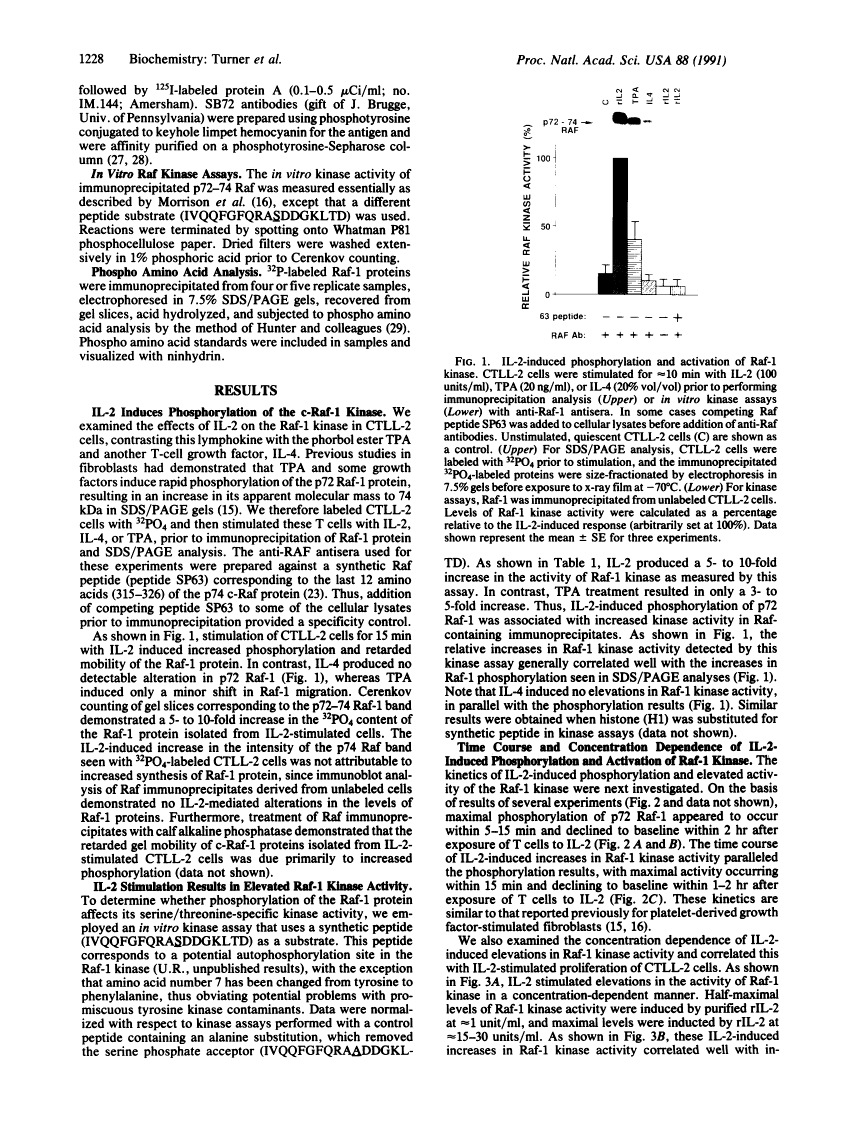
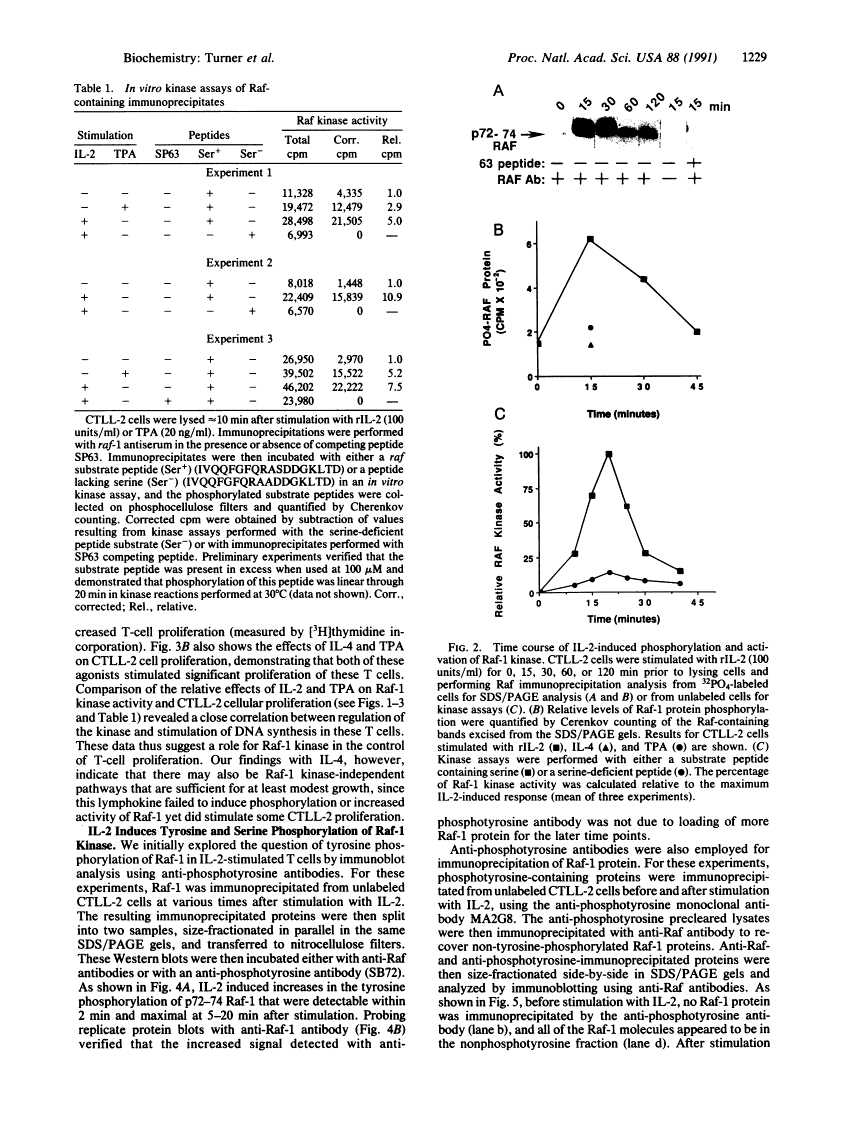
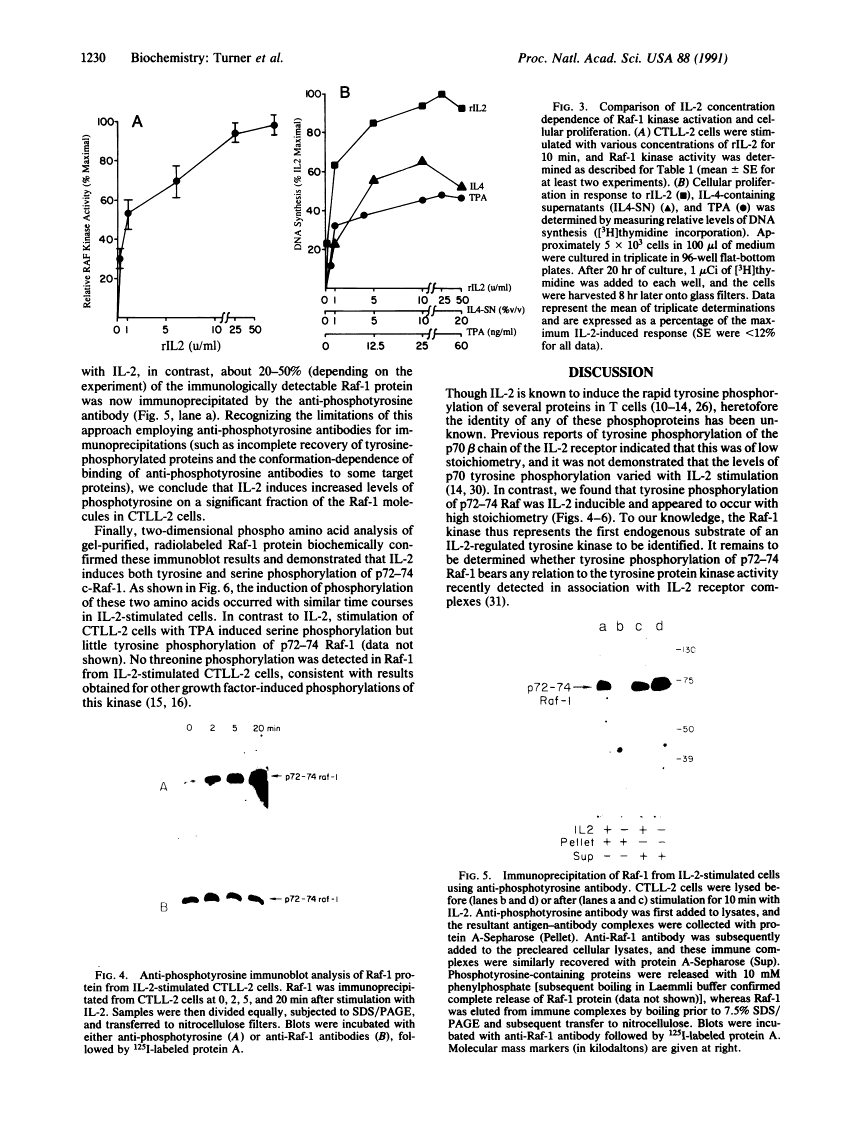
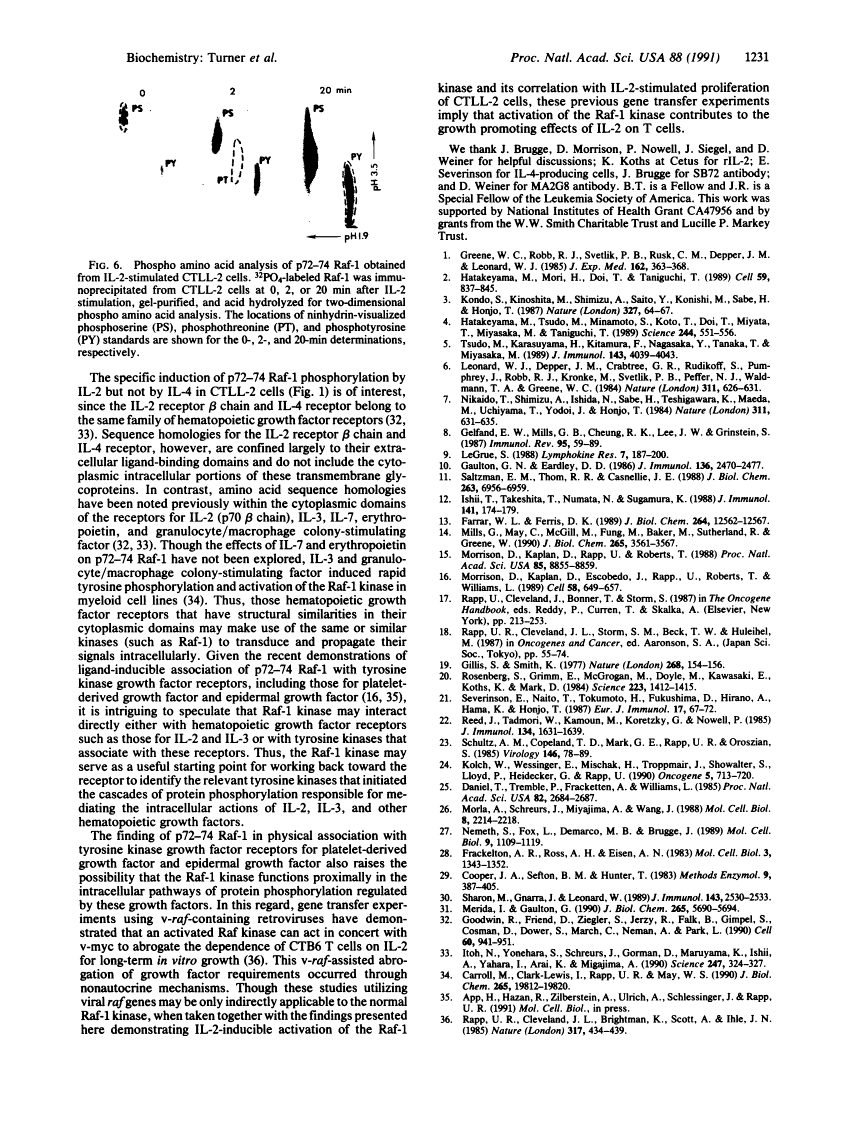
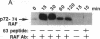Abstract
Free full text

Interleukin 2 induces tyrosine phosphorylation and activation of p72-74 Raf-1 kinase in a T-cell line.
Abstract
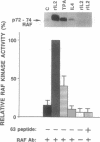
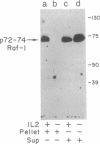
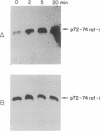
Interleukin 2 (IL-2) is a lymphokine, produced by T cells upon antigenic or mitogenic stimulation, that is a critical regulator of T-cell proliferation. Although the binding of IL-2 to its receptor has been well characterized, the molecular mechanisms by which IL-2 transmits its signal from the membrane to the interior of the cell are poorly understood. Like most other growth factors, IL-2 causes rapid phosphorylation of proteins within its target cells. Unlike many other growth factors, however, the known subunits of the IL-2 receptor lack tyrosine-specific kinase activity, and little is known about the kinases whose activities are regulated by IL-2. Here we show that IL-2 (but not IL-4) induces rapid phosphorylation of the p72-74 serine/threonine-specific kinase encoded by the c-Raf-1 protooncogene in an IL-2-dependent murine T-cell line, CTLL-2, and that this phosphorylation is associated with increased kinase activity in p72-74 Raf-1-containing immune complexes. The concentration dependence of IL-2-mediated elevations in Raf-1 kinase activity correlated well with IL-2-stimulated proliferation of CTLL-2 cells. Furthermore, much of the IL-2-stimulated phosphorylation of p72-74 Raf-1 occurred on tyrosines. To our knowledge, the Raf-1 kinase represents the first endogenous substrate of an IL-2-regulated tyrosine kinase to be identified.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.2M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Greene WC, Robb RJ, Svetlik PB, Rusk CM, Depper JM, Leonard WJ. Stable expression of cDNA encoding the human interleukin 2 receptor in eukaryotic cells. J Exp Med. 1985 Jul 1;162(1):363–368. [Europe PMC free article] [Abstract] [Google Scholar]
- Hatakeyama M, Mori H, Doi T, Taniguchi T. A restricted cytoplasmic region of IL-2 receptor beta chain is essential for growth signal transduction but not for ligand binding and internalization. Cell. 1989 Dec 1;59(5):837–845. [Abstract] [Google Scholar]
- Kondo S, Kinoshita M, Shimizu A, Saito Y, Konishi M, Sabe H, Honjo T. Expression and functional characterization of artificial mutants of interleukin-2 receptor. Nature. 1987 May 7;327(6117):64–67. [Abstract] [Google Scholar]
- Hatakeyama M, Tsudo M, Minamoto S, Kono T, Doi T, Miyata T, Miyasaka M, Taniguchi T. Interleukin-2 receptor beta chain gene: generation of three receptor forms by cloned human alpha and beta chain cDNA's. Science. 1989 May 5;244(4904):551–556. [Abstract] [Google Scholar]
- Tsudo M, Karasuyama H, Kitamura F, Nagasaka Y, Tanaka T, Miyasaka M. Reconstitution of a functional IL-2 receptor by the beta-chain cDNA. A newly acquired receptor transduces negative signal. J Immunol. 1989 Dec 15;143(12):4039–4043. [Abstract] [Google Scholar]
- Leonard WJ, Depper JM, Crabtree GR, Rudikoff S, Pumphrey J, Robb RJ, Krönke M, Svetlik PB, Peffer NJ, Waldmann TA, et al. Molecular cloning and expression of cDNAs for the human interleukin-2 receptor. Nature. 1984 Oct 18;311(5987):626–631. [Abstract] [Google Scholar]
- Nikaido T, Shimizu A, Ishida N, Sabe H, Teshigawara K, Maeda M, Uchiyama T, Yodoi J, Honjo T. Molecular cloning of cDNA encoding human interleukin-2 receptor. Nature. 1984 Oct 18;311(5987):631–635. [Abstract] [Google Scholar]
- Gelfand EW, Mills GB, Cheung RK, Lee JW, Grinstein S. Transmembrane ion fluxes during activation of human T lymphocytes: role of Ca2+, Na+/H+ exchange and phospholipid turnover. Immunol Rev. 1987 Feb;95:59–87. [Abstract] [Google Scholar]
- LeGrue SJ. Does interleukin 2 stimulus-response coupling result in generation of intracellular second messengers? Lymphokine Res. 1988 Summer;7(2):187–200. [Abstract] [Google Scholar]
- Gaulton GN, Eardley DD. Interleukin 2-dependent phosphorylation of interleukin 2 receptors and other T cell membrane proteins. J Immunol. 1986 Apr 1;136(7):2470–2477. [Abstract] [Google Scholar]
- Saltzman EM, Thom RR, Casnellie JE. Activation of a tyrosine protein kinase is an early event in the stimulation of T lymphocytes by interleukin-2. J Biol Chem. 1988 May 25;263(15):6956–6959. [Abstract] [Google Scholar]
- Ishii T, Takeshita T, Numata N, Sugamura K. Protein phosphorylation mediated by IL-2/IL-2 receptor beta-chain interaction. J Immunol. 1988 Jul 1;141(1):174–179. [Abstract] [Google Scholar]
- Farrar WL, Ferris DK. Two-dimensional analysis of interleukin 2-regulated tyrosine kinase activation mediated by the p70-75 beta subunit of the interleukin 2 receptor. J Biol Chem. 1989 Jul 25;264(21):12562–12567. [Abstract] [Google Scholar]
- Mills GB, May C, McGill M, Fung M, Baker M, Sutherland R, Greene WC. Interleukin 2-induced tyrosine phosphorylation. Interleukin 2 receptor beta is tyrosine phosphorylated. J Biol Chem. 1990 Feb 25;265(6):3561–3567. [Abstract] [Google Scholar]
- Morrison DK, Kaplan DR, Rapp U, Roberts TM. Signal transduction from membrane to cytoplasm: growth factors and membrane-bound oncogene products increase Raf-1 phosphorylation and associated protein kinase activity. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8855–8859. [Europe PMC free article] [Abstract] [Google Scholar]
- Morrison DK, Kaplan DR, Escobedo JA, Rapp UR, Roberts TM, Williams LT. Direct activation of the serine/threonine kinase activity of Raf-1 through tyrosine phosphorylation by the PDGF beta-receptor. Cell. 1989 Aug 25;58(4):649–657. [Abstract] [Google Scholar]
- Gillis S, Smith KA. Long term culture of tumour-specific cytotoxic T cells. Nature. 1977 Jul 14;268(5616):154–156. [Abstract] [Google Scholar]
- Rosenberg SA, Grimm EA, McGrogan M, Doyle M, Kawasaki E, Koths K, Mark DF. Biological activity of recombinant human interleukin-2 produced in Escherichia coli. Science. 1984 Mar 30;223(4643):1412–1414. [Abstract] [Google Scholar]
- Severinson E, Naito T, Tokumoto H, Fukushima D, Hirano A, Hama K, Honjo T. Interleukin 4 (IgG1 induction factor): a multifunctional lymphokine acting also on T cells. Eur J Immunol. 1987 Jan;17(1):67–72. [Abstract] [Google Scholar]
- Reed JC, Tadmori W, Kamoun M, Koretzky G, Nowell PC. Suppression of interleukin 2 receptor acquisition by monoclonal antibodies recognizing the 50 KD protein associated with the sheep erythrocyte receptor on human T lymphocytes. J Immunol. 1985 Mar;134(3):1631–1639. [Abstract] [Google Scholar]
- Schultz AM, Copeland TD, Mark GE, Rapp UR, Oroszlan S. Detection of the myristylated gag-raf transforming protein with raf-specific antipeptide sera. Virology. 1985 Oct 15;146(1):78–89. [Abstract] [Google Scholar]
- Kolch W, Weissinger E, Mischak H, Troppmair J, Showalter SD, Lloyd P, Heidecker G, Rapp UR. Probing structure and function of the raf protein kinase domain with monoclonal antibodies. Oncogene. 1990 May;5(5):713–720. [Abstract] [Google Scholar]
- Daniel TO, Tremble PM, Frackelton AR, Jr, Williams LT. Purification of the platelet-derived growth factor receptor by using an anti-phosphotyrosine antibody. Proc Natl Acad Sci U S A. 1985 May;82(9):2684–2687. [Europe PMC free article] [Abstract] [Google Scholar]
- Morla AO, Schreurs J, Miyajima A, Wang JY. Hematopoietic growth factors activate the tyrosine phosphorylation of distinct sets of proteins in interleukin-3-dependent murine cell lines. Mol Cell Biol. 1988 May;8(5):2214–2218. [Europe PMC free article] [Abstract] [Google Scholar]
- Nemeth SP, Fox LG, DeMarco M, Brugge JS. Deletions within the amino-terminal half of the c-src gene product that alter the functional activity of the protein. Mol Cell Biol. 1989 Mar;9(3):1109–1119. [Europe PMC free article] [Abstract] [Google Scholar]
- Frackelton AR, Jr, Ross AH, Eisen HN. Characterization and use of monoclonal antibodies for isolation of phosphotyrosyl proteins from retrovirus-transformed cells and growth factor-stimulated cells. Mol Cell Biol. 1983 Aug;3(8):1343–1352. [Europe PMC free article] [Abstract] [Google Scholar]
- Cooper JA, Sefton BM, Hunter T. Detection and quantification of phosphotyrosine in proteins. Methods Enzymol. 1983;99:387–402. [Abstract] [Google Scholar]
- Sharon M, Gnarra JR, Leonard WJ. The beta-chain of the IL-2 receptor (p70) is tyrosine-phosphorylated on YT and HUT-102B2 cells. J Immunol. 1989 Oct 15;143(8):2530–2533. [Abstract] [Google Scholar]
- Merida I, Gaulton GN. Protein tyrosine phosphorylation associated with activation of the interleukin 2 receptor. J Biol Chem. 1990 Apr 5;265(10):5690–5694. [Abstract] [Google Scholar]
- Goodwin RG, Friend D, Ziegler SF, Jerzy R, Falk BA, Gimpel S, Cosman D, Dower SK, March CJ, Namen AE, et al. Cloning of the human and murine interleukin-7 receptors: demonstration of a soluble form and homology to a new receptor superfamily. Cell. 1990 Mar 23;60(6):941–951. [Abstract] [Google Scholar]
- Itoh N, Yonehara S, Schreurs J, Gorman DM, Maruyama K, Ishii A, Yahara I, Arai K, Miyajima A. Cloning of an interleukin-3 receptor gene: a member of a distinct receptor gene family. Science. 1990 Jan 19;247(4940):324–327. [Abstract] [Google Scholar]
- Carroll MP, Clark-Lewis I, Rapp UR, May WS. Interleukin-3 and granulocyte-macrophage colony-stimulating factor mediate rapid phosphorylation and activation of cytosolic c-raf. J Biol Chem. 1990 Nov 15;265(32):19812–19817. [Abstract] [Google Scholar]
- Rapp UR, Cleveland JL, Brightman K, Scott A, Ihle JN. Abrogation of IL-3 and IL-2 dependence by recombinant murine retroviruses expressing v-myc oncogenes. Nature. 1985 Oct 3;317(6036):434–438. [Abstract] [Google Scholar]
Associated Data
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.88.4.1227
Read article for free, from open access legal sources, via Unpaywall:
https://www.pnas.org/content/pnas/88/4/1227.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1073/pnas.88.4.1227
Article citations
Lymphoma and myeloma cell resistance to cytotoxic agents and ionizing radiations is not affected by exposure to anti-IL-6 antibody.
PLoS One, 4(11):e8026, 30 Nov 2009
Cited by: 14 articles | PMID: 19956602 | PMCID: PMC2779452
Second nature: biological functions of the Raf-1 "kinase".
FEBS Lett, 579(15):3271-3277, 23 Mar 2005
Cited by: 89 articles | PMID: 15943972
Review
Impaired TCR-mediated induction of Ki67 by naive CD4+ T cells is only occasionally corrected by exogenous IL-2 in HIV-1 infection.
J Immunol, 171(10):5208-5214, 01 Nov 2003
Cited by: 16 articles | PMID: 14607921
RAF antisense oligonucleotide as a tumor radiosensitizer.
Oncogene, 22(37):5876-5884, 01 Sep 2003
Cited by: 30 articles | PMID: 12947394
Review
IL-2 activation of a PI3K-dependent STAT3 serine phosphorylation pathway in primary human T cells.
Cell Signal, 15(6):625-636, 01 Jun 2003
Cited by: 50 articles | PMID: 12681450
Go to all (62) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Interleukin 2 regulates Raf-1 kinase activity through a tyrosine phosphorylation-dependent mechanism in a T-cell line.
Proc Natl Acad Sci U S A, 90(12):5544-5548, 01 Jun 1993
Cited by: 18 articles | PMID: 7685905 | PMCID: PMC46757
Interleukin-2 activation of STAT5 requires the convergent action of tyrosine kinases and a serine/threonine kinase pathway distinct from the Raf1/ERK2 MAP kinase pathway.
EMBO J, 15(8):1902-1913, 01 Apr 1996
Cited by: 81 articles | PMID: 8617237 | PMCID: PMC450109
Interleukin-13 induces rapid tyrosine phosphorylation and activation of Raf-1 kinase in human monocytic progenitor cell line U937.
Biochem Biophys Res Commun, 206(1):103-111, 01 Jan 1995
Cited by: 6 articles | PMID: 7529495
Protooncogene-encoded protein kinases in interleukin-2 signal transduction.
Semin Immunol, 5(5):327-336, 01 Oct 1993
Cited by: 4 articles | PMID: 8260649
Review