Abstract
Purpose
In 2009, the US Food and Drug Administration (FDA) mandated a label change for leukotriene inhibitors (LTIs) to include neuropsychiatric adverse events (eg, depression and suicidality) as a precaution. This study investigated how this label change affected the use of LTIs and other asthma controller medications, mental health visits, and suicide attempts.Methods
We analyzed data (2005-2010) from 5 large health plans in the US Population-Based Effectiveness in Asthma and Lung Diseases (PEAL) Network. The study cohort included children and adolescents (n = 30,000), young adults (n = 20,000), and adults (n = 90,000) with asthma. We used interrupted time series to examine changes in rates of LTI dispensings, non-LTI dispensings, mental health visits, and suicide attempts (using a validated algorithm based on a combination of diagnoses of injury or poisoning and psychiatric conditions).Findings
The label change was associated with abrupt reductions in LTI use among all age groups (relative reductions of 8.3%, 15.1%, and 6.0% among adolescents, young adults, and adults, respectively, compared with expected rates at 1 year after the warnings). Although we detected immediate offset increases in non-LTI asthma medication use, these increases were not sustained among adolescents and young adults. There were small increases in mental health visits among LTI users.Implications
The FDA label change for LTIs communicated possible risk of neuropsychiatric events. Communication and enhanced awareness may have increased reporting of mental health symptoms among young adults and adults. It is important to assess intended and unintended consequences of FDA warnings and label changes.Free full text

Asthma Treatments and Mental Health Visits After a Food and Drug Administration Label Change for Leukotriene Inhibitors
Abstract
Purpose
In 2009, the US Food and Drug Administration (FDA) mandated a label change for leukotriene inhibitors (LTIs) to include neuropsychiatric adverse events (eg, depression and suicidality) as a precaution. This study investigated how this label change affected the use of LTIs and other asthma controller medications, mental health visits, and suicide attempts.
Methods
We analyzed data (2005–2010) from 5 large health plans in the US Population-Based Effectiveness in Asthma and Lung Diseases (PEAL) Network. The study cohort included children and adolescents (n = 30,000), young adults (n = 20,000), and adults (n = 90,000) with asthma. We used interrupted time series to examine changes in rates of LTI dispensings, non-LTI dispensings, mental health visits, and suicide attempts (using a validated algorithm based on a combination of diagnoses of injury or poisoning and psychiatric conditions).
Findings
The label change was associated with abrupt reductions in LTI use among all age groups (relative reductions of 8.3%, 15.1%, and 6.0% among adolescents, young adults, and adults, respectively, compared with expected rates at 1 year after the warnings). Although we detected immediate offset increases in non-LTI asthma medication use, these increases were not sustained among adolescents and young adults. There were small increases in mental health visits among LTI users.
Implications
The FDA label change for LTIs communicated possible risk of neuropsychiatric events. Communication and enhanced awareness may have increased reporting of mental health symptoms among young adults and adults. It is important to assess intended and unintended consequences of FDA warnings and label changes.
INTRODUCTION
During the last decade, there has been increasing recognition of the potential for certain prescription medications to increase the risk of psychiatric symptoms and suicidality.1 Primarily on the basis of spontaneous adverse drug event reports (eg, via the MedWatch system), the US Food and Drug Administration (FDA) has issued communications and warnings about psychiatric symptoms and suicidality for whole classes of medications. These warnings initially included selective serotonin receptor inhibitors and were later expanded to all antidepressants, antiepileptic drugs, the smoking-cessation drug varenicline,2 and leukotriene inhibitors (LTIs).
LTIs include montelukast, zafirlukast, and zileuton in the United States and are the second most commonly used controller medications for asthma after inhaled corticosteroids. LTIs are also used for seasonal allergies. The FDA first issued alerts in March 20083 and reviewed postmarketing reports of patients taking LTIs and clinical trial data submitted by manufacturers. Most reported neuropsychiatric adverse events were associated with montelukast. Although these data do not suggest that LTIs are associated with suicidality, these clinical trials were not designed specifically to examine neuropsychiatric events.
In June 2009, the FDA required manufacturers of LTIs to include neuropsychiatric adverse events as a precaution on the drug label.4 In the risk communications, the FDA recommended that “patients and prescribers should monitor for the possibility of neuropsychiatric events associated with these agents.”3 Neuropsychiatric events include agitation, aggression, anxiety, sleep disorder, depression, and suicidal thinking and behavior (including suicide and suicide attempts).4 The widespread use of LTIs heightened the concern about the potential association with suicide. At the same time, excessive caution could mean that effective therapies are withheld from patients who could benefit from them. The aims of this study were to examine the effects of the FDA label change for LTIs on patterns of treatment for asthma, mental health visits, and suicide attempts.
METHODS
Data Sources
This study included individuals with asthma in the Population-Based Effectiveness in Asthma and Lung Diseases (PEAL) Network.5–7 Five health plans from this network participated in this study: Harvard Pilgrim Health Care, HealthPartners, Kaiser Permanente Northern California, Kaiser Permanente Georgia, and Kaiser Permanente Northwest. Electronic data from subjects from each of the 5 sites were pooled to form the PEAL Data Warehouse, which includes information on demographic characteristics, health plan enrollment, dispensings of medications, and use of inpatient and outpatient health care services. The institutional review board at each site approved this study.
Study Design and Cohorts
Randomized controlled trials are not able to evaluate nationwide policy changes such as FDA warnings. We used an interrupted times series design,8–12 which can provide strong evidence of causal effects because it controls for prepolicy secular trends in study outcomes. The approach measures whether a policy causes abrupt changes in the level and/or the preexisting trend (slope) of study outcomes.8–12 To calculate population-level time series, we created a rolling cohort from January 2005 through December 2010. For every month, we identified and included health plan members who had (1) continuous enrollment for the past 12 months, (2) continuous enrollment for the current month, (3) at least one outpatient or inpatient visit in the past 12 months with a diagnosis of asthma (International Classification of Diseases, Ninth Revision (ICD-9) code for asthma [493.x]), and (4) no history of chronic obstructive pulmonary diseases (ICD-9 codes 491, 492, and 496); cystic fibrosis (ICD-9 code 277.0x); bronchiectasis (ICD-9 code 494); pulmonary hypertension or embolism (ICD-9 codes 416.0 and 415.1); bronchopulmonary dysplasia (ICD-9 code 770.7); or congestive heart failure (ICD-9 code 428) in the past 12 months. We excluded subjects with these comorbid illnesses, as we have done in previous studies,13,14 because asthma medications can be used for these chronic illnesses, which are distinct from asthma. These criteria were consistent with recent studies of FDA warnings among the asthma population using similar data.15 We included children and adolescents (ages 5–17 years), young adults (ages 18–29 years), and adults (ages 30–64 years) because the prevalence of suicidality is higher among young adults aged 18 through 29 years than among adults 30 years and older based on the US Centers for Disease Control and Prevention data.16 In addition, we identified users of LTIs; for each month, we identified and included individuals who had a dispensing for a LTI agent in the previous 6 months and met the above study criteria.
Outcome Measures
Among the rolling cohorts of asthmatic patients, we calculated the monthly percentage of individuals dispensed an LTI, dispensed a non-LTI medication, or who had a mental health visit. We also calculated the quarterly percentage of patients who were medically treated for suicide attempt. To construct these measures, for each month, we identified individuals with asthma who (1) were dispensed an LTI medication, (2) were dispensed a non-LTI asthma controller medication (inhaled corticosteroids and inhaled corticosteroids with long-acting β-agonists), and (3) who had a mental health visit (defined using Current Procedural Terminology psychiatric codes [908xx series] and ICD-9 codes [290– 314]17). For each quarter, we identified asthmatic patients hospitalized or treated in the emergency department for suicide attempts using a validated algorithm18 based on a combination of diagnoses of injury or poisoning and psychiatric conditions; this algorithm has a positive predictive value of 87.8% for suicide attempts.18 Although encounters for suicide attempts can be identified in administrative databases using external cause of injury codes (E-codes), they are known to be incompletely captured in commercial plan databases19; thus, we used a previously validated algorithm.18
Statistical Analysis
We used segmented regression models8 to estimate cohort-level changes in our outcome measures from the prelabel change period (January 1, 2005, to June 30, 2009) to the postlabel change period (July 1, 2009, to December 31, 2010). We analyzed each age group separately and adjusted for baseline (control) trends and for seasonal trends in statistical models. The models included a binary indicator to estimate the immediate-level change in outcome measures and a term to estimate the trend change. We also estimated absolute and relative differences (with 95% CIs)20 in observed versus predicted rates at 1-year postlabel change (ie, in June 2010) to represent the full effect of the label change. For parsimony, we excluded nonsignificant (P ≥ 0.20) time-series terms in a reverse stepwise fashion; exclusion of nonsignificant terms did not meaningfully change the coefficients on the remaining terms.8 We controlled for significant autocorrelation terms in the models. We conducted all statistical analyses using SAS statistical software, version 9.3 (SAS Institute, Cary, North Carolina).
RESULTS
The rolling cohorts included approximately 30,000 children and adolescents, 20,000 young adults, and 90,000 adults per month with asthma; the key characteristics were stable over time (Table I). Table II presents estimated changes in levels and trends in study outcomes from segmented regression models. Table III provides absolute and relative changes in study outcomes at 1 year after the label change.
Table I
Characteristics of study cohorts including individuals with asthma from 5 health plans in the nationwide Population-Based Effectiveness in Asthma and Lung Diseases Network.
| Prelabel change | Postlabel change | ||||
|---|---|---|---|---|---|
| Characteristic | Jan 2006 | Jan 2007 | Jan 2008 | Jul 2009 | Jul 2010 |
| Total No. | 125,577 | 136,799 | 136,223 | 149,913 | 161,351 |
| Adolescents, % | 22 | 21 | 18 | 19 | 20 |
| Young adults, % | 16 | 16 | 16 | 16 | 16 |
| Adults, % | 63 | 63 | 66 | 65 | 63 |
| Adolescents, No. | 27,280 | 28,862 | 24,596 | 28,593 | 32,576 |
| Female, % | 47 | 46 | 46 | 47 | 46 |
| High SES,*% | 52 | 52 | 52 | 52 | 51 |
| Young adults, No. | 19,541 | 21,733 | 21,936 | 24,595 | 26,472 |
| Female, % | 62 | 61 | 62 | 62 | 62 |
| High SES,*% | 54 | 54 | 54 | 55 | 54 |
| Adults, No. | 78,756 | 86,204 | 89,691 | 96,725 | 102,303 |
| Female, % | 67 | 66 | 67 | 67 | 67 |
| High SES,*% | 55 | 55 | 55 | 55 | 55 |
SES = socioeconomic status.
Table II
Parameter estimates, 95% CIs, and P values from the most parsimonious segmented regression models predicting monthly percentages of medication use and mental health visits and quarterly percentages of suicide attempts by age group.
| Parameter | Estimate (95% CI) | P |
|---|---|---|
| Adolescents | ||
| LTI dispensings | ||
| Immediate impact | −0.23 (−0.44 to −0.01) | 0.0400 |
| Trend | 0 | |
| Non-LTI dispensings | ||
| Immediate impact | 0.54 (0.20–0.89) | 0.0026 |
| Trend | −0.02 (−0.06 to 0.01) | 0.1448 |
| Asthma drug dispensings | ||
| Immediate impact | 0.37 (−0.03 to 0.77) | 0.0674 |
| Trend | −0.04 (−0.07 to −0.01) | 0.0232 |
| Mental health visits (LTI users) | ||
| Immediate impact | 0.25 (0.01–0.49) | 0.0454 |
| Trend | 0 | |
| Suicide attempts | ||
| Immediate impact | 0 | |
| Trend | 0 | |
| Young adults | ||
| LTI dispensings | ||
| Immediate impact | −0.02 (−0.03 to −0.01) | 0.0013 |
| Trend | 0 | |
| Non-LTI dispensings | ||
| Immediate impact | 0.46 (0.14–0.79) | 0.0058 |
| Trend | −0.04 (−0.06 to −0.01) | 0.0082 |
| Asthma drug dispensings | ||
| Immediate impact | 0.40 (0.07–0.74) | 0.0191 |
| Trend | −0.05 (−0.08 to −0.02) | 0.0006 |
| Mental health visits (LTI users) | ||
| Immediate impact | 0 | |
| Trend | 0 | |
| Suicide attempts | ||
| Immediate impact | 0.03 (0.01–0.05) | 0.0032 |
| Trend | 0 | |
| Adults | ||
| LTI dispensings | ||
| Immediate impact | 0 | |
| Trend | −0.01 (−0.02 to 0.00) | 0.0095 |
| Non-LTI dispensings | ||
| Immediate impact | 0.44 (0.03–0.84) | 0.0351 |
| Trend | 0 | |
| Asthma drug dispensing | ||
| Immediate impact | 0.39 (0.00–0.79) | 0.0503 |
| Trend | 0 | |
| Mental health visits (LTI users) | ||
| Immediate impact | 0.61 (0.30–0.91) | 0.0002 |
| Trend | 0 | |
| Suicide attempts | ||
| Immediate impact | 0 | |
| Trend | 0.002 (0.001–0.003) | 0.001 |
LTI = leukotriene inhibitor.
Table III
Absolute and relative changes in medication use, mental health visits, and suicide attempts by 1 year after the warnings compared with expected rates derived from baseline trend.
| Parameter | Absolute Change (95% CI), % | Relative Change (95% CI), % |
|---|---|---|
| Adolescents | ||
| LTI dispensings | −0.23 (−0.44 to −0.01) | −8.31 (−15.92 to −0.71) |
| Non-LTI dispensings | 0.25 (−0.13 to 0.63) | 5.11 (−2.83 to 13.05) |
| Asthma drug dispensings | −0.09 (−0.40 to 0.22) | −1.26 (−5.66 to 3.15) |
| Mental health visits (LTI users) | 0.25 (0.01–0.49) | 3.56 (0.10–7.01) |
| Suicide attempts | 0 | 0 |
| Young adults | ||
| LTI dispensings | −0.26 (−0.41 to −0.11) | −15.07 (−22.89 to −7.25) |
| Non-LTI dispensings | 0.02 (−0.23 to 0.28) | 0.47 (−4.59 to 5.54) |
| Asthma drug dispensings | −0.19 (−0.46 to 0.07) | −3.10 (−7.21 to 1.00) |
| Mental health visits (LTI users) | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) |
| Suicide attempts | 0.03 (0.01–0.05) | 66.31 (9.83–122.79) |
| Adults | ||
| LTI dispensings | −0.17 (−0.29 to −0.04) | −6.00 (−10.34 to −1.66) |
| Non-LTI dispensings | 0.44 (0.04–0.83) | 4.59 (0.27–8.92) |
| Asthma drug dispensings | 0.39 (0.01–0.78) | 3.49 (−0.03 to 7.00) |
| Mental health visits (LTI users) | 0.61 (0.31–0.91) | 8.16 (3.91–12.40) |
| Suicide attempts | 0.01 (0.00–0.01) | 23.47 (10.95–35.99) |
LTI = leukotriene inhibitor.
Effects of the Label Change Among Children and Adolescents
Immediately before the label change, 2.79% of children and adolescents used LTIs, 5.77% used non-LTI asthma controller medications, 7.69% of LTI users had mental health visits, and 0.05% were medically treated for suicide attempts. After the label change, there was a small shift in use from LTIs to non-LTI asthma medications (Figure 1A), with LTI use decreasing 0.23 percentage points (95% CI, −0.44 to −0.01) and non-LTI use increasing 0.54 percentage points (95% CI, 0.20–0.89; Table II). These changes led to a relative reduction of 8.31% in LTI use (95% CI, −15.92 to −0.71; Table III) without significant offset increases in non-LTI medication use at 1 year after the label change. Immediately after the label change, mental health visits increased 0.25 percentage points (95% CI, 0.01–0.49; Figure 1B) among LTI users, leading to a relative increase of 3.56% (95% CI, 0.10–7.01). We did not observe changes in suicide attempts (Figure 1C).
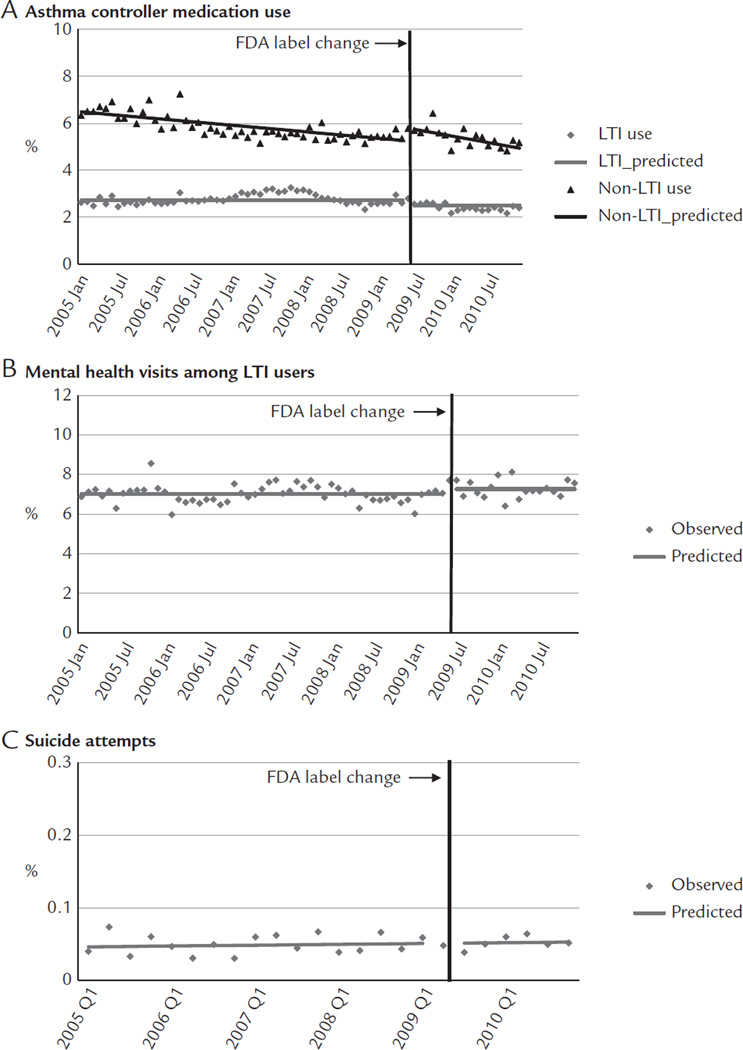
Rates of medication use (A), mental health visits (B), and suicide attempts (C) before and after the Food and Drug Administration (FDA) label change among adolescents enrolled in 5 health plans in the nationwide Population-Based Effectiveness in Asthma and Lung Diseases Network. LTI = leukotriene inhibitor.
Effects of the Label Change Among Young Adults
Immediately before the label change, approximately 1.68% of young adults used LTIs, 5.81% used non-LTI asthma controller medications, 7.94% of LTI users had mental health visits, and 0.06% were medically treated for suicide attempts. After the label change, there was a small shift in use from LTIs to non-LTI asthma medications (Figure 2A). The label change was associated with a small decrease of 0.02 percentage points per month in the trend of LTIs (95% CI, −0.03 to −0.01; Table II). This reduction was offset by increases in non-LTI medication use, with an immediate increase of 0.46 percentage points (95% CI, 0.14–0.79), although this increase was not sustained because there was a reduction of 0.04 percentage points per month (95% CI, −0.06 to −0.01). These changes led to a relative reduction of 15.1% in LTI use (95% CI, −22.89 to −7.25; Table III) without apparent offset increases in non-LTI medication use at 1 year after the label change. We did not detect changes in mental health visits among LTI users (Figure 2B). Immediately after the label change, there was a small increase of 0.03 percentage points (95% CI, 0.01–0.05) in suicide attempts (Figure 2C).
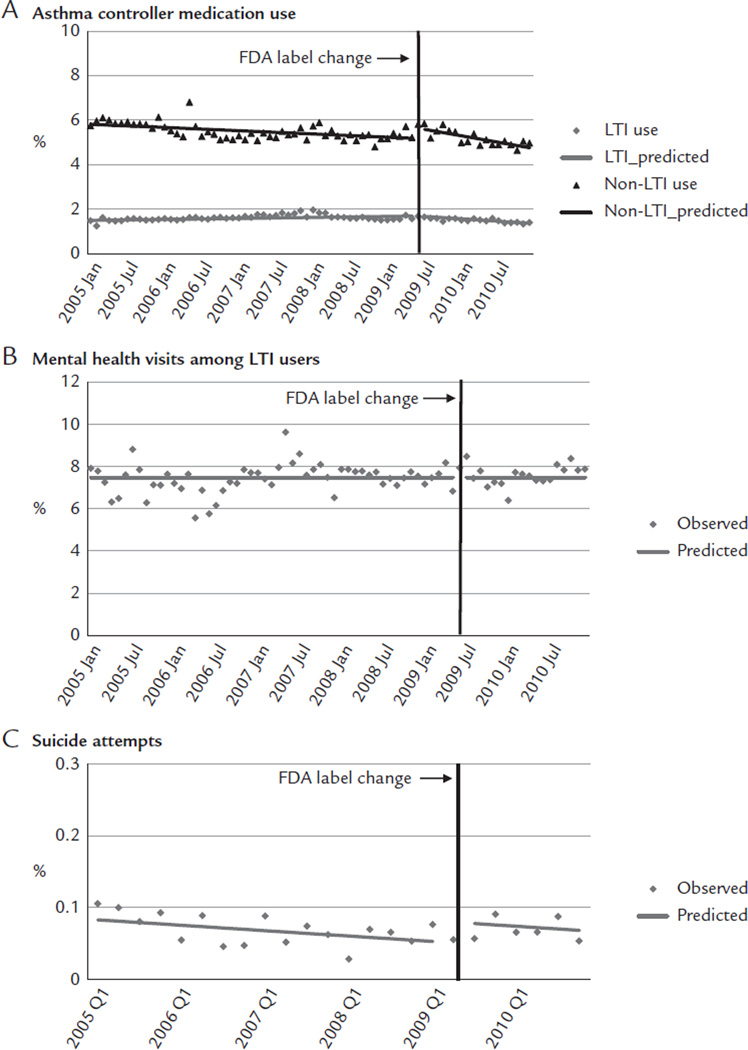
Rates of medication use (A), mental health visits (B), and suicide attempts (C) before and after the Food and Drug Administration (FDA) label change among young adults enrolled in 5 health plans in the nationwide Population-Based Effectiveness in Asthma and Lung Diseases Network. LTI = leukotriene inhibitor.
Effects of the Label Change Among Adults
Immediately before the label change, approximately 2.72% of adults used LTIs, 10.53% used non-LTI asthma controller medications, 8.32% of LTI users had mental health visits, and 0.03% were medically treated for suicide attempts. After the label change, there was a small shift in use from LTIs to non-LTI asthma medications (Figure 3A). The label change was associated with a small decrease of 0.01 percentage points per month in the trend of LTIs (95% CI, −0.02 to −0.004; Table II). This reduction was offset by an immediate increase of 0.44 percentage points (95% CI, 0.03–0.84) in use of non-LTI asthma medications. These changes led to a relative reduction of 6.00% in LTI use (95% CI, −10.34 to −1.66; Table III) that was offset by a relative increase of 4.59% in non-LTI medication use (95% CI, 0.27–8.92) at 1 year after the label change. Immediately after the label change, mental health visits increased 0.61 percentage points (95% CI, 0.30–0.91; Figure 3B) among LTI users, leading to a relative increase of 8.16% (95% CI, 3.91–12.40). After the label change, there was a small increase of 0.002 percentage points per month (95% CI, 0.001–0.003) in suicide attempts (Figure 3C).
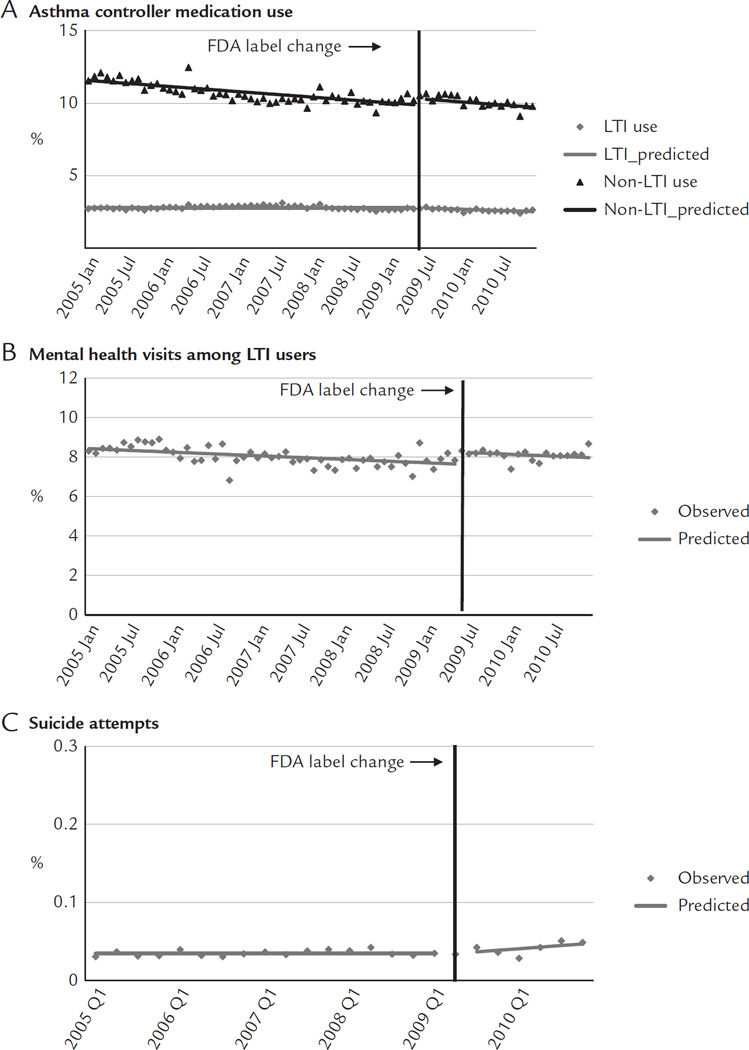
Rates of medication use (A), mental health visits (B), and suicide attempts (C) before and after the Food and Drug Administration (FDA) label change among adults enrolled in 5 health plans in the nationwide Population-based Effectiveness in Asthma and Lung Diseases Network. LTI = leukotriene inhibitor.
DISCUSSION
Our study of treatment patterns among a large and geographically diverse population of patients with asthma revealed small effects associated with the 2009 FDA label change for LTIs. After the label change, there were small reductions in LTI use among all age groups. Our study also assessed the extent of substitution with alternative treatments. Although we detected immediate offset increases in non-LTI medication use, there were no increases in trend. Among adolescents and adults, there were small increases in mental health visits for LTI users after the label change. There were some apparent small increases in suicide attempts, measured by a proxy based on diagnoses of injury or poisoning and psychiatric conditions, among young adults and adults. The observed small increases in mental health visits and suicide attempts may be explained by increased reporting of mental health symptoms.
The lack of substantial changes after the FDA label change for LTIs is not surprising. First, to date, there is no definitive evidence of an association between LTIs and suicide based on FDA’s review of adverse event data from manufacturer-conducted placebo-controlled clinical trials4 or observational studies.21,22 In the case of LTIs, the intent of the label change was to provide clinicians and patients with updated information necessary to make rational treatment decisions. Minimal changes in LTI use and mental health visits are likely appropriate responses. Second, the FDA only required labeling changes to communicate the risk of neuropsychiatric events associated with LTIs. The type of FDA communications used may influence the level of public awareness and the extent of change in prescriber behavior. These communications range from minor revisions to the label of the drug to boxed warnings when risks are particularly severe; boxed warnings are FDA’s strongest warning given to a drug or drug class.
Lay media can play an important role in changing medication use and health outcomes because messages in FDA drug risk communications may be oversimplified and distorted through media channels.23 Furthermore, the publicity itself may affect the response to FDA label changes and warnings.24,25 We previously found that the widely publicized FDA warnings for antidepressants and suicidality led to substantial reductions in antidepressant use and simultaneous increases in suicide attempts by poisonings among youth,12 suggesting that excessive caution might have resulted in reduced use of effective therapies for patients who could benefit from them. Compared with antidepressants, the safety concern about LTIs and FDA label change received little media attention; thus, there were no substantial changes after the FDA warnings.
Strengths of this study include the large number of individuals from across the country studied over a longitudinal period of 6 years. Furthermore, this is the first study to investigate changes in use of LTIs and other asthma controller medications, mental health visits, and suicide attempts after an FDA-mandated label change for LTIs. The FDA has moved toward broader and more proactive communication of drug warnings for marketed drugs in recent years. Risk communications can have intended and unintended effects, making such studies important.24
Several limitations deserve mention. First, our follow-up period was 18 months, so we could not observe changes in outcomes beyond this period. Second, our outcomes may not have been fully captured because we elected not to use deliberate self-harm E-codes because they are incompletely coded across sites participating in this study and in commercial plan databases generally.19 Third, our sample comprised insured populations (commercial plans and public insurers); the findings may not reflect behavior among uninsured patients. Fourth, because suicide attempts are extremely rare, even with our large sample, we could not stratify our analysis of suicide attempts by key characteristics, such as sex, socioeconomic status, and race/ethnicity, to better understand the impact of FDA’s label change among select patient groups. Notwithstanding these limitations, given our study’s interrupted time series design,8–12 we provide evidence that the FDA label change led to small, short-term reductions in LTI use, but these reductions were not offset by increases in non-LTI asthma medication use among adolescents and young adults. Less optimal use of asthma controller medications may influence asthma-related adverse outcomes (eg, asthma-related hospitalization). Further studies are needed to examine effects of this label change–induced change in asthma controller medication use on asthma-related clinical outcomes.
Because knowledge of the risk of drugs is continually evolving, it is often necessary for the FDA and manufacturers to update a product’s label with new information on the safety profile. Regulators face the challenge of designing clear risk messages that are maximally effective, are specific, and do not become risks themselves. The FDA’s drug risk communications exist within a sea of information (including lay media) that may influence the behavior of patients and clinicians. Active surveillance is needed to allow timely detection of unintended effects of drug risk communications.
CONCLUSIONS
The FDA’s label change for LTIs that communicated the possible risk of neuropsychiatric events led to reduced use of LTIs without corresponding increases in non-LTI medication use among young people. Further research is warranted to study the impact of reduced asthma controller medication use on clinical outcomes. This label change may have also increased reporting of mental health symptoms among young adults and adults. Risk communications may affect behavior of medication use and related health services. It is important to monitor intended and unintended consequences of FDA warnings and label changes.
Acknowledgments
C.Y. Lu, F. Zhang, and S.B. Soumerai developed study question and contributed to study design. A.C. Wu, T. Lieu, and M.D. Lakoma assisted with data acquisition. T. Lieu and S.B. Soumerai obtained funding. C.Y. Lu and F. Zhang contributed to statistical analysis. C.Y. Lu, M.D. Lakoma, F. Zhang, S.B. Soumerai, and A.C. Wu contributed to interpretation of data. C.Y. Lu drafted and revised manuscript. All authors provided revisions to drafts of manuscript. All authors approved the final version of the manuscript. All authors are grateful to Caitlin Lupton for assistance with literature searches.
The PEAL Network is supported by Agency for Healthcare Research and Quality grant 1R01HS019669 (principal investigator: S.B. Soumerai). S.B. Soumerai was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases grant 1P30-DK092924 from the Health Delivery Systems Center for Diabetes Translational Research. C.Y. Lu, F. Zhang, and S.B. Soumerai were supported in part by cooperative agreement U19MH092201 with the US National Institute of Mental Health. The sponsor had no role in the design and conduct of the study; analysis, and interpretation of the data; the preparation of the manuscript; or decision to submit the manuscript for publication. This paper is subject to the National Institutes of Health Public Access Policy (http://publicaccess.nih.gov/).
Footnotes
CONFLICTS OF INTEREST
All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. M.G. Butler reports contracts with the FDA and Novartis outside the submitted work. V. Fung reports financial interest in Merck. The authors have indicated that they have no other conflicts of interest regarding the content of this article.
REFERENCES
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.clinthera.2015.03.027
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc5101625?pdf=render
Citations & impact
Impact metrics
Article citations
Neuropsychiatric events associated with montelukast in patients with asthma: a systematic review.
Eur Respir Rev, 32(169):230079, 27 Sep 2023
Cited by: 3 articles | PMID: 37758273 | PMCID: PMC10523155
Review Free full text in Europe PMC
Multitargeted Herbal Prescription So Shiho Tang: A Scoping Review on Biomarkers for the Evaluation of Therapeutic Effects.
Pharmaceuticals (Basel), 16(10):1371, 27 Sep 2023
Cited by: 3 articles | PMID: 37895842 | PMCID: PMC10610176
Review Free full text in Europe PMC
Use of assisted reproductive technologies before and after the Artificial Reproduction Act in Taiwan.
PLoS One, 13(11):e0206208, 01 Nov 2018
Cited by: 4 articles | PMID: 30383814 | PMCID: PMC6211666
Counter-Point: Staying Honest When Policy Changes Backfire.
Med Care, 56(5):384-390, 01 May 2018
Cited by: 3 articles | PMID: 29634631 | PMCID: PMC5898649
The Effects of Asthma and Bullying on Suicidal Behaviors Among US Adolescents.
J Sch Health, 88(10):762-767, 01 Oct 2018
Cited by: 0 articles | PMID: 30203476 | PMCID: PMC6134875
Go to all (8) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Changes in antidepressant use by young people and suicidal behavior after FDA warnings and media coverage: quasi-experimental study.
BMJ, 348:g3596, 18 Jun 2014
Cited by: 92 articles | PMID: 24942789 | PMCID: PMC4062705
Risk of suicide attempt in asthmatic children and young adults prescribed leukotriene-modifying agents: a nested case-control study.
J Allergy Clin Immunol, 130(2):368-375, 12 Jun 2012
Cited by: 25 articles | PMID: 22698520
Montelukast, a leukotriene receptor antagonist, for the treatment of persistent asthma in children aged 2 to 5 years.
Pediatrics, 108(3):E48, 01 Sep 2001
Cited by: 174 articles | PMID: 11533366
Association between leukotriene-modifying agents and suicide: what is the evidence?
Drug Saf, 34(7):533-544, 01 Jul 2011
Cited by: 29 articles | PMID: 21663330
Review
Funding
Funders who supported this work.
AHRQ HHS (2)
Grant ID: R01 HS019669
Grant ID: 1R01HS019669
NIDDK NIH HHS (2)
Grant ID: 1P30-DK092924
Grant ID: P30 DK092924
NIMH NIH HHS (2)
Grant ID: U19 MH092201
Grant ID: U19MH092201





