Abstract
Free full text

Light regulates expression of brain-derived neurotrophic factor mRNA in rat visual cortex.
Abstract
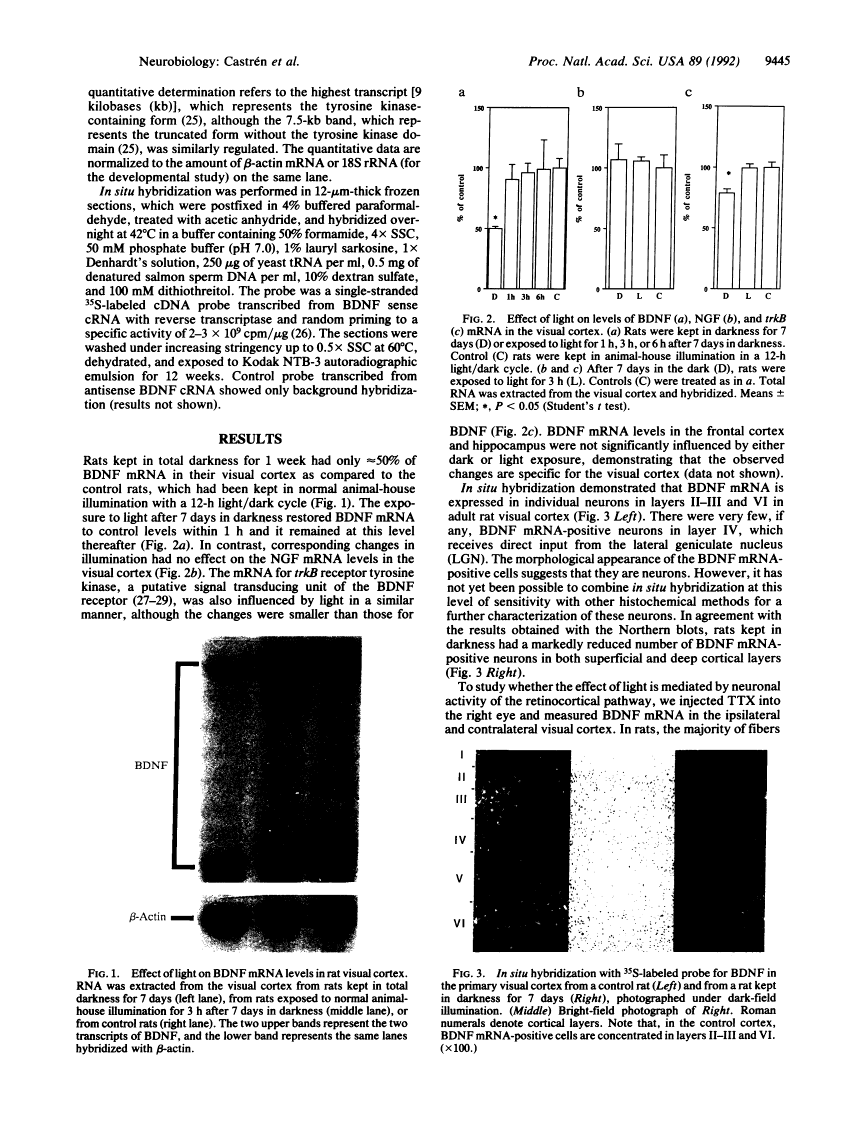
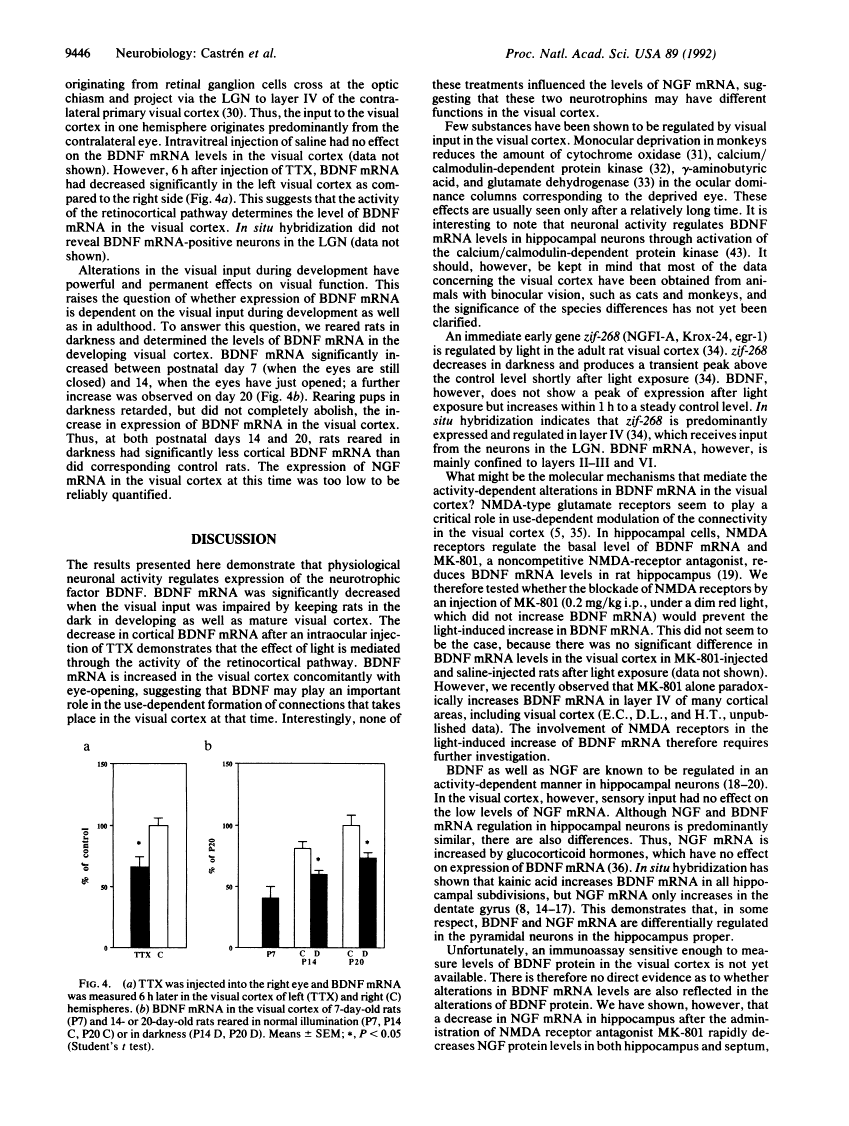
Specific sensory input has profound transient and long-lasting effects on the function of corresponding sensory cortical areas both during development and in adulthood. To study whether neurotrophic factors might play a role in such processes, we investigated the effects of light on the nerve growth factor and brain-derived neurotrophic factor (BDNF) mRNA levels in rat visual cortex. Keeping adult rats in the dark or preventing normal activity of retinal ganglion cells by intraocular injection of tetrodotoxin significantly decreased the levels of BDNF mRNA in the visual cortex but not in other cortical areas. Exposure to light after a period in darkness rapidly restored the mRNA to control levels. These alterations in visual input had no effect on nerve growth factor mRNA. The mRNA of trkB, the putative signal-transducing receptor unit for BDNF, was also decreased in darkness, although less than BDNF mRNA. BDNF mRNA levels increased in the visual cortex of newborn rats after eye-opening. This increase is retarded, although not completely abolished, by rearing the pups in darkness. Thus, the levels of BDNF mRNA are rapidly regulated by sensory input during development and in adulthood. BDNF may therefore play an important role in formation and in activity-dependent modulation of specific connections in the visual cortex.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.1M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Wiesel TN, Hubel DH. Comparison of the effects of unilateral and bilateral eye closure on cortical unit responses in kittens. J Neurophysiol. 1965 Nov;28(6):1029–1040. [Abstract] [Google Scholar]
- Hubel DH, Wiesel TN. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol. 1970 Feb;206(2):419–436. [Abstract] [Google Scholar]
- Kaas JH, Krubitzer LA, Chino YM, Langston AL, Polley EH, Blair N. Reorganization of retinotopic cortical maps in adult mammals after lesions of the retina. Science. 1990 Apr 13;248(4952):229–231. [Abstract] [Google Scholar]
- Gilbert CD, Wiesel TN. Receptive field dynamics in adult primary visual cortex. Nature. 1992 Mar 12;356(6365):150–152. [Abstract] [Google Scholar]
- Kleinschmidt A, Bear MF, Singer W. Blockade of "NMDA" receptors disrupts experience-dependent plasticity of kitten striate cortex. Science. 1987 Oct 16;238(4825):355–358. [Abstract] [Google Scholar]
- Ayer-LeLievre C, Olson L, Ebendal T, Seiger A, Persson H. Expression of the beta-nerve growth factor gene in hippocampal neurons. Science. 1988 Jun 3;240(4857):1339–1341. [Abstract] [Google Scholar]
- Whittemore SR, Friedman PL, Larhammar D, Persson H, Gonzalez-Carvajal M, Holets VR. Rat beta-nerve growth factor sequence and site of synthesis in the adult hippocampus. J Neurosci Res. 1988 Aug;20(4):403–410. [Abstract] [Google Scholar]
- Gall CM, Isackson PJ. Limbic seizures increase neuronal production of messenger RNA for nerve growth factor. Science. 1989 Aug 18;245(4919):758–761. [Abstract] [Google Scholar]
- Bandtlow CE, Meyer M, Lindholm D, Spranger M, Heumann R, Thoenen H. Regional and cellular codistribution of interleukin 1 beta and nerve growth factor mRNA in the adult rat brain: possible relationship to the regulation of nerve growth factor synthesis. J Cell Biol. 1990 Oct;111(4):1701–1711. [Europe PMC free article] [Abstract] [Google Scholar]
- Ernfors P, Wetmore C, Olson L, Persson H. Identification of cells in rat brain and peripheral tissues expressing mRNA for members of the nerve growth factor family. Neuron. 1990 Oct;5(4):511–526. [Abstract] [Google Scholar]
- Hofer M, Pagliusi SR, Hohn A, Leibrock J, Barde YA. Regional distribution of brain-derived neurotrophic factor mRNA in the adult mouse brain. EMBO J. 1990 Aug;9(8):2459–2464. [Europe PMC free article] [Abstract] [Google Scholar]
- Phillips HS, Hains JM, Laramee GR, Rosenthal A, Winslow JW. Widespread expression of BDNF but not NT3 by target areas of basal forebrain cholinergic neurons. Science. 1990 Oct 12;250(4978):290–294. [Abstract] [Google Scholar]
- Wetmore C, Ernfors P, Persson H, Olson L. Localization of brain-derived neurotrophic factor mRNA to neurons in the brain by in situ hybridization. Exp Neurol. 1990 Aug;109(2):141–152. [Abstract] [Google Scholar]
- Ballarín M, Ernfors P, Lindefors N, Persson H. Hippocampal damage and kainic acid injection induce a rapid increase in mRNA for BDNF and NGF in the rat brain. Exp Neurol. 1991 Oct;114(1):35–43. [Abstract] [Google Scholar]
- Ernfors P, Bengzon J, Kokaia Z, Persson H, Lindvall O. Increased levels of messenger RNAs for neurotrophic factors in the brain during kindling epileptogenesis. Neuron. 1991 Jul;7(1):165–176. [Abstract] [Google Scholar]
- Gall C, Murray K, Isackson PJ. Kainic acid-induced seizures stimulate increased expression of nerve growth factor mRNA in rat hippocampus. Brain Res Mol Brain Res. 1991 Jan;9(1-2):113–123. [Abstract] [Google Scholar]
- Isackson PJ, Huntsman MM, Murray KD, Gall CM. BDNF mRNA expression is increased in adult rat forebrain after limbic seizures: temporal patterns of induction distinct from NGF. Neuron. 1991 Jun;6(6):937–948. [Abstract] [Google Scholar]
- Zafra F, Hengerer B, Leibrock J, Thoenen H, Lindholm D. Activity dependent regulation of BDNF and NGF mRNAs in the rat hippocampus is mediated by non-NMDA glutamate receptors. EMBO J. 1990 Nov;9(11):3545–3550. [Europe PMC free article] [Abstract] [Google Scholar]
- Zafra F, Castrén E, Thoenen H, Lindholm D. Interplay between glutamate and gamma-aminobutyric acid transmitter systems in the physiological regulation of brain-derived neurotrophic factor and nerve growth factor synthesis in hippocampal neurons. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10037–10041. [Europe PMC free article] [Abstract] [Google Scholar]
- Lu B, Yokoyama M, Dreyfus CF, Black IB. Depolarizing stimuli regulate nerve growth factor gene expression in cultured hippocampal neurons. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6289–6292. [Europe PMC free article] [Abstract] [Google Scholar]
- Zilles K, Wree A, Schleicher A, Divac I. The monocular and binocular subfields of the rat's primary visual cortex: a quantitative morphological approach. J Comp Neurol. 1984 Jul 1;226(3):391–402. [Abstract] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. [Abstract] [Google Scholar]
- Lindholm D, Heumann R, Hengerer B, Thoenen H. Interleukin 1 increases stability and transcription of mRNA encoding nerve growth factor in cultured rat fibroblasts. J Biol Chem. 1988 Nov 5;263(31):16348–16351. [Abstract] [Google Scholar]
- Klein R, Conway D, Parada LF, Barbacid M. The trkB tyrosine protein kinase gene codes for a second neurogenic receptor that lacks the catalytic kinase domain. Cell. 1990 May 18;61(4):647–656. [Abstract] [Google Scholar]
- Middlemas DS, Lindberg RA, Hunter T. trkB, a neural receptor protein-tyrosine kinase: evidence for a full-length and two truncated receptors. Mol Cell Biol. 1991 Jan;11(1):143–153. [Europe PMC free article] [Abstract] [Google Scholar]
- Schnürch H, Risau W. Differentiating and mature neurons express the acidic fibroblast growth factor gene during chick neural development. Development. 1991 Apr;111(4):1143–1154. [Abstract] [Google Scholar]
- Soppet D, Escandon E, Maragos J, Middlemas DS, Reid SW, Blair J, Burton LE, Stanton BR, Kaplan DR, Hunter T, et al. The neurotrophic factors brain-derived neurotrophic factor and neurotrophin-3 are ligands for the trkB tyrosine kinase receptor. Cell. 1991 May 31;65(5):895–903. [Abstract] [Google Scholar]
- Squinto SP, Stitt TN, Aldrich TH, Davis S, Bianco SM, Radziejewski C, Glass DJ, Masiakowski P, Furth ME, Valenzuela DM, et al. trkB encodes a functional receptor for brain-derived neurotrophic factor and neurotrophin-3 but not nerve growth factor. Cell. 1991 May 31;65(5):885–893. [Abstract] [Google Scholar]
- Klein R, Nanduri V, Jing SA, Lamballe F, Tapley P, Bryant S, Cordon-Cardo C, Jones KR, Reichardt LF, Barbacid M. The trkB tyrosine protein kinase is a receptor for brain-derived neurotrophic factor and neurotrophin-3. Cell. 1991 Jul 26;66(2):395–403. [Europe PMC free article] [Abstract] [Google Scholar]
- Wong-Riley M, Carroll EW. Effect of impulse blockage on cytochrome oxidase activity in monkey visual system. Nature. 1984 Jan 19;307(5948):262–264. [Abstract] [Google Scholar]
- Hendry SH, Kennedy MB. Immunoreactivity for a calmodulin-dependent protein kinase is selectively increased in macaque striate cortex after monocular deprivation. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1536–1540. [Europe PMC free article] [Abstract] [Google Scholar]
- Hendry SH, Jones EG. Reduction in number of immunostained GABAergic neurones in deprived-eye dominance columns of monkey area 17. Nature. 1986 Apr 24;320(6064):750–753. [Abstract] [Google Scholar]
- Worley PF, Christy BA, Nakabeppu Y, Bhat RV, Cole AJ, Baraban JM. Constitutive expression of zif268 in neocortex is regulated by synaptic activity. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5106–5110. [Europe PMC free article] [Abstract] [Google Scholar]
- Constantine-Paton M, Cline HT, Debski E. Patterned activity, synaptic convergence, and the NMDA receptor in developing visual pathways. Annu Rev Neurosci. 1990;13:129–154. [Abstract] [Google Scholar]
- Lindholm Dan, Castrén Eero, Hengerer Bastian, Zafra Francisco, Berninger Benedikt, Thoenen Hans. Differential Regulation of Nerve Growth Factor (NGF) Synthesis in Neurons and Astrocytes by Glucocorticoid Hormones. Eur J Neurosci. 1992;4(5):404–410. [Abstract] [Google Scholar]
- Domenici L, Berardi N, Carmignoto G, Vantini G, Maffei L. Nerve growth factor prevents the amblyopic effects of monocular deprivation. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8811–8815. [Europe PMC free article] [Abstract] [Google Scholar]
- Kaplan DR, Martin-Zanca D, Parada LF. Tyrosine phosphorylation and tyrosine kinase activity of the trk proto-oncogene product induced by NGF. Nature. 1991 Mar 14;350(6314):158–160. [Abstract] [Google Scholar]
- Klein R, Jing SQ, Nanduri V, O'Rourke E, Barbacid M. The trk proto-oncogene encodes a receptor for nerve growth factor. Cell. 1991 Apr 5;65(1):189–197. [Abstract] [Google Scholar]
- Vazquez ME, Ebendal T. Messenger RNAs for trk and the low-affinity NGF receptor in rat basal forebrain. Neuroreport. 1991 Oct;2(10):593–596. [Abstract] [Google Scholar]
- Rodriguez-Tébar A, Dechant G, Barde YA. Binding of brain-derived neurotrophic factor to the nerve growth factor receptor. Neuron. 1990 Apr;4(4):487–492. [Abstract] [Google Scholar]
- Klein R, Martin-Zanca D, Barbacid M, Parada LF. Expression of the tyrosine kinase receptor gene trkB is confined to the murine embryonic and adult nervous system. Development. 1990 Aug;109(4):845–850. [Abstract] [Google Scholar]
Associated Data
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.89.20.9444
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc50148?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1073/pnas.89.20.9444
Article citations
Neuron and astrocyte specific 5mC and 5hmC signatures of BDNF's receptor, TrkB.
Front Mol Neurosci, 17:1463437, 29 Aug 2024
Cited by: 0 articles | PMID: 39268252 | PMCID: PMC11390696
Circadian rhythm of brain-derived neurotrophic factor in serum and plasma.
Exp Physiol, 109(10):1755-1767, 06 Aug 2024
Cited by: 1 article | PMID: 39105714 | PMCID: PMC11442779
Fractal Phototherapy in Maximizing Retina and Brain Plasticity.
Adv Neurobiol, 36:585-637, 01 Jan 2024
Cited by: 0 articles | PMID: 38468055
A human in vitro neuronal model for studying homeostatic plasticity at the network level.
Stem Cell Reports, 18(11):2222-2239, 19 Oct 2023
Cited by: 1 article | PMID: 37863044 | PMCID: PMC10679660
Psycho-Cognitive Profile and NGF and BDNF Levels in Tears and Serum: A Pilot Study in Patients with Graves' Disease.
Int J Mol Sci, 24(9):8074, 29 Apr 2023
Cited by: 0 articles | PMID: 37175781 | PMCID: PMC10178719
Go to all (310) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Mismatch between BDNF mRNA and protein expression in the developing visual cortex: the role of visual experience.
Eur J Neurosci, 13(4):709-721, 01 Feb 2001
Cited by: 48 articles | PMID: 11207806
Dark rearing blocks the developmental down-regulation of brain-derived neurotrophic factor messenger RNA expression in layers IV and V of the rat visual cortex.
Neuroscience, 88(2):393-403, 01 Jan 1999
Cited by: 20 articles | PMID: 10197762
Rapid regulation of brain-derived neurotrophic factor mRNA within eye-specific circuits during ocular dominance column formation.
J Neurosci, 20(4):1470-1483, 01 Feb 2000
Cited by: 64 articles | PMID: 10662837 | PMCID: PMC6772351
Complexity in the modulation of neurotrophic factor mRNA expression by early visual experience.
Brain Res Dev Brain Res, 143(2):225-232, 01 Jul 2003
Cited by: 6 articles | PMID: 12855194