJ, AB, CP, and MAS were employees of, and stockholders in, Eli Lilly and Company at the time the experiments were conducted. The work by RC, SS, and FW was partially supported by grant NIH/NIAAA AA014351.
References
- Arnold JM, Roberts DC. A critique of fixed and progressive ratio schedules used to examine the neural substrates of drug reinforcement. Pharmacol Biochem Behav. 1997;57:441–447. [Abstract] [Google Scholar]
- Berthele A, Platzer S, Dworzak D, Schadrack J, Mahal B, Buttner A, Assmus HP, Wurster K, Zieglgansberger W, Conrad B, Tolle TR. [3H]-nociceptin ligand-binding and nociceptin opioid receptor mrna expression in the human brain. Neuroscience. 2003;121:629–640. [Abstract] [Google Scholar]
- Cenci MA, Kalen P, Mandel RJ, Bjorklund A. Regional differences in the regulation of dopamine and noradrenaline release in medial frontal cortex, nucleus accumbens and caudate-putamen: a microdialysis study in the rat. Brain Res. 1992;581:217–228. [Abstract] [Google Scholar]
- Chang SD, Mascarella SW, Spangler SM, Gurevich VV, Navarro HA, Carroll FI, Bruchas MR. Quantitative Signaling and Structure-Activity Analyses Demonstrate Functional Selectivity at the Nociceptin/Orphanin FQ Opioid Receptor. Mol Pharmacol. 2015;88:502–511. [Europe PMC free article] [Abstract] [Google Scholar]
- Ciccocioppo R, Economidou D, Fedeli A, Angeletti S, Weiss F, Heilig M, Massi M. Attenuation of ethanol self-administration and of conditioned reinstatement of alcohol-seeking behaviour by the antiopioid peptide nociceptin/orphanin FQ in alcohol-preferring rats. Psychopharmacology (Berl) 2004;172:170–178. [Europe PMC free article] [Abstract] [Google Scholar]
- Ciccocioppo R, Panocka I, Polidori C, Regoli D, Massi M. Effect of nociceptin on alcohol intake in alcohol-preferring rats. Psychopharmacology (Berl) 1999;141:220–224. [Abstract] [Google Scholar]
- Ciccocioppo R, Stopponi S, Economidou D, Kuriyama M, Kinoshita H, Heilig M, Roberto M, Weiss F, Teshima K. Chronic treatment with novel brain-penetrating selective NOP receptor agonist MT-7716 reduces alcohol drinking and seeking in the rat. Neuropsychopharmacology. 2014;39:2601–2610. [Europe PMC free article] [Abstract] [Google Scholar]
- Corbani M, Gonindard C, Meunier JC. Ligand-regulated internalization of the opioid receptor-like 1: a confocal study. Endocrinology. 2004;145:2876–2885. [Abstract] [Google Scholar]
- Curro D, Yoo JH, Anderson M, Song I, Del Valle J, Owyang C. Molecular cloning of the orphanin FQ receptor gene and differential tissue expression of splice variants in rat. Gene. 2001;266:139–145. [Abstract] [Google Scholar]
- Dautzenberg FM, Wichmann J, Higelin J, Py-Lang G, Kratzeisen C, Malherbe P, Kilpatrick GJ, Jenck F. Pharmacological characterization of the novel nonpeptide orphanin FQ/nociceptin receptor agonist Ro 64-6198: rapid and reversible desensitization of the ORL1 receptor in vitro and lack of tolerance in vivo. J Pharmacol Exp Ther. 2001;298:812–819. [Abstract] [Google Scholar]
- de Guglielmo G, Martin-Fardon R, Teshima K, Ciccocioppo R, Weiss F. MT-7716, a potent NOP receptor agonist, preferentially reduces ethanol seeking and reinforcement in post-dependent rats. Addict Biol. 2015;20:643–651. [Europe PMC free article] [Abstract] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–5278. [Europe PMC free article] [Abstract] [Google Scholar]
- Economidou D, Fedeli A, Fardon RM, Weiss F, Massi M, Ciccocioppo R. Effect of novel nociceptin/orphanin FQ-NOP receptor ligands on ethanol drinking in alcohol-preferring msP rats. Peptides. 2006;27:3299–3306. [Europe PMC free article] [Abstract] [Google Scholar]
- Economidou D, Hansson AC, Weiss F, Terasmaa A, Sommer WH, Cippitelli A, Fedeli A, Martin-Fardon R, Massi M, Ciccocioppo R, Heilig M. Dysregulation of nociceptin/orphanin FQ activity in the amygdala is linked to excessive alcohol drinking in the rat. Biol Psychiatry. 2008;64:211–218. [Europe PMC free article] [Abstract] [Google Scholar]
- Gavioli EC, Calo G. Antidepressant- and anxiolytic-like effects of nociceptin/orphanin FQ receptor ligands. Naunyn Schmiedebergs Arch Pharmacol. 2006;372:319–330. [Abstract] [Google Scholar]
- Gavioli EC, Calo G. Nociceptin/orphanin FQ receptor antagonists as innovative antidepressant drugs. Pharmacol Ther. 2013;140:10–25. [Abstract] [Google Scholar]
- Kenakin T. Functional selectivity and biased receptor signaling. J Pharmacol Exp Ther. 2011;336:296–302. [Abstract] [Google Scholar]
- Kimura Y, Fujita M, Hong J, Lohith TG, Gladding RL, Zoghbi SS, Tauscher JA, Goebl N, Rash KS, Chen Z, Pedregal C, Barth VN, Pike VW, Innis RB. Brain and whole-body imaging in rhesus monkeys of 11C-NOP-1A, a promising PET radioligand for nociceptin/orphanin FQ peptide receptors. J Nucl Med. 2011;52:1638–1645. [Europe PMC free article] [Abstract] [Google Scholar]
- Koob GF, Buck CL, Cohen A, Edwards S, Park PE, Schlosburg JE, Schmeichel B, Vendruscolo LF, Wade CL, Whitfield TW, Jr, George O. Addiction as a stress surfeit disorder. Neuropharmacology. 2014;76(Pt B):370–382. [Europe PMC free article] [Abstract] [Google Scholar]
- Kuzmin A, Kreek MJ, Bakalkin G, Liljequist S. The nociceptin/orphanin FQ receptor agonist Ro 64-6198 reduces alcohol self-administration and prevents relapse-like alcohol drinking. Neuropsychopharmacology. 2007;32:902–910. [Abstract] [Google Scholar]
- Kuzmin A, Sandin J, Terenius L, Ogren SO. Acquisition, expression, and reinstatement of ethanol-induced conditioned place preference in mice: effects of opioid receptor-like 1 receptor agonists and naloxone. J Pharmacol Exp Ther. 2003;304:310–318. [Abstract] [Google Scholar]
- Lambert DG. The nociceptin/orphanin FQ receptor: a target with broad therapeutic potential. Nat Rev Drug Discov. 2008;7:694–710. [Abstract] [Google Scholar]
- Lohith TG, Zoghbi SS, Morse CL, Araneta MF, Barth VN, Goebl NA, Tauscher JT, Pike VW, Innis RB, Fujita M. Brain and whole-body imaging of nociceptin/orphanin FQ peptide receptor in humans using the PET ligand 11C-NOP-1A. J Nucl Med. 2012;53:385–392. [Europe PMC free article] [Abstract] [Google Scholar]
- Martin-Fardon R, Ciccocioppo R, Massi M, Weiss F. Nociceptin prevents stress-induced ethanol- but not cocaine-seeking behavior in rats. Neuroreport. 2000;11:1939–1943. [Abstract] [Google Scholar]
- Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, Butour JL, Guillemot JC, Ferrara P, Monsarrat B, et al. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–535. [Abstract] [Google Scholar]
- Murphy NP, Ly HT, Maidment NT. Intracerebroventricular orphanin FQ/nociceptin suppresses dopamine release in the nucleus accumbens of anaesthetized rats. Neuroscience. 1996;75:1–4. [Abstract] [Google Scholar]
- Neal CR, Jr, Mansour A, Reinscheid R, Nothacker HP, Civelli O, Akil H, Watson SJ., Jr Opioid receptor-like (ORL1) receptor distribution in the rat central nervous system: comparison of ORL1 receptor mRNA expression with (125)I-[(14)Tyr]-orphanin FQ binding. J Comp Neurol. 1999a;412:563–605. [Abstract] [Google Scholar]
- Neal CR, Jr, Mansour A, Reinscheid R, Nothacker HP, Civelli O, Watson SJ., Jr Localization of orphanin FQ (nociceptin) peptide and messenger RNA in the central nervous system of the rat. J Comp Neurol. 1999b;406:503–547. [Abstract] [Google Scholar]
- Olianas MC, Dedoni S, Boi M, Onali P. Activation of nociceptin/orphanin FQ-NOP receptor system inhibits tyrosine hydroxylase phosphorylation, dopamine synthesis, and dopamine D(1) receptor signaling in rat nucleus accumbens and dorsal striatum. J Neurochem. 2008;107:544–556. [Abstract] [Google Scholar]
- Pike VW, Rash KS, Chen Z, Pedregal C, Statnick MA, Kimura Y, Hong J, Zoghbi SS, Fujita M, Toledo MA, Diaz N, Gackenheimer SL, Tauscher JT, Barth VN, Innis RB. Synthesis and evaluation of radioligands for imaging brain nociceptin/orphanin FQ peptide (NOP) receptors with positron emission tomography. J Med Chem. 2011;54:2687–2700. [Europe PMC free article] [Abstract] [Google Scholar]
- Post A, Smart T, Krikke-Workel J, Witkin J, Statnick M, Harmer C, Dawson G, Mohs R. The efficacy and safety of LY2940094, a selective nociceptin receptor antagonist, in patients with major depressive disorder: A randomized, double-blind, placebo-controlled study. Neuropsychopharmacology. 2014;39:S291–S472. [Google Scholar]
- Post A, Smart TS, Krikke-Workel J, Dawson GR, Harmer CJ, Browning M, Jackson K, Kakar R, Mohs R, Statnick M, Wafford K, McCarthy A, Barth V, Witkin JM. A Selective Nociceptin Receptor Antagonist to Treat Depression: Evidence from Preclinical and Clinical Studies. Neuropsychopharmacology 2015 [Europe PMC free article] [Abstract] [Google Scholar]
- Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, Grandy DK, Langen H, Monsma FJ, Jr, Civelli O. Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science. 1995;270:792–794. [Abstract] [Google Scholar]
- Rizzi A, Gavioli EC, Marzola G, Spagnolo B, Zucchini S, Ciccocioppo R, Trapella C, Regoli D, Calo G. Pharmacological characterization of the nociceptin/orphanin FQ receptor antagonist SB-612111 [(−)-cis-1-methyl-7-[[4-(2,6-dichlorophenyl)piperidin-1-yl]methyl]-6,7,8,9-tetrah ydro-5H-benzocyclohepten-5-ol]: in vivo studies. J Pharmacol Exp Ther. 2007;321:968–974. [Abstract] [Google Scholar]
- Rorick-Kehn LM, Witkin JM, Statnick MA, Eberle EL, McKinzie JH, Kahl SD, Forster BM, Wong CJ, Li X, Crile RS, Shaw DB, Sahr AE, Adams BL, Quimby SJ, Diaz N, Jimenez A, Pedregal C, Mitch CH, Knopp KL, Anderson WH, Cramer JW, McKinzie DL. LY2456302 is a novel, potent, orally-bioavailable small molecule kappa-selective antagonist with activity in animal models predictive of efficacy in mood and addictive disorders. Neuropharmacology. 2014;77:131–144. [Abstract] [Google Scholar]
- Spampinato S, Baiula M. Agonist-regulated endocytosis and desensitization of the human nociceptin receptor. Neuroreport. 2006;17:173–177. [Abstract] [Google Scholar]
- Spampinato S, Baiula M, Calienni M. Agonist-regulated internalization and desensitization of the human nociceptin receptor expressed in CHO cells. Current drug targets. 2007;8:137–146. [Abstract] [Google Scholar]
- Statnick MA, Chen Y, Ansonoff M, Witkin JM, Rorick-Kehn L, Suter TM, Song M, Hu C, Lafuente C, Jimenez A, Benito A, Diaz N, Martinez-Grau MA, Toledo MA, Pintar JE. A Novel Nociceptin Receptor Antagonist LY2940094 Inhibits Excessive Feeding Behavior in Rodents: A Possible Mechanism for the Treatment of Binge Eating Disorder. J Pharmacol Exp Ther. 2016;356:493–502. [Abstract] [Google Scholar]
- Toledo MA, Pedregal C, Lafuente C, Diaz N, Martinez-Grau MA, Jimenez A, Benito A, Torrado A, Mateos C, Joshi EM, Kahl SD, Rash KS, Mudra DR, Barth VN, Shaw DB, McKinzie D, Witkin JM, Statnick MA. Discovery of a novel series of orally active nociceptin/orphanin FQ (NOP) receptor antagonists based on a dihydrospiro(piperidine-4,7′-thieno[2,3-c]pyran) scaffold. J Med Chem. 2014;57:3418–3429. [Abstract] [Google Scholar]
- Waller MB, McBride WJ, Lumeng L, Li TK. Induction of dependence on ethanol by free-choice drinking in alcohol-preferring rats. Pharmacol Biochem Behav. 1982;16:501–507. [Abstract] [Google Scholar]
- Weiss F, Koob GF. Drug addiction: functional neurotoxicity of the brain reward systems. Neurotox Res. 2001;3:145–156. [Abstract] [Google Scholar]
- Witkin J, Statnick M, Rorick-Kehn L, Barth V, Wafford K, Pintar J, Perry K, Toledo M, Diaz N, Lafuente C, Jimenez A, Benito A, Martinez-Grau M, Pedregal C. A novel nociceptin-1 receptor antagonist produces novel antidepressant- and anxiolytic-related effects in rodents under review. [Google Scholar]
- Witkin JM, Statnick MA, Rorick-Kehn LM, Pintar JE, Ansonoff M, Chen Y, Tucker RC, Ciccocioppo R. The biology of Nociceptin/Orphanin FQ (N/OFQ) related to obesity, stress, anxiety, mood, and drug dependence. Pharmacol Ther. 2014;141:283–299. [Europe PMC free article] [Abstract] [Google Scholar]
- Zaveri NT. The nociceptin/orphanin FQ receptor (NOP) as a target for drug abuse medications. Curr Top Med Chem. 2011;11:1151–1156. [Europe PMC free article] [Abstract] [Google Scholar]
Full text links
Read article at publisher's site: https://doi.org/10.1111/acer.13052
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc4844902?pdf=render
Citations & impact
Impact metrics
Article citations
Effect of chronic delivery of the NOP/MOR partial agonist AT-201 and NOP antagonist J-113397 on heroin relapse in a rat model of opioid maintenance.
Psychopharmacology (Berl), 241(12):2497-2511, 13 Sep 2024
Cited by: 0 articles | PMID: 39269500 | PMCID: PMC11569015
Development of a genetically encoded sensor for probing endogenous nociceptin opioid peptide release.
Nat Commun, 15(1):5353, 25 Jun 2024
Cited by: 4 articles | PMID: 38918403 | PMCID: PMC11199706
Pharmacological blockage of NOP receptors decreases ventral tegmental area dopamine neuronal activity through GABAB receptor-mediated mechanism.
Neuropharmacology, 248:109866, 15 Feb 2024
Cited by: 0 articles | PMID: 38364970
Regulation of N-type calcium channels by nociceptin receptors and its possible role in neurological disorders.
Mol Brain, 15(1):95, 24 Nov 2022
Cited by: 2 articles | PMID: 36434658 | PMCID: PMC9700961
Review Free full text in Europe PMC
The life and times of endogenous opioid peptides: Updated understanding of synthesis, spatiotemporal dynamics, and the clinical impact in alcohol use disorder.
Neuropharmacology, 225:109376, 11 Dec 2022
Cited by: 2 articles | PMID: 36516892 | PMCID: PMC10548835
Review Free full text in Europe PMC
Go to all (35) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
NOP receptor antagonism attenuates reinstatement of alcohol-seeking through modulation of the mesolimbic circuitry in male and female alcohol-preferring rats.
Neuropsychopharmacology, 46(12):2121-2131, 20 Jul 2021
Cited by: 8 articles | PMID: 34285372 | PMCID: PMC8505627
Occupancy of Nociceptin/Orphanin FQ Peptide Receptors by the Antagonist LY2940094 in Rats and Healthy Human Subjects.
Drug Metab Dispos, 44(9):1536-1542, 27 Jun 2016
Cited by: 9 articles | PMID: 27353045
The nociceptin/orphanin FQ receptor agonist SR-8993 as a candidate therapeutic for alcohol use disorders: validation in rat models.
Psychopharmacology (Berl), 233(19-20):3553-3563, 11 Aug 2016
Cited by: 16 articles | PMID: 27515665 | PMCID: PMC5021736
The biology of Nociceptin/Orphanin FQ (N/OFQ) related to obesity, stress, anxiety, mood, and drug dependence.
Pharmacol Ther, 141(3):283-299, 01 Nov 2013
Cited by: 109 articles | PMID: 24189487 | PMCID: PMC5098338
Review Free full text in Europe PMC
Funding
Funders who supported this work.
Eli Lilly and Co., Indianapolis, IN, USA
NIAAA NIH HHS (1)
Grant ID: R01 AA014351
NIH/NIAAA (1)
Grant ID: AA014351





