Abstract
Free full text

Sleep Dysfunction and Gastrointestinal Diseases
Abstract
Sleep deprivation and impaired sleep quality have been associated with poor health outcomes. Many patients experience sleep disturbances, which can increase the risk of medical conditions such as hypertension, obesity, stroke, and heart disease as well as increase overall mortality. Recent studies have suggested that there is a strong association between sleep disturbances and gastrointestinal diseases. Proinflammatory cytokines, such as tumor necrosis factor, interleukin-1, and interleukin-6, have been associated with sleep dysfunction. Alterations in these cytokines have been seen in certain gastrointestinal diseases, such as gastroesophageal reflux disease, inflammatory bowel disease, liver disorders, and colorectal cancer. It is important for gastroenterologists to be aware of the relationship between sleep disorders and gastrointestinal illnesses to ensure good care for patients. This article reviews the current research on the interplay between sleep disorders, immune function, and gastrointestinal diseases.
There has been a recent surge of research evaluating sleep disturbances and their effect on overall health. Sleep disorders are estimated to affect 50 to 70 million Americans.1 Patients with sleep deprivation experience a reduction in productivity and quality of life along with an increase in accidents and errors in the workplace.2 Sleep disorders have been linked to neurocognitive effects such as slower response time, impaired attention, and increased likelihood of falling asleep at work.2 Most studies suggest that the daily sleep requirement for adults is 7 to 9 hours per night. The cumulative long-term effects of sleep deprivation have been linked to an increased risk of a wide array of comorbidities, including hypertension, diabetes, obesity, stroke, and heart attack.1 More importantly, long-term sleep deprivation has been found to increase overall morbidity and mortality.
Recent studies have suggested a strong association between sleep disturbances and gastrointestinal diseases.3-6 Although it is evident that sleep disturbances are often found in patients with gastrointestinal disease, it is difficult to determine the cause and effect of the disturbances. There is a unique interplay between certain gastrointestinal diseases and sleep. Poor sleep has been shown to result in the exacerbation of gastrointestinal symptoms. Conversely, many gastrointestinal diseases affect the sleep-wake cycle and lead to poor sleep.
This article will discuss the relationship between sleep and various gastrointestinal illnesses, including gastroesophageal reflux disease (GERD), peptic ulcer disease (PUD), irritable bowel syndrome (IBS), inflammatory bowel disease (IBD), colorectal cancer, and liver disease.
The Physiology of Sleep
Sleep is a state characterized by changes in the level of consciousness, unresponsiveness to the surrounding environment, and inactivity of voluntary muscles. Sleep restores people physically and psychologically. Sleep is divided by polysomnographic criteria into rapid eye movement (REM) sleep and non-rapid eye movement (NREM) sleep. NREM sleep constitutes approximately 75% to 80% of total sleep time, whereas REM sleep accounts for the remaining 20% to 25% of total sleep time.1 REM sleep is associated with dreaming, learning, and memory consolidation.1
The sleep-wake system is regulated by 2 processes that create the circadian rhythm of sleep and wakefulness: one that promotes sleep (process S) and one that maintains wakefulness (process C).7 These processes are used to predict the timing and duration of sleep. Process S, the homeostatic sleep drive, builds with sustained wakefulness and peaks before bedtime as the need for sleep increases. Additionally, process S is involved in inhibiting neuronal communication in the hypothalamus, which turns off arousal mechanisms during sleep.1 Disorders that affect process S promote insomnia.7 Clock-dependent alertness, known as process C, is responsible for promoting alertness, physical activity, muscle tone, and hormone secretion. Process C promotes wakefulness.
The circadian rhythm originates in the suprachiasmatic nucleus (SCN) in the anterior hypothalamus and functions as a person’s biological clock. This biological clock is a 24-hour clock that may be regulated by the timing of melatonin secretion. The SCN regulates melatonin, cortisol, and a person’s core body temperature. Alterations of the SCN lead to abnormalities in the circadian rhythm and changes in a person’s sleep-wake cycles.8
The Autonomic and Enteric Nervous Systems
The connection between the brain and the gastrointestinal system is imperative to the regulation of the digestive tract and maintenance of the gut immune system. The gut-brain axis works through mechanisms that involve immune activation, intestinal permeability, and enteroendocrine signaling.9 This bidirectional network involves the central nervous system (CNS), autonomic nervous system, and enteric nervous system (ENS). The network incorporates sympathetic and parasympathetic activity, which drive afferent signals through enteric and vagal pathways to the CNS and efferent signals from the CNS to the intestine.9 There are neural and hormonal influences that allow the brain to modulate the activity of intestinal cells such as interstitial cells of Cajal, enterochromaffin cells, and smooth muscle cells.9 This dynamic relationship between the brain and the gastrointestinal system involves feedback loops, which also influence the circadian rhythm and sleep regulation pathways. This suggests that there is a relationship between sleep disturbances and physiologic changes of the gastrointestinal tract.
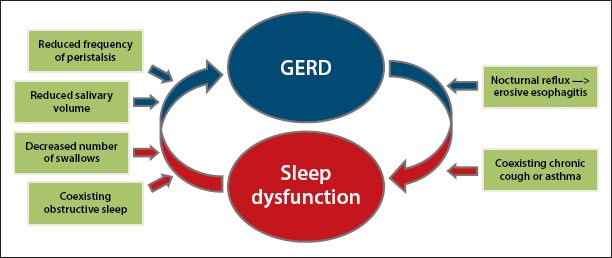
From the initiation of mastication to forward propagation of food, along with enzymatic breakdown of food and the absorption of key nutrients, the act of digestion is a complex system. Prior studies have suggested that changes occur in the digestive process during sleep. During sleep, the esophagus becomes susceptible to gastric acid injury, as there are a decreased number of swallows, reduced salivary volume, and decreased frequency of peristalsis, leading to increased gastric acid reflux. Salivary production declines from 0.5 mL/minute when awake to nearly 0 mL/minute in sleep.10 Swallowing rates decrease from approximately 25 times per hour when awake to 5 times per hour during sleep.11 This results in the prolongation of acid clearance and loss of esophageal protective mechanisms, leading to an increased risk of mucosal damage and esophagitis.12
The stomach plays 2 important roles in digestion: the acidification of ingested food and the forward propulsion of food into the duodenum. Acid secretion can be highly variable but shows a circadian pattern with peak secretion between 10 PM and 2 AM.13 This peak in acid secretion, however, may not correlate with gastric pH during NREM and REM sleep. Watanabe and colleagues showed that intragastric pH values were significantly lower in awake patients than patients in NREM and REM sleep.14
The migrating motor complexes (MMCs) are the waves of electrical activity that move through the gastrointestinal tract in a regular cycle. The cycling of the MMCs is controlled by the ENS, which is an independent system that mediates activity in the absence of CNS input. Kumar and colleagues evaluated the synchrony between NREM/ REM sleep and enteric MMCs and found that during sleep, there is a significant reduction in the MMC cycle length.15 However, the distribution of MMCs in NREM and REM sleep was consistent with a random distribution.15 Orr and Chen demonstrated that the amplitude of the gastric cycle declines in NREM sleep and returns in REM sleep.16 The small bowel functions primarily to absorb nutrients and propel the food bolus toward the colon. Small intestinal motility diminishes during sleep.17
The colon is primarily involved in water absorption and temporary containment of intestinal material. Colonic contractions are significantly reduced during the night and are nearly eliminated during NREM sleep.18 REM sleep, however, is associated with increased colonic pressure. To maintain fecal continence during sleep, the internal anal sphincter retains active pressure independent of external sphincter activity, and anal canal pressure remains above rectal pressure.19
The Immune System and Gastrointestinal Diseases
Cytokines are key mediators of the immune system and work to induce and control inflammation. Research shows that the sleep-wake cycle is affected by the release of cytokines, specifically interleukin (IL)-1, IL-6, and tumor necrosis factor (TNF), which increase fatigue and promote sleep.20 Conversely, sleep loss has been shown to activate IL-1 and TNF.16
There is a strong body of evidence that supports changes in cytokine release, specifically an increase in proinflammatory cytokines, in most gastrointestinal disorders. Changes in serum cytokine levels with these underlying diseases will affect sleep, as cytokines are major modulators of the sleep-wake cycle.20-22 Vgontzas and colleagues studied 25 young healthy subjects and restricted their sleep from 8 hours per night to 6 hours per night for 1 week.23 The 24-hour secretion of IL-6 increased in men and women and TNF increased in men.23 This study supports the key role that cytokines play in the modulation of the sleep-wake cycle.
IL-8 has been extensively studied in GERD and is expressed in high amounts in the affected mucosa of GERD patients. Additionally, IL-1 and IL-6 are known to be key mediators in the control of inflammatory responses in patients with GERD.24,25 Cytokines are central in the recruitment and activation of inflammatory cells in patients with Helicobacter pylori—induced ulcers. A recent study has shown that patients with IBS had significantly higher serum levels of proinflammatory cytokines, such as IL-1, IL-6, and TNF, when compared with healthy individuals.26 In an animal study, Tang and colleagues demonstrated that chronic sleep deprivation can exacerbate colonic inflammation in mice and should, therefore, be considered a possible contributory factor to disease flares in IBD patients.27
Serum levels of multiple cytokines, including TNF, IL-6, and IL-8, as well as levels of vascular endothelial growth factor have been shown to be elevated in patients with colorectal cancer.28 Cytokines are not only produced by tumor cells, but also produced by surrounding cells such as macrophages and mast cells, which are recruited by the tumor. Additionally, IL-6 is a growth factor for colon cancer and promotes tumor cell growth and survival.29
Gastroesophageal Reflux Disease and Sleep
Sleep disturbances are commonly reported in patients with GERD (Figure). Approximately 10% to 20% of individuals in the United States complain of weekly GERD symptoms.30 A Gallup survey revealed that among people who experience weekly heartburn, 79% report nocturnal symptoms. Among those, 63% reported that symptoms affected their ability to sleep, and 40% believed that nocturnal heartburn impaired their ability to function the following day.30 Furthermore, Jansson and colleagues performed a population study of over 6500 patients in Norway and found that the risk of GERD symptoms in patients with insomnia was 3 times greater than in those with no sleep complaints.31 These studies suggest that most GERD patients have nocturnal symptoms resulting in an increased risk of sleep disturbances.
Nocturnal reflux differs from reflux during arousal, as there is a decrease in swallowing and salivary secretion during the night as well as decreased esophageal peristalsis and prolonged esophageal clearance, which lead to increased gastric acid reflux.32 There is a correlation between nocturnal reflux symptoms and increased risks of erosive esophagitis and sleep disorders. Chen and colleagues evaluated over 3000 patients with GERD symptoms and found a 2-fold increase in the risk of sleep disturbance among patients experiencing GERD.5 A significant proportion of patients with reflux had endoscopic evidence of erosive esophagitis (554/653 patients, 84%). In addition, Chen and colleagues showed that there was an increase in the severity of erosive changes with reduced quality of sleep.5 This suggests that reduced quality of sleep in patients with high-grade esophagitis may cause an increased risk of Barrett esophagus and esophageal adenocarcinoma.
Studies have suggested that there may be a relationship between obstructive sleep apnea (OSA) and GERD. GERD has been estimated to affect 58% to 62% of patients with OSA.33 Demeter and Pap suggested that sleep apnea causes negative intrathoracic pressure, resulting in intermittent airway obstruction as the intrathoracic pressure decreases.33 This induces transient lower esophageal sphincter relaxation along with reduced esophageal clearance mechanisms, predisposing patients to increased GERD.33 However, it has not been possible to replicate these findings in further studies. A study by Kuribayashi and colleagues showed that during obstructive events, there is a compensatory increase in the upper and lower esophageal sphincter pressure, which is a protective mechanism, and no reflux events were seen during apnea.34 A study conducted by Shepherd and colleagues showed a positive relationship between nighttime heartburn symptoms and the risk of sleep apnea.35
Furthermore, the literature suggests a relationship between obesity as it relates to increased risks of GERD and OSA. Fisher and colleagues studied 30 morbidly obese patients being evaluated for bariatric surgery.36 This study found a significant relationship between elevated body mass index (BMI) and increased esophageal acid exposure and episodes of GERD.36 Furthermore, obesity predisposes patients to OSA. A study conducted by Kerr and colleagues demonstrated a dramatic reduction in GERD frequency in OSA patients treated with nasal continuous positive airway pressure.37 In patients with coexisting OSA and GERD, health care providers should ensure that OSA is adequately treated with nasal continuous positive pressure. This is increasingly important in patients who are intolerant of proton pump inhibitors and/or H2 blockers.
Peptic Ulcer Disease and Sleep
Data on the relationship between sleep disorders and the risk of PUD are sparse. Most of the data regarding the incidence of PUD have been obtained from studies on shift workers. A Japanese study by Segawa and colleagues noted the prevalence of gastric ulcers (2.38% vs 1.38%, respectively) for shift workers and day workers.38 For duodenal ulcers, the prevalence was 1.37% in shift workers and 0.69% in day workers.38 The relative risk for peptic ulcers was 2.18 for shift workers compared with day workers.38 Possible etiologies for the higher risk of PUD in shift workers may be unpredictable timing of meals, sleep dysfunction, work stress, and concomitant use of nonsteroidal anti-inflammatory drugs.
Patients with OSA are at a higher risk for primary sleep disorders. These patients undergo episodes of intermittent hypoxia causing systemic inflammation, oxidative stress, and sympathetic activation, which, in turn, have been linked to cardiovascular comorbidities. Shiao and colleagues evaluated 35,000 patients and found that patients with sleep apnea experienced a 2.4-fold higher risk of peptic ulcer bleeding.39 More research is warranted to further evaluate the relationship between PUD and sleep disorders.
Irritable Bowel Syndrome, Functional Dyspepsia, and Sleep
IBS is defined as abdominal discomfort associated with changes in bowel habits. Emerging research on the pathophysiology of IBS has included bowel dysmotility, small bowel bacterial overgrowth, autonomic dysfunction, visceral hyperalgesia, and microscopic inflammation.40 Functional dyspepsia refers to discomfort in the upper abdomen that may be described as postprandial heaviness, early satiety, bloating, nausea, and/or epigastric pain. Emerging insight into the pathophysiology of functional dyspepsia suggests that dysmotility, visceral hypersensitivity, and the immune system all play a role in the pathogenesis of the condition.41 IBS is a common disorder that affects a significant percentage of the general population. It is likely that patients with IBS and functional dyspepsia have increased nocturnal autonomic arousal, which may result in poor sleep. It has also been postulated that patients with IBS spend more time in REM sleep, which is characterized by arousals and stimulatory effects on colonic motility, which may lead to sleep disturbance.42
Numerous studies have shown that patients with IBS and/or functional dyspepsia report poor sleep. Morito and colleagues studied 2936 patients and found that the presence of functional dyspepsia and IBS was a statistically significant risk factor for poor sleep.43 Fass and colleagues found that 57.2% of patients studied with IBS and/or functional dyspepsia reported abdominal pain and/or discomfort that awakened them from sleep, resulting in disrupted sleep. Overall, patients with functional dyspepsia, with and without IBS symptoms, reported sleep disturbances more often than healthy subjects. The reported sleep changes in patients with only IBS symptoms, however, were no different from healthy subjects in this study.44 Moreover, the increase in the prevalence of sleep disturbance was directly related to the patient’s perceived intensity of their gastrointestinal symptoms. In a small study, Heitkemper and colleagues found reductions in nighttime melatonin and tryptophan levels in women with IBS, which may contribute to poor sleep quality in this population.45
Although most studies have used questionnaires or patient reporting to establish sleep changes, few studies have used polysomnography to quantify changes in sleep in IBS patients. Elsenbruch and colleagues also found that patients with IBS reported poor sleep significantly more than healthy controls.46 There was no objective difference between the 2 groups when using polysomnography as a marker of sleep quality. Both groups had a similar number of arousals, amount of slow wave sleep, and amount of REM sleep.46 This study supports the notion that sleep disturbances in patients with IBS may be related to a hyperresponsiveness or exaggerated reaction to normal stimuli rather than actual changes in sleep architecture.
It has recently been suggested that microscopic colonic inflammation may play a role in the pathophysiology of IBS. Studies have shown an increased number of lymphocytic inflammatory cells specifically in patients with postinfectious IBS. As discussed previously, it has been demonstrated that sleep deprivation results in upregulation of the immune system.47 It is possible that sleep deprivation leads to an increase in microscopic inflammation in the bowel, which may, in turn, result in gastrointestinal symptoms.
Small intestinal bacterial overgrowth (SIBO) is a syndrome defined by an increased amount of bacteria in the small bowel. There have been a number of studies that show patients with IBS having abnormal lactulose breath test results, suggesting that SIBO is closely related to IBS. Given this relationship, treatment with the antibiotics neomycin or rifaximin (Xifaxan, Salix) may improve symptoms of IBS. Pimentel and colleagues found that 10 days of treatment with rifaximin resulted in a statistically significant improvement in IBS symptoms compared with placebo in a randomized, double-blinded study, and symptom improvement was sustained through 10 weeks.48 Moreover, it has been postulated that patients with IBS and/or SIBO have increased sleep disturbance. Weinstock and colleagues also found that restless leg syndrome, which leads to disrupted sleep, was seen more often in patients with IBS and SIBO compared with the general population.49 It is essential to consider concurrent SIBO in IBS patients with reported sleep changes.
Not only does research support IBS causing changes in sleep, but several studies have shown that poor sleep affects underlying IBS symptoms or causes functional dyspepsia. Buchanan and colleagues demonstrated that self-reported poor sleep quality significantly predicted higher next-day abdominal pain, anxiety, and fatigue. Furthermore, poor sleep efficiency, according to actigraphy, significantly predicted worsening next-day anxiety and fatigue.50
Vege and colleagues conducted a population-based study evaluating 2269 patients and found that among the patients who complained of poor sleep, 33.3% met the criteria for IBS and 21.3% for functional dyspepsia.51 The association between IBS and sleep disturbance was statistically significant. Wells and colleagues studied 205 resident physicians working overnight calls and found that 19% met Rome III criteria for IBS.52 This study showed that IBS was more frequent in residents with fewer hours of sleep while on call. For every fewer hour of sleep while on call, there was a 32% increase in the risk of IBS.52 Similarly, a study conducted by Kim and colleagues found that the incidence of IBS in rotating night shift workers was significantly higher than in daytime workers.53
Given the strong association between poor sleep and IBS, Song and colleagues performed the first randomized, controlled trial evaluating the effect of melatonin in patients with IBS.54 The researchers found that 2 weeks of treatment with melatonin significantly reduced reported symptoms in IBS patients. There was no difference seen in polysomnography between the patients treated with melatonin and those treated with placebo.54 Furthermore, Chojnacki and colleagues suggested that after 6 months of melatonin administration, 50% of the study population with constipation-predominant IBS experienced improvement in constipation.55 Saha and colleagues also showed significant improvement in quality of life in patients with IBS who were treated with melatonin compared with the placebo group.56
Inflammatory Bowel Disease and Sleep
Sleep disturbances in IBD has been a major area of focus over the past several years. Multiple theories have been proposed regarding the etiology of sleep dysfunction in patients with IBD. Nocturnal symptoms of abdominal pain, rectal urgency, and diarrhea can result in sleep disturbances. Medications involved in IBD management, including corticosteroids, have been associated with sleep disturbances. Alternately, sleep disturbances have been shown to impact immune function and the development of inflammation. Sleep deprivation causes an upregulation of the immune function, which, in turn, activates inflammatory cells and increases the risk of infection.4 The activation of the immune system is associated with an increase in proinflammatory cytokines, such as IL-1, IL-6, and TNF, along with an elevation in C-reactive protein (CRP) levels. Animal studies have shown that sleep restriction has been associated with an increase in these proinflammatory cytokines, which are markers of activity in IBD. Furthermore, Tang and colleagues’ mouse model of colitis and the effects of sleep deprivation on colitis found that acute and chronic intermittent sleep deprivation worsen the severity of colonic inflammation.27 Studies on sleep dysfunction have yielded similar results in other chronic inflammatory conditions, such as HIV, rheumatoid arthritis, and systemic lupus erythematosus. The chronic inflammation in IBD and resulting disturbed sleep create a vicious cycle with a negative feedback loop. Poor sleep leads to the production of inflammatory cytokines, which, in turn, worsens colitis, and the cycle continues.
Several tools have been used to evaluate sleep disturbances in humans. Buysse and colleagues developed a sleep evaluation tool, the Pittsburgh Sleep Quality Index (PSQI), which is a 19-item self-rated questionnaire that assesses sleep quality and disturbances over a 1-month period.57 This index has a sensitivity of 90% and specificity of 86% for distinguishing good sleepers from poor sleepers.57 Recently, increased attention has been given to the relationship between sleep disturbances and disease activity in IBD. Ali and colleagues conducted a prospective observational cohort study confirming the quality of sleep and disease activity in IBD using the PSQI scale.3 All patients with active disease had an abnormal PSQI score. Of the 30 patients with histologic evidence of inflammation on endoscopic evaluation, all were found to have abnormal PSQI scores independent of clinical disease activity (odds ratio, 6.0; P<.0001). The study concluded that an abnormal PSQI score has a positive predictive value of 83% for histologic inflammation, which suggests a strong association between poor sleep quality and clinically active IBD.3 Similar findings were seen in a cohort study conducted by Graff and colleagues, which showed that patients with active IBD (77%) experienced worse sleep than those with inactive IBD (49%).58 However, this study utilized laboratory markers such as CRP as markers of disease activity without endoscopic evaluation.
These studies support the premise that disease activity in IBD contributes to disturbed sleep; however, few studies have examined the role of sleep disturbances on the risk of relapse in IBD. Ananthakrishnan and colleagues conducted a longitudinal Internet-based cohort study of 3173 patients that showed that Crohn’s disease patients in remission with reported impaired sleep had a 2-fold increased risk of active disease at 6 months.6 There was no change in disease activity seen in ulcerative colitis patients who reported impaired sleep.6 Studies involving rheumatoid arthritis patients have shown that therapy with anti-TNF drugs have improved sleep quality.59 It is difficult to ascertain, however, whether the medication directly improved sleep or whether control of inflammation resulted in improved sleep. These studies suggest that sleep quality may be a modifiable risk factor to prevent symptomatic flare in IBD patients.
Melatonin has been studied as a potential treatment in IBD.60 Animal models have shown that melatonin provides anti-inflammatory benefits with reduction in the severity of mucosal inflammation. Rakhimova conducted a study evaluating the use of melatonin in IBD patients and showed improvement in inflammation in 78% of Crohn’s disease patients and 88% of ulcerative colitis patients.61 This study shows promising results with the use of melatonin to improve sleep, resulting in potential improvement in gastrointestinal symptoms and inflammation in a variety of disease states. However, further research is needed to explore the treatment of underlying sleep dysfunction with the goal of improving disease activity in IBD.
Colorectal Cancer and Sleep
There has been recent research to support an increase in risk for colon cancer with changes in sleep. Both decreased sleep duration and increased sleep duration have been associated with colorectal cancer in recent studies. There have been many proposed mechanisms for the potential increased risk. Release of inflammatory cytokines in patients with sleep changes can potentially set the stage for the development of colorectal cancer. Additionally, both shorter sleep duration and longer sleep duration have been associated with obesity, which is an independent risk factor for colorectal cancer.62,63
Thompson and colleagues studied 1240 patients and found that those with fewer than 6 hours of sleep per night had close to a 50% increased risk of colorectal adenomas compared with patients who slept 7 hours or more.62 In this study, there was no association between quality of sleep and colorectal adenomas. Jiao and colleagues studied post-menopausal women and found that there was an increased risk of colorectal cancer in those who slept fewer than 6 hours per night or more than 9 hours per night.63 Furthermore, Patel and colleagues found increased levels of CRP and IL-6 with prolonged sleep duration.64 This study found a statistically significant increase in CRP levels and IL-6 levels for each hour past the mean reported sleep duration of 7.6 hours. Additionally, for each hour of sleep duration under 7.6 hours, the TNF levels increased by 8%.64 These studies demonstrate cytokine release with subsequent systemic inflammation potentially increasing the risk for colorectal cancer in patients with sleep deprivation.
Zhang and colleagues found that men who slept more than 9 hours per night had an increased risk of colorectal cancer compared with those who slept 7 hours per night.65 This risk was increased if a patient was overweight with a BMI greater than 25 or was a regular snorer. However, the findings for women in this study were not statistically significant. Having a longer sleep duration increases cortisol secretion along with insulin resistance, resulting in obesity, which is an independent risk factor for colorectal cancer.65 Moreover, a longer sleep duration leads to increased proinflammatory cytokine release, specifically of IL-1 and TNF, which play a role in new tumor growth. Snoring results in intermittent hypoxia, which can also promote tumor growth.66
Given the assumed disruption of sleep in night shift workers, there have been multiple studies on these individuals and the risk of colorectal cancer. For example, Schernhammer and colleagues found an increased risk for colorectal cancer in individuals working rotating night shifts at least 3 nights per month for 15 or more years.67
Liver Disease and Sleep
Sleep changes in patients with cirrhosis complicated by hepatic encephalopathy have been well studied. Although explored less, sleep changes can also be seen in patients with chronic liver disease without hepatic encephalopathy. Abnormalities in the circadian rhythm are thought to be responsible for sleep abnormalities seen in chronic liver disease. There are multiple factors that contribute to these changes in the circadian process. First, cirrhotic patients may have changes in the function of the SCN, leading to dysfunction in the sleep-wake cycle.68 There may also be changes in melatonin secretion with delayed melatonin peak during the night, leading to sleep disruption.69,70 Impaired hepatic metabolism of melatonin in cirrhosis has also been described leading to elevated daytime levels of melatonin and sleep disruption.71
The inversion of the sleep-wake cycle with excessive daytime sleepiness and restless nights is well documented in patients with hepatic encephalopathy and thought to be one of the first signs of having this condition. The buildup of ammonia leads to changes in multiple neurotransmitters, including dopamine, gamma-aminobutyric acid, and nitric oxide, resulting in changes in brain activity that can be seen on electroencephalogram (EEG). On EEG, triphasic waves are associated with stage 1 hepatic encephalopathy, as is slowing of the background alpha rhythm. In patients with higher levels of ammonia and advanced hepatic encephalopathy, the EEG slows, with delta waves present in comatose patients with stage IV hepatic encephalopathy.72
Multiple studies have shown that patients with cirrhosis have difficulty obtaining restful and adequate sleep.8,73,74 Cordoba and colleagues studied patients with cirrhosis, without hepatic encephalopathy or alcohol use, and found that 47.7% reported unsatisfactory sleep when compared with healthy subjects.8 Their findings were confirmed with actigraphy. Specifically, cirrhotic patients complained of shorter sleep times (<6 hours per night), more frequent nocturnal awakenings, and more difficulty falling asleep, along with a preference for evening activities due to morning fatigue.8 Sleep disturbances were likely secondary to a malfunctioning circadian system controlled by the SCN. This is different from the sleep pattern inversion seen in patients with hepatic encephalopathy.8 These findings were confirmed in a study conducted by Montagnese and colleagues, which showed that cirrhotic patients had poorer sleep quality and took a longer time to fall asleep compared with healthy study controls.70 Tarter and colleagues found that 35% of nonalcoholic cirrhotic patients reported difficulties with sleep.74 These studies demonstrate sleep disturbances in cirrhotic patients without hepatic encephalopathy.
Hepatitis C virus (HCV) infection is a major cause of liver disease worldwide. According to the Centers for Disease Control and Prevention, approximately 2.7 million people in the United States are chronically infected with HCV, and 29,718 new cases were reported in 2013.75 According to the World Health Organization, approximately 150,000 new cases occur annually in the United States and Western Europe combined.76 Sleep disturbances have been described in studies of patients with chronic HCV infection. Carlson and colleagues found that 63% of patients with chronic HCV infection had sleep disturbances with no differences seen between patients with or without underlying psychiatric conditions.77 Additionally, a significant portion of patients with HCV infection have neurocognitive symptoms, such as weakness, malaise, fatigue, changes in concentration, and/or quality-of-life impairment, also called brain fog. These changes are independent of the severity of the liver disease.78
OSA affects approximately 3% to 7% of adult men and 2% to 5% of adult women in the general population.79 OSA plays a role in many gastrointestinal diseases. Multiple studies have shown that hypoxia secondary to OSA may lead to an increase in fat accumulation in the liver causing nonalcoholic fatty liver disease (NAFLD) and/or hepatic inflammation leading to nonalcoholic steatohepatitis. Additionally, studies have shown that OSA may lead to sleep disturbances in cirrhosis and further confuse the interpretation of sleep changes often seen in cirrhotic patients.80 Obesity is a well-studied risk factor for both sleep apnea and NAFLD.21,81-83
Future Implications
Sleep disorders have a strong impact on gastrointestinal diseases, and, conversely, many gastrointestinal disease processes influence the sleep-wake cycle and sleep quality. This is a connection that has previously been overlooked. Given the recent research that has shown a strong interplay, clinicians should not ignore the relationship between gastrointestinal diseases and sleep.
Obesity plays a significant role in multiple gastrointestinal disease processes, such as GERD, hepatic steatosis, and potentially colon cancer. Obesity is a major risk factor for sleep apnea, resulting in poor sleep, which can, in turn, lead to gastrointestinal disease. A strong focus on weight reduction can improve existing symptoms in patients with underlying gastrointestinal conditions. Furthermore, weight reduction can potentially reduce the risk of a patient developing certain disease states.
Research has shown that treating a patient’s underlying sleep disorder may result in improvement in their gastrointestinal symptoms. Furthermore, control of gastrointestinal disease states will result in improved sleep quality. It is, therefore, important for gastroenterologists to take a detailed sleep history and equally important for sleep specialists to identify any underlying gastrointestinal diseases in order to better care for patients.
References
Articles from Gastroenterology & Hepatology are provided here courtesy of Millenium Medical Publishing
Citations & impact
Impact metrics
Article citations
Sleep quality disparities in different pregnancy trimesters in low- and middle-income countries: a systematic review and meta-analysis.
BMC Pregnancy Childbirth, 24(1):627, 01 Oct 2024
Cited by: 0 articles | PMID: 39354392 | PMCID: PMC11446071
Review Free full text in Europe PMC
The Bidirectional Relationship Between Sleep Disturbance and Functional Dyspepsia: A Systematic Review to Understand Mechanisms and Implications on Management.
Cureus, 16(8):e66098, 03 Aug 2024
Cited by: 0 articles | PMID: 39229406 | PMCID: PMC11370990
Review Free full text in Europe PMC
The association of dietary inflammatory index with sleep outcomes: A systematic review.
Health Promot Perspect, 14(2):136-147, 29 Jul 2024
Cited by: 0 articles | PMID: 39291049 | PMCID: PMC11403336
Review Free full text in Europe PMC
The impact of diet, exercise, and sleep on Helicobacter pylori infection with different occupations: a cross-sectional study.
BMC Infect Dis, 24(1):692, 11 Jul 2024
Cited by: 0 articles | PMID: 38992594 | PMCID: PMC11241877
Circadian rhythm regulates the function of immune cells and participates in the development of tumors.
Cell Death Discov, 10(1):199, 27 Apr 2024
Cited by: 4 articles | PMID: 38678017 | PMCID: PMC11055927
Review Free full text in Europe PMC
Go to all (40) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Sleep, immunity and inflammation in gastrointestinal disorders.
World J Gastroenterol, 19(48):9231-9239, 01 Dec 2013
Cited by: 72 articles | PMID: 24409051 | PMCID: PMC3882397
Review Free full text in Europe PMC
Wake-up Call to Clinicians: The Impact of Sleep Dysfunction on Gastrointestinal Health and Disease.
J Clin Gastroenterol, 52(3):194-203, 01 Mar 2018
Cited by: 6 articles | PMID: 29189428
Review
Gastrointestinal physiology and digestive disorders in sleep.
Curr Opin Pulm Med, 15(6):571-577, 01 Nov 2009
Cited by: 11 articles | PMID: 19797956
Review
The effect of sleep on gastrointestinal functioning in common digestive diseases.
Lancet Gastroenterol Hepatol, 5(6):616-624, 01 Jun 2020
Cited by: 50 articles | PMID: 32416862
Review