Abstract
Free full text

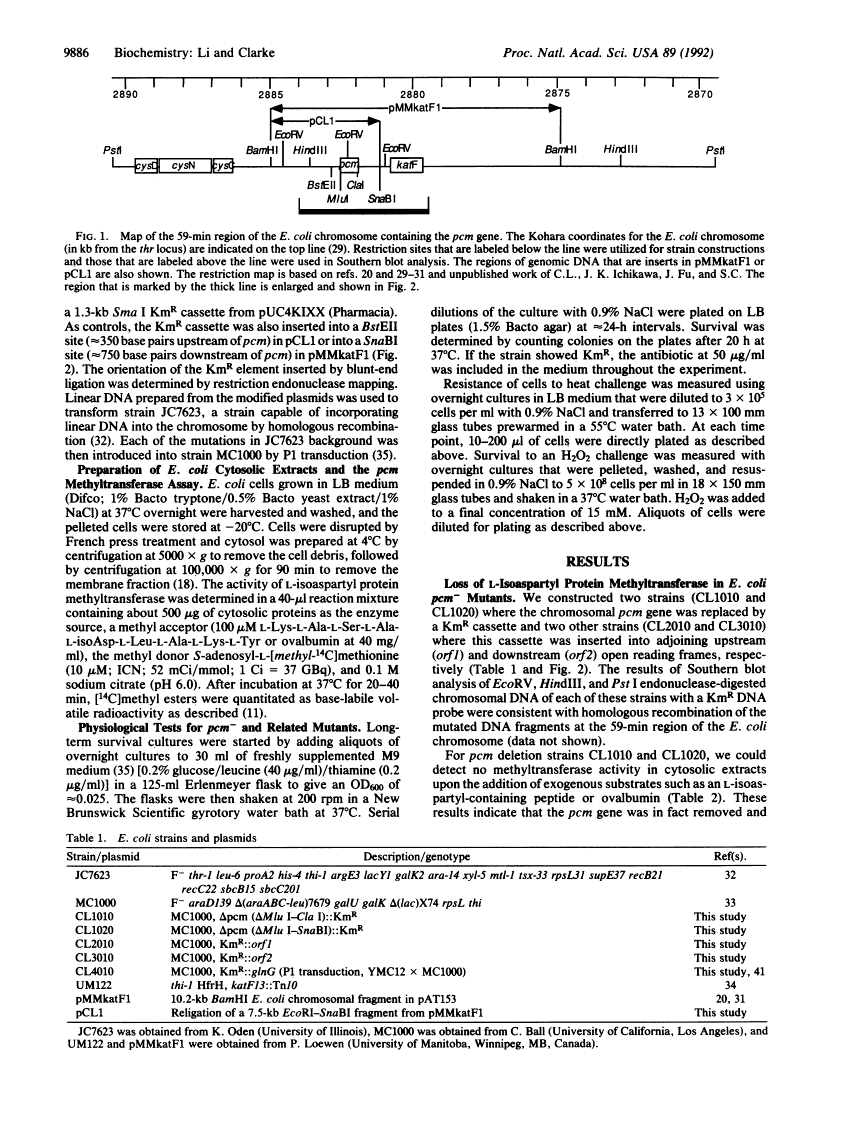
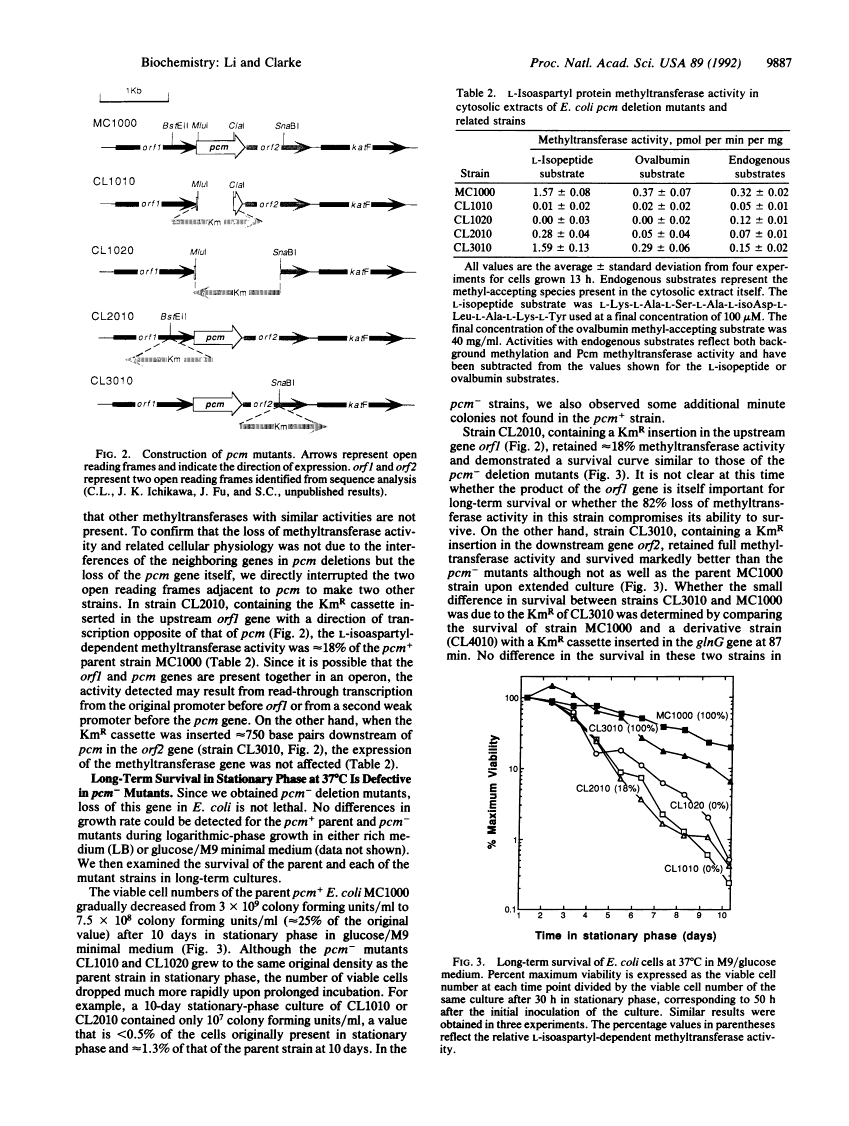
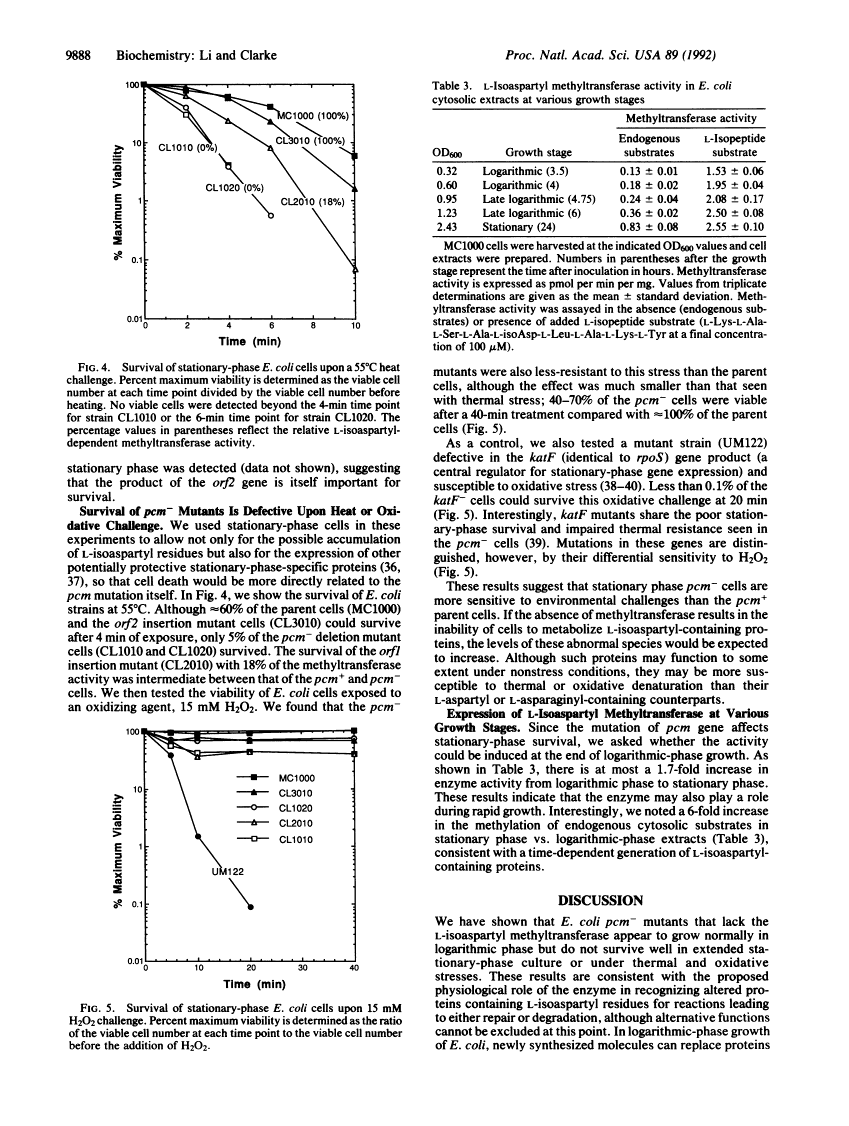
A protein methyltransferase specific for altered aspartyl residues is important in Escherichia coli stationary-phase survival and heat-shock resistance.
Abstract
Proteins are subject to spontaneous degradation reactions including the deamidation, isomerization, and racemization of asparaginyl and aspartyl residues. A major product of these reactions, the L-isoaspartyl residue, is recognized with high affinity by the protein-L-isoaspartate(D-aspartate) O-methyltransferase (EC 2.1.1.77). This enzyme catalyzes the methyl esterification of the L-isoaspartyl residue in a reaction that can initiate its conversion to the normal aspartyl configuration. To directly study the physiological role of this methyltransferase, especially with respect to the potential repair of isomerized aspartyl residues in aging proteins, we examined the ability of the bacterium Escherichia coli to survive in the absence of its activity. We utilized gene disruption techniques to replace the chromosomal copy of the pcm gene that encodes the methyltransferase with a kanamycin-resistance cassette to produce mutants that have no detectable L-isoaspartyl methyltransferase activity. Although no changes in exponential-phase growth were observed, pcm- mutants did not survive well upon extended culture into stationary phase or upon heat challenge at 55 degrees C. These results provide genetic evidence for a role of the L-isoaspartyl methyltransferase in the metabolism of altered proteins that can accumulate in aging cells and limit their viability.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.0M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Stadtman ER. Protein modification in aging. J Gerontol. 1988 Sep;43(5):B112–B120. [Abstract] [Google Scholar]
- Harding JJ, Beswick HT, Ajiboye R, Huby R, Blakytny R, Rixon KC. Non-enzymic post-translational modification of proteins in aging. A review. Mech Ageing Dev. 1989 Oct;50(1):7–16. [Abstract] [Google Scholar]
- Geiger T, Clarke S. Deamidation, isomerization, and racemization at asparaginyl and aspartyl residues in peptides. Succinimide-linked reactions that contribute to protein degradation. J Biol Chem. 1987 Jan 15;262(2):785–794. [Abstract] [Google Scholar]
- Patel K, Borchardt RT. Chemical pathways of peptide degradation. III. Effect of primary sequence on the pathways of deamidation of asparaginyl residues in hexapeptides. Pharm Res. 1990 Aug;7(8):787–793. [Abstract] [Google Scholar]
- Johnson BA, Langmack EL, Aswad DW. Partial repair of deamidation-damaged calmodulin by protein carboxyl methyltransferase. J Biol Chem. 1987 Sep 5;262(25):12283–12287. [Abstract] [Google Scholar]
- George-Nascimento C, Lowenson J, Borissenko M, Calderón M, Medina-Selby A, Kuo J, Clarke S, Randolph A. Replacement of a labile aspartyl residue increases the stability of human epidermal growth factor. Biochemistry. 1990 Oct 16;29(41):9584–9591. [Abstract] [Google Scholar]
- Clarke S. Protein carboxyl methyltransferases: two distinct classes of enzymes. Annu Rev Biochem. 1985;54:479–506. [Abstract] [Google Scholar]
- Barten DM, O'Dea RF. The function of protein carboxylmethyltransferase in eucaryotic cells. Life Sci. 1990;47(3):181–194. [Abstract] [Google Scholar]
- Lowenson JD, Clarke S. Recognition of D-aspartyl residues in polypeptides by the erythrocyte L-isoaspartyl/D-aspartyl protein methyltransferase. Implications for the repair hypothesis. J Biol Chem. 1992 Mar 25;267(9):5985–5995. [Abstract] [Google Scholar]
- McFadden PN, Clarke S. Conversion of isoaspartyl peptides to normal peptides: implications for the cellular repair of damaged proteins. Proc Natl Acad Sci U S A. 1987 May;84(9):2595–2599. [Europe PMC free article] [Abstract] [Google Scholar]
- Johnson BA, Murray ED, Jr, Clarke S, Glass DB, Aswad DW. Protein carboxyl methyltransferase facilitates conversion of atypical L-isoaspartyl peptides to normal L-aspartyl peptides. J Biol Chem. 1987 Apr 25;262(12):5622–5629. [Abstract] [Google Scholar]
- Galletti P, Ciardiello A, Ingrosso D, Di Donato A, D'Alessio G. Repair of isopeptide bonds by protein carboxyl O-methyltransferase: seminal ribonuclease as a model system. Biochemistry. 1988 Mar 8;27(5):1752–1757. [Abstract] [Google Scholar]
- O'Connor CM, Clarke S. Specific recognition of altered polypeptides by widely distributed methyltransferases. Biochem Biophys Res Commun. 1985 Nov 15;132(3):1144–1150. [Abstract] [Google Scholar]
- Johnson BA, Ngo SQ, Aswad DW. Widespread phylogenetic distribution of a protein methyltransferase that modifies L-isoaspartyl residues. Biochem Int. 1991 Jul;24(5):841–847. [Abstract] [Google Scholar]
- O'Connor CM, Germain BJ. Kinetic and electrophoretic analysis of transmethylation reactions in intact Xenopus laevis oocytes. J Biol Chem. 1987 Jul 25;262(21):10404–10411. [Abstract] [Google Scholar]
- Li C, Clarke S. Distribution of an L-isoaspartyl protein methyltransferase in eubacteria. J Bacteriol. 1992 Jan;174(2):355–361. [Europe PMC free article] [Abstract] [Google Scholar]
- Ingrosso D, Fowler AV, Bleibaum J, Clarke S. Sequence of the D-aspartyl/L-isoaspartyl protein methyltransferase from human erythrocytes. Common sequence motifs for protein, DNA, RNA, and small molecule S-adenosylmethionine-dependent methyltransferases. J Biol Chem. 1989 Nov 25;264(33):20131–20139. [Abstract] [Google Scholar]
- Fu JC, Ding L, Clarke S. Purification, gene cloning, and sequence analysis of an L-isoaspartyl protein carboxyl methyltransferase from Escherichia coli. J Biol Chem. 1991 Aug 5;266(22):14562–14572. [Abstract] [Google Scholar]
- McFadden PN, Clarke S. Methylation at D-aspartyl residues in erythrocytes: possible step in the repair of aged membrane proteins. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2460–2464. [Europe PMC free article] [Abstract] [Google Scholar]
- Lowenson JD, Clarke S. Spontaneous degradation and enzymatic repair of aspartyl and asparaginyl residues in aging red cell proteins analyzed by computer simulation. Gerontology. 1991;37(1-3):128–151. [Abstract] [Google Scholar]
- Roszak DB, Colwell RR. Survival strategies of bacteria in the natural environment. Microbiol Rev. 1987 Sep;51(3):365–379. [Europe PMC free article] [Abstract] [Google Scholar]
- Matin A, Auger EA, Blum PH, Schultz JE. Genetic basis of starvation survival in nondifferentiating bacteria. Annu Rev Microbiol. 1989;43:293–316. [Abstract] [Google Scholar]
- Matin A. The molecular basis of carbon-starvation-induced general resistance in Escherichia coli. Mol Microbiol. 1991 Jan;5(1):3–10. [Abstract] [Google Scholar]
- Siegele DA, Kolter R. Life after log. J Bacteriol. 1992 Jan;174(2):345–348. [Europe PMC free article] [Abstract] [Google Scholar]
- Beck E, Ludwig G, Auerswald EA, Reiss B, Schaller H. Nucleotide sequence and exact localization of the neomycin phosphotransferase gene from transposon Tn5. Gene. 1982 Oct;19(3):327–336. [Abstract] [Google Scholar]
- Mazodier P, Cossart P, Giraud E, Gasser F. Completion of the nucleotide sequence of the central region of Tn5 confirms the presence of three resistance genes. Nucleic Acids Res. 1985 Jan 11;13(1):195–205. [Europe PMC free article] [Abstract] [Google Scholar]
- Kohara Y, Akiyama K, Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987 Jul 31;50(3):495–508. [Abstract] [Google Scholar]
- Leyh TS, Taylor JC, Markham GD. The sulfate activation locus of Escherichia coli K12: cloning, genetic, and enzymatic characterization. J Biol Chem. 1988 Feb 15;263(5):2409–2416. [Abstract] [Google Scholar]
- Mulvey MR, Sorby PA, Triggs-Raine BL, Loewen PC. Cloning and physical characterization of katE and katF required for catalase HPII expression in Escherichia coli. Gene. 1988 Dec 20;73(2):337–345. [Abstract] [Google Scholar]
- Winans SC, Elledge SJ, Krueger JH, Walker GC. Site-directed insertion and deletion mutagenesis with cloned fragments in Escherichia coli. J Bacteriol. 1985 Mar;161(3):1219–1221. [Europe PMC free article] [Abstract] [Google Scholar]
- Loewen PC, Triggs BL. Genetic mapping of katF, a locus that with katE affects the synthesis of a second catalase species in Escherichia coli. J Bacteriol. 1984 Nov;160(2):668–675. [Europe PMC free article] [Abstract] [Google Scholar]
- Jenkins DE, Schultz JE, Matin A. Starvation-induced cross protection against heat or H2O2 challenge in Escherichia coli. J Bacteriol. 1988 Sep;170(9):3910–3914. [Europe PMC free article] [Abstract] [Google Scholar]
- Jenkins DE, Chaisson SA, Matin A. Starvation-induced cross protection against osmotic challenge in Escherichia coli. J Bacteriol. 1990 May;172(5):2779–2781. [Europe PMC free article] [Abstract] [Google Scholar]
- Mulvey MR, Loewen PC. Nucleotide sequence of katF of Escherichia coli suggests KatF protein is a novel sigma transcription factor. Nucleic Acids Res. 1989 Dec 11;17(23):9979–9991. [Europe PMC free article] [Abstract] [Google Scholar]
- Lange R, Hengge-Aronis R. Identification of a central regulator of stationary-phase gene expression in Escherichia coli. Mol Microbiol. 1991 Jan;5(1):49–59. [Abstract] [Google Scholar]
- McCann MP, Kidwell JP, Matin A. The putative sigma factor KatF has a central role in development of starvation-mediated general resistance in Escherichia coli. J Bacteriol. 1991 Jul;173(13):4188–4194. [Europe PMC free article] [Abstract] [Google Scholar]
- Backman K, Chen YM, Magasanik B. Physical and genetic characterization of the glnA--glnG region of the Escherichia coli chromosome. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3743–3747. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.89.20.9885
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc50238?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Article citations
The protein carboxymethyltransferase-dependent aspartate salvage pathway plays a crucial role in the intricate metabolic network of Escherichia coli.
Sci Adv, 10(6):eadj0767, 09 Feb 2024
Cited by: 0 articles | PMID: 38335294 | PMCID: PMC10857468
Isolation and Identification of Protein L-Isoaspartate-O-Methyltransferase (PIMT) Interacting Proteins in Salmonella Typhimurium.
Curr Microbiol, 77(5):695-701, 01 May 2020
Cited by: 1 article | PMID: 31263924
Predominance of Anaerobic, Spore-Forming Bacteria in Metabolically Active Microbial Communities from Ancient Siberian Permafrost.
Appl Environ Microbiol, 85(15):e00560-19, 18 Jul 2019
Cited by: 16 articles | PMID: 31152014 | PMCID: PMC6643238
Trouble is coming: Signaling pathways that regulate general stress responses in bacteria.
J Biol Chem, 294(31):11685-11700, 13 Jun 2019
Cited by: 117 articles | PMID: 31197038 | PMCID: PMC6682744
Review Free full text in Europe PMC
Protein repair L-isoaspartyl methyltransferase 1 (PIMT1) in rice improves seed longevity by preserving embryo vigor and viability.
Plant Mol Biol, 89(4-5):475-492, 05 Oct 2015
Cited by: 21 articles | PMID: 26438231
Go to all (44) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The L-isoaspartyl protein repair methyltransferase enhances survival of aging Escherichia coli subjected to secondary environmental stresses.
J Bacteriol, 180(10):2623-2629, 01 May 1998
Cited by: 51 articles | PMID: 9573145 | PMCID: PMC107212
Targeted gene disruption of the Caenorhabditis elegans L-isoaspartyl protein repair methyltransferase impairs survival of dauer stage nematodes.
Arch Biochem Biophys, 348(2):320-328, 01 Dec 1997
Cited by: 25 articles | PMID: 9434744
Protein L-isoaspartyl methyltransferase from the nematode Caenorhabditis elegans: genomic structure and substrate specificity.
Biochemistry, 34(34):10794-10806, 01 Aug 1995
Cited by: 28 articles | PMID: 7662659
Aging as war between chemical and biochemical processes: protein methylation and the recognition of age-damaged proteins for repair.
Ageing Res Rev, 2(3):263-285, 01 Jul 2003
Cited by: 177 articles | PMID: 12726775
Review