Abstract
Rationale
The implementation of mass spectrometry (MS)-based metabolomics is advancing many areas of biomedical research. The time associated with traditional chromatographic methods for resolving metabolites prior to mass analysis has limited the potential to perform large-scale, highly powered metabolomics studies and clinical applications.Methods
Here we describe a three-minute method for the rapid profiling of central metabolic pathways through UHPLC/MS, tracing experiments in vitro and in vivo, and targeted quantification of compounds of interest using spiked in heavy labeled standards.Results
This method has shown to be linear, reproducible, selective, sensitive, and robust for the semi-targeted analysis of central carbon and nitrogen metabolism. Isotopically labeled internal standards are used for absolute quantitation of steady-state metabolite levels and de novo synthesized metabolites in tracing studies. We further propose exploratory applications to biofluids, cell and tissue extracts derived from relevant biomedical/clinical samples.Conclusions
While limited to the analysis of central carbon and nitrogen metabolism, this method enables the analysis of hundreds of samples per day derived from diverse biological matrices. This approach makes it possible to analyze samples from large patient populations for translational research, personalized medicine, and clinical metabolomics applications. Copyright © 2017 John Wiley & Sons, Ltd.Free full text

A Three-Minute Method for high-throughput quantitative metabolomics and quantitative tracing experiments of central carbon and nitrogen pathways
Abstract
RATIONALE
The implementation of mass spectrometry-based metabolomics is advancing many areas of biomedical research. The time associated with traditional chromatographic methods for resolving metabolites prior to mass analysis has limited the potential to perform large scale, highly powered metabolomics studies and clinical applications.
METHODS
Here we describe a three-minute method for the rapid profiling of central metabolic pathways through UHPLC-MS, tracing experiments in vitro and in vivo, and targeted quantification of compounds of interest using spiked in heavy labeled standards.
RESULTS
This method has shown to be linear, reproducible, selective, sensitive, and robust for the semi-targeted analysis of central carbon and nitrogen metabolism. Isotopically-labeled internal standards are used for absolute quantitation of steady-state metabolite levels and de novo synthesized metabolites in tracing studies. We further propose exploratory applications to biofluids, cell and tissue extracts derived from relevant biomedical/clinical samples.
CONCLUSIONS
While limited to the analysis of central carbon and nitrogen metabolism, this method enables the analysis of hundreds of samples per day derived from diverse biological matrices. This approach makes it possible to analyze samples from large patient populations for translational research, personalized medicine, and clinical metabolomics applications.
Introduction
Mass spectrometry (MS)-based metabolomics has recently become a key analytical readout for biomedical investigations, especially in fields of cancer research and precision medicine. Improvements in the mass accuracy and resolving capabilities of MS instrumentation have allowed this technique to complement and, often times, replace nuclear magnetic resonance (NMR)-based approaches [1][2] for metabolomics studies due to increased sensitivity, flexibility, and decreased sample requirements.[1] However, MS-based metabolomics is still limited by time-consuming analytical workflows and issues with robustness,[1] both of which are essential factors for the successful execution of high-throughput platforms for hypothesis generation/testing[3] and translational clinical applications.[4] While nanochip detection kits are poised to provide an innovative tool for the quantitative real-time assessment of a limited number of compounds in the future,[5] MS-based workflows that enable more extensive coverage of the metabolome in under 10 minutes have already been described and implemented.[6] Although additional methodology using flow-injection time-of-flight MS (TOF-MS) offers rapid real-time analytical readouts for a theoretical limit of 1,000 samples per day,[7–9] this technique is applicable primarily to liquid-based matrices and sacrifices chromatographic separation of metabolites.
We recently described a three-minute method that combines ultra-high performance liquid chromatography (UHPLC) and MS to perform high-throughput quantitative analyses of amino acids in numerous biological matrices including biofluids, cell and tissue extracts.[10] Here, we describe a fast method that utilizes UHPLC-MS to provide expanded coverage of key metabolic pathways while maintaining analytical times that are comparable to flow-injection TOF-MS.[8,9] Characterization of approximately 400 analytical standards has demonstrated the capability of this high-throughput approach to quantitatively analyze central carbon and nitrogen metabolism, including pathways such as glycolysis, the pentose phosphate pathway (PPP), the tricarboxylic acid (TCA) cycle, the urea cycle, amino acid, purine and pyrimidine metabolism. We demonstrate the biomedical utility of this approach by profiling clinically-relevant samples such as packed red blood cell (RBC) concentrates used for transfusion, biofluids (plasma, mesenteric lymph and bronchoalveolar lavage fluid – BALF), and lung tissue biopsies isolated from rats that have undergone severe trauma and hemorrhagic shock.
Analytical platforms that infer rates of glycolysis and mitochondrial metabolism through the measurement of oxygen consumption and extracellular acidification rates have become widely utilized due to their relatively low costs and ease of use.[11] However, these techniques do not directly measure small molecule targets and therefore provide a limited insight into the specific utilization and coordination of central metabolic pathways.[11] Here we demonstrate the utility of our method to directly measure steady-state levels and perform tracing experiments in vitro (e.g. RBC stored under blood bank conditions) and in vivo (e.g. bolus chase in animal models) using different stable isotope substrates (e.g. 13C1,2,3-glucose and 13C15N-glutamine). Tracing experiments provide investigators with a tool to mechanistically investigate metabolic fluxes by kinetically elucidating the incorporation of isotopically labeled carbon and nitrogen atoms into downstream metabolites.[12–15] Moreover, we demonstrate the suitability of this approach to perform absolute quantitation of endogenous metabolites, as well as quantitation of de novo synthesized metabolites in stable isotope tracing experiments using internal isotopically-labeled standards that have identical chemical properties but unique molecular weights relative to the endogenous unlabeled and de novo synthesized isotopologues. Taken together, this rapid method paves the way for cost-effective and ultra-high-throughput semi-targeted metabolomics and quantitative tracing experiments, as demonstrated by the analysis of approximately 2,000 samples over three distinct applications.
Materials and Methods
A significantly more detailed version of this section is provided as Supporting information - Materials and Methods extended. All procedures performed in this study were in accordance with the ethical standards of the Ethics Committee of the University of Colorado Denver and in conformity with the Declarations of Helsinki.
UHPLC-MS Setup
Analyses were performed using a Vanquish UHPLC system (Thermo Fisher Scientific, San Jose, CA, USA) coupled online to a Q Exactive mass spectrometer (Thermo Fisher Scientific, San Jose, CA, USA). Samples were resolved over a Kinetex C18 column, 2.1 × 150 mm, 1.7 μm particle size (Phenomenex, Torrance, CA, USA) equipped with a guard column (SecurityGuard™ Ultracartridge – UHPLC C18 for 2.1 mm ID Columns – AJO-8782 – Phenomenex, Torrance, CA, USA) at 25°C using an isocratic condition of 5% acetonitrile, 95% water, and 0.1% formic acid flowed at 250 μl/min. The Q Exactive mass spectrometer (Thermo Fisher Scientific, San Jose, CA, USA) was operated independently in positive or negative ion mode, scanning in Full MS mode (2 μscans) from 60 to 900 m/z at 70,000 resolution, with 4 kV spray voltage, 15 shealth gas, 5 auxiliary gas. Calibration was performed prior to analysis using the PierceTM Positive and Negative Ion Calibration Solutions (Thermo Fisher Scientific). Acquired data was then converted from .raw to .mzXML file format using Mass Matrix (Cleveland, OH, USA). Metabolite assignments, isotopologue distributions, and correction for expected natural abundances of deuterium, 13C, and 15N isotopes were performed using MAVEN (Princeton, NJ, USA).[16]
Graphs, heat maps and statistical analyses (either T-Test or ANOVA) were prepared with GraphPad Prism 5.0 (GraphPad Software, Inc, La Jolla, CA) and GENE-E (Broad Institute, MA).
Metabolite standard characterization
Coverage and selectivity
Experimental retention times for over 400 analytical standards (MSMLS, IROATech, Bolton, MA, USA) were determined over a four and three-minute isocratic method (conditions described above – Supplementary Table 1). Compounds were resuspended 240 μl of 5% methanol (v/v) according to the manufacturer’s instructions (Supplementary Table 1). Standards were then diluted 1:9 (v/v) into either 0.1% formic acid or a metabolite extraction solution consisting of methanol, acetonitrile, water (5:3:2, v/v/v), and 10 μl were injected for UHPLC-MS analysis.
Robustness of chromatography and quantitation
Reproducibility of chromatographic retention time, pressure, mass accuracy, and absence of compound carry over in blank runs was characterized over 1500 non-consecutive runs of packed RBCs and supernatants (as detailed below) spanning across six days of analysis. Technical mixes were prepared by pooling RBC extracts from each of the 4 donors at day 2 of storage. Technical mixes were injected every 15 runs and the analysis was repeated after 4 days of data acquisition. Comparison of 10 technical mixes from day 1 to day 2 or from day 4 to day 5 were analyzed to assess intra and inter-day technical variability, expressed as coefficients of variation (CV = mean/standard deviation) to monitor analytical variables.
Quantitative analysis and matrix effects
To asses inter-day reproducibility and the linearity of quantitation, standards were analyzed at concentrations ranging over 4 orders of magnitude, from 0.5 to 5000 pmol per injection (detailed in Supporting information - Sigma Aldrich, St. Louis, MO, USA). Ion suppression effects were determined with the use of uniformly labeled 13C6-glucose that was spiked into extraction solution at four different concentrations ranging over four orders of magnitude (0.1, 1, 10, 100, 1000 μM) prior to plasma extraction (described below in Applications). Plasma samples from 3 control rats (see below) were run either undiluted or diluted two-fold with extraction solution.
Reproducibility
To further characterize stability and reproducibility, CVs were determined for the quantitation of heavy-labeled and xenometabolite standards added to plasma prior to extraction. These standards included eighteen 13C15N-amino acids at a final concentration of 0.25 μM (Cambridge Isotopes Laboratories, Inc., Tewksbury, MA) and 5-fluorouracil at a final concentration of 2.5 μM (Sigma Aldrich, St. Louis, MO, USA).
Applications
Rat model of trauma/hemorrhagic shock
Samples were kindly provided by Dr. Anthony Bacon and Anne Slaughter (University of Colorado Denver).
Biofluids
Briefly, nonlethal trauma/hemorrhagic shock was induced in Sprague Dawley rats (n = 4) by laparotomy and controlled hemorrhage from the femoral artery until the rats achieved mean arterial pressure (MAP) equal to 30 mm Hg (MAP 30), as previously described [17]. Plasma, mesenteric lymph and bronchoalveolar lavage fluid (BALF) samples (20 μl) were collected before hemorrhagic shock (Shock) and immediately after (30 minutes of shock at MAP 30), either in presence or absence of mesenteric lymph diversion (MLD) through cannulation of the mesenteric artery
Samples (20 μl) of biofluids (plasma, mesenteric lymph, or BALF) were extracted in 480 μl of ice cold extraction solution by vortex for 30 minutes at 4 °C, as reported.1 Insoluble proteins and lipids were pelleted by centrifugation at 4°C for 10 minutes at 10,000g and supernatants were collected and stored at −80°C until subsequent analysis.
In vivo bolus chase of 13C15N-glutamine in rat lung biopsies
Heavy labeled lung biopsies were isolated from Shock/Sham (n = 8 per group) rats generated as described above. At the 15 min time point, Shock and Sham rats received a 1 mL/kg intravenous infusion of 13C515N2-glutamine (Product no. CNLM-1275-H-0.1, Cambridge Isotope Laboratories, Inc., Tewksbury, MA) in physiological solution (35 mM) at a rate of 0.5 mL/min. Animals remained in shock for a total of 45 min (30 min after bolus injection). Ten mg of lung tissues were ground to powder in liquid nitrogen, then extracted in ice-cold lysis/extraction buffer at a concentration of 10 mg/mL, before vortexing and centrifugation as described above.
Packed red blood cell collection and storage
Packed RBC units, kindly provided by Dr. Tatsuro Yoshida (New Health Sciences, Inc., Cambridge, MA), were collected and stored as previously reported [18]. Samples (0.5 ml) were obtained through sterile couplers at day 2, 7, 14, 21, 28, 35 and 42 [18]. RBCs and supernatants were separated by centrifugation and extracted in ice-cold metabolite extraction solution at 1:9 and 1:24 (v/v) dilutions, respectively, as previously described [18].
Method Comparison to gradient-based C18 and HILIC methods
Extracts of RBC (10 μl) and AS-3 supernatant (20 μl) were analyzed with the three-minute method and two gradient-based methods using a C18 or HILIC [19,20]. The two alternative chromatographic methods consisted of (i) a 9 minute method on a Kinetex C18 column, 2.1 × 150 mm, 1.7 μm particle size (Phenomenex, Torrance, CA, USA) run at 400 μl/min and 30°C (mobile phases – A: H2O, 0.1% formic acid; B: acetonitrile, 0.1% formic acid); or (ii) a 15 min method on an Acquity UPLC BEH Amide Column, 2.1 × 100 mm, 1.8 μm particle size (Waters, Milford, MA, USA) run at 350 μl/min and 35°C (mobile phases – A: 5% acetonitrile, 10 mM ammonium acetate, pH 10.0; B: 95% acetonitrile, 10 mM ammonium acetate, pH 10.0 adjusted with MS grade ammonium hydroxide). Further analytical details are extensively described in the Supporting information document.
In vitro labeling experiments
Packed RBCs (n =4) were collected, processed and stored in CP2D-AS-3, as described above. Before processing, AS-3 (containing 55 mM dextrose in 100 ml of solution) was supplemented with 11 mM 13C1,2,3-glucose (Product no. CLM-4673-PK - Cambridge Isotope Laboratories, Inc., Tewksbury, MA). Samples were collected on a weekly basis at storage day 2, 7, 14, 21, 28, 35 and 42.
Quantitative tracing experiments
Packed RBCs incubated with 13C1,2,3-glucose (see previous paragraph) were extracted with the addition of a standard mixture containing 18 13C,15N-labeled amino acids at a final concentration of 0.25 μM (Product no. MSK-A2-1.2 - Cambridge Isotope Laboratories, Inc., Tewksbury, MA).
Results and Discussion
We recently described a rapid method to analyze the amino acid profiles of distinct biological matrices such as biofluids, cell and tissue extracts in only three minutes.[10] Here we expanded upon this method to detect and profile a wide range of compounds associated with central carbon and nitrogen metabolic pathways.
Selectivity and reproducibility
Over 400 pure analytical standards ranging from 72 to 666 Da were tested and characterized using an isocratic gradient, providing retention times for each compound (Supplementary Table 1). While aqueous dilution of standards into 0.1% formic acid improved chromatographic separation and detection over a four-minute window, dilution of the same compounds into the organic metabolite extraction solution (methanol/acetonitrile/water 5:3:2 v/v/v) [10,15,17,18,21] provided enhanced compound coverage, increased signal/noise ratios, and sensitivity within a shorter three-minute retention time window. Separation was achieved for key metabolites from pathways such as glycolysis, TCA cycle, glutaminolysis/glutathione homeostasis, and polyamine metabolism (Figure 1C). Despite the relatively short chromatographic resolution window, this method provided baseline separation of compound classes (Figure 1C, 1G). We observed resolution of structural and positional isomers including, but not limited, to 2,3-cAMP and 3,5-cAMP (Figure 1D); L-leucine, L-isoleucine, and norleucine; 3-aminoisobutanoate and 4-aminobutanoate; fumarate and maleic acid; betaine, L-valine, and norvaline; noradrenaline and pyridoxine; D-glucoronolactone and ascorbate; citrate and isocitrate (Supplementary Table 1).
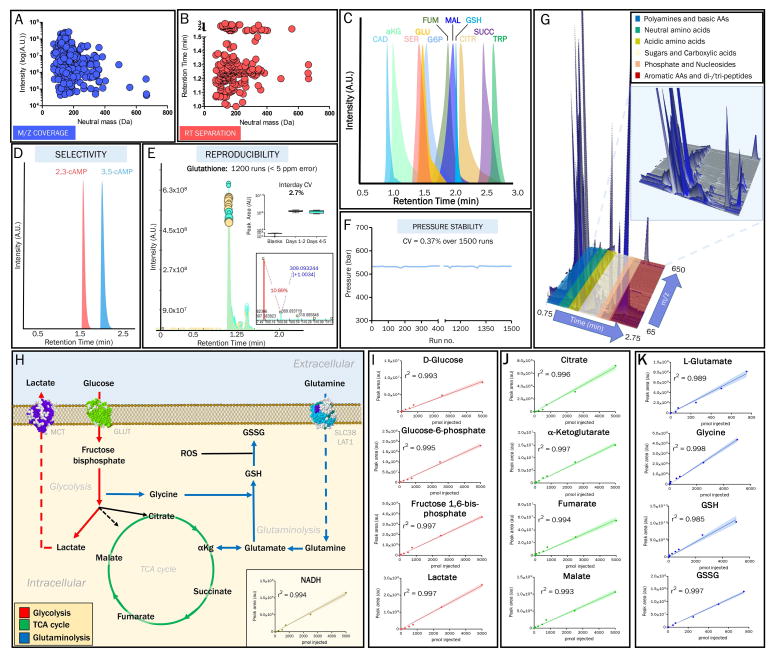
Using a library of ~400 compounds, the retention times and peak areas were determined when diluted in aqueous and organic solutions (Supplementary Table 1). Results are depicted as 2-dimensional plots showing (A) neutral mass vs. ion intensity, or (B) neutral mass vs. chromatographic retention time. In C, metabolites critical to central carbon and nitrogen metabolism are resolved during the isochratic elution.. In D, resolution of isobaric isomers such as 2,3-cAMP and 3,5-cAMP was observed. Reproducibility of peak shape, retention time, and ion signal intensity is shown in 10 replicate injections of a technical mixture over the course of 1200 analytical runs spanning over 6 non-consecutive days (E). (F) The stability of system pressure indicates a lack of excessive analyte accumulation on the column during 1500 runs. In G, we provide a representative 3D chromatogram showing separation of different hydrophilic compound classes over a three minute run. Linearity of quantitation (r2 > 0.98) for key metabolites in central carbon and nitrogen pathways (H) was assessed over 4 orders of magnitude using a 9-point standard curve. Representative metabolites are shown for glycolysis (red - I), TCA cycle (green - J), and glutaminolysis/glutathione homeostasis (GSH/GSSG – reduced/oxidized glutathione) (blue - K).
We assessed reproducibility of this method by analyzing the coefficients of variation (CV) for analytical parameters such as retention time, peak shape, and ion intensity (peak area top and peak area integration) of identified metabolites in a technical replicate mixture injected every 15 samples over the course of 1200 analytical runs during six non-consecutive days. An example is presented in Figure 1E, showing an inter day peak area CV = 2.7% for glutathione. Although this method does not incorporate a washing/re-equilibration segment, carry over of compounds were not observed in blank runs before and after 1500 sample runs spanning over 6 days. In support, the column operational pressure was maintained during this time period (Figure 1F) indicating that the guard column contributes to the stability of column performance by retaining relatively hydrophobic compounds. In addition, we determined retention time CVs of 0.28% and extremely stable system pressure (CV=0.37%) throughout the entire analysis (Figure 1E, 1F). MS signal was also stable during this extensive analysis as demonstrated by preservation of sub-5 ppm mass accuracy for all standard calibration mixtures analyzed both prior to and post-analysis (data not shown). We observed < 0.5% average deviation from the theoretical ion intensity for the natural abundance of the M+1 ion (13C1) in glutathione (Figure 1E inset), indicating MS signal stability that is a key analytical feature for both the identification of compounds on the basis of the chemical formulas as well as for quantitative and tracing experiments.[22]
Relative and absolute quantitative analysis with external calibration curves and heavy-labeled spiked in standards
We next characterized the performance of this method with respect to quantitative analyses. Relative levels of metabolites were assessed across samples by characterizing signal linearity in serial dilutions of select compounds from central pathways (Figure 1H). Signal linearity was assessed using linear regression analysis (r2), and was confirmed over four orders of magnitude that were tested for compounds reported in Figure 1I–K (Supplementary Table 2), with the lowest limit of detection (LLOD) = 100 fmol and the lowest limit of quantitation (LLOQ) = 500 fmol. By further validating signal reproducibility using internal standards such as the xenometabolite 5-fluorouracil (Figure 2A) and isotopically-labeled 13C515N1-L-valine (Figure 2B), we then confirmed signal linearity in the presence of a complex matrix by determining an r2 > 0.98 for 13C6-glucose that was exogenously added to human plasma as an internal standard at four log orders of concentration (0.1 – 1000 μM) before extraction (Figure 2C and 2D). This experiment was performed over two non-consecutive days to demonstrate inter-day reproducibility of the linearity of quantifications (Figure 2D).
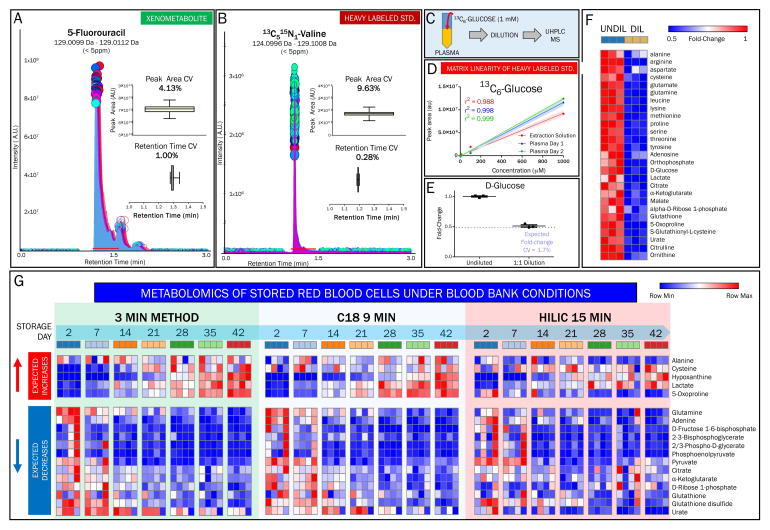
To show reproducibility of internal standards, coefficients of variation (CV = average/standard deviation) for peak area and retention time were calculated for (A) the xenometabolite 5-fluorouracil and (B) isotopically-labeled 13C5,15N1-valine. (C) Interday variability, matrix effects on ionization, and signal linearity were determined by extracting plasma with the addition of 1 mM 13C6-glucose. (D) Signal linearity is shown with a 5-point serial dilution in plasma over 4 orders of magnitude. Dilutions on two non-consecutive days and in extraction solution alone show interday reproducibility and matrix effects, respectively. (E) Dilution of plasma 1:1 (v/v) with extraction solution demonstrates linearity of fold change calculations for the relative quantitation for glucose, (F) and for additional compounds in central carbon and nitrogen pathways. Fold change values are depicted using a heat map, with blue representing a fold change of 0.5 and red representing a fold change of 1. (G) Red blood cell extracts sampled over a 42 day storage period were analyzed using the three-minute method and conventionl gradient-based methods using a C18 (9 minute method) and amide HILIC (15 minute method). Metabolite abundances are depicted as heat maps to facilitate comparison between methods of technical reproducibility and expected trends based on previously published results.
Human plasma, either undiluted or diluted two-fold in extraction solution (Figure 2E), was tested with the three-minute method to determine linearity of quantitation, recovery, and matrix-dependent ion suppression. Minor deviations from the expected fold changes of 2 were observed, with a CV < 2% for glucose and other representative metabolites of key pathways (Figure 2E and 2F).
Comparison to gradient-based methods: metabolomics of stored RBCs
Next, we tested the suitability of this method for applications requiring high-throughput relative and absolute quantitation of target compounds in clinically-relevant samples. Metabolic analyses of RBCs are clinically relevant in that blood biochemical testing is a hallmark of clinical biochemistry. Indeed, RBCs are by far the most abundant host cell in the human body (25 out of 30 trillion host cells in the human body are RBCs) and their metabolism mirrors states of health and disease. In addition, transfusion of packed RBCs is a life-saving intervention for patients with hematological malignancies and hemoglobinopathies, but also for surgical patients or those suffering from traumatic injuries with hemorrhage. While RBC storage is a logistic necessity to make approximately 108 million units available for millions of recipients worldwide every year, refrigerated storage of RBCs negatively affects RBC metabolism and physiology, the effects of which are referred to as the storage lesion. RBC metabolism during storage under blood bank conditions has been extensively characterized by our group using LC-MS, [18,21,23–25] and by others via NMR and gas chromatography-MS (GC-MS)-based approaches.[26,27] Here, four units of RBCs were sampled during storage in Additive Solution (AS)-3 (Nutricel, Haemonetics) on a weekly basis until the expiration date of the units (42 days upon collection and processing). Samples were analyzed with the three-minute method and conventional gradient-based methods using either a C18 or HILIC column.[19,20] It is worth noting that the HILIC method was validated in-house using the same IROA standard library (Supplementary Table 1). Samples were run in randomized order, while technical mixes (pool of day 0 samples from each of the four units) were run every 15 samples to ensure stability of the system under each analytical condition (Supplementary Table 3). Metabolite levels were graphed as a heat map in order to facilitate comparison with the existing literature that extensively describes the metabolic impairments associated with the storage lesion (Figure 2G, a vectorial version of the heat maps including metabolite names is provided in Supplementary Figure 1).[18,21,26,27] The storage lesion is in part characterized by the progressive accumulation of lactate, increased purine catabolism, depletion of intermediates of the pentose phosphate pathway (PPP), and impaired glutathione synthesis. Decreased levels of citrate, adenine, urate, ribose phosphate, glutamine, reduced and oxidized glutathione (GSH and GSSG), and increased levels of hypoxanthine and 5-oxoproline are also observed in stored RBCs.[18,21,26,27] Recently, we identified 8 metabolic markers of the RBC storage lesion that were validated by two independent laboratories with at least three different analytical setups.[21] Comparison of the results for those biomarkers (three representative markers are shown in Supplementary Figure 2) generated by the three methods used here indicates that the methods are indeed comparable among each other and are consistent with previously observed RBC metabolic trends during storage [18,21,26,27]. For example, accumulation of the glycolytic catabolite lactate and purine oxidation product hypoxanthine has been shown using C18 and HILIC-based UHPLC-MS and NMR by independent laboratories.[18,21,26,27] and here confirmed with all methods (Supplementary Figure 2). Analogously, all three methods highlighted comparable storage-dependent citrate consumption in RBCs stored in AS-3 (Supplementary Figure 2), consistent with recent reports identifying a role for citrate metabolism in stored RBCs and suggesting this metabolite as a marker of the RBC storage lesion[21,24]. Furthermore, the three-minute method maintained superior reproducibility across technical replicates relative to the amide HILIC column despite lacking a conventional elution gradient, indicated by the analysis of technical mixes run every 15 samples during the entire analysis (Supplementary Table 3).
Applicability of the method in different biological matrices
In the last eighteen months we have applied this method to thousands of samples from a variety of biological matrices and species ranging from E. coli to humans (Figure 3A). We present here a few representative applications that demonstrate the suitability of this method to assess metabolic phenotypes in diverse biological matrices, including cultured cells, human and mouse biofluids and tissue extracts.

(A) This method has been used to analyze cells, tissues and biofluids from a wide range of species. (B) As an example of biofluid analysis, plasma, mesenteric lymph, and bronchiolar lavage fluid (BALF) were isolated from rats hemorrhaged until shock (Shock group) at a mean arterial pressure equal to 30 mmHg (MAP 30), or unhemorrhaged (Sham group), with and without a mesenteric lymph diversion (MLD). (C) Metabolite abundances in plasma, mesenteric lymph, and BALF are graphed as heat maps with blue representing the row minimum and red representing the row maximum. The abundances of multiple metabolites are affected by shock and MLD, (D) such as the case with the pro-inflammatory metabolite succinate.
Applications 1: The metabolic effects of mesenteric lymph diversion to treat trauma/hemorrhagic shock, as assessed in the clinically relevant biofluids of lymph, BALF, and plasma
As noted above, the transfusion of packed RBCs is a life-saving intervention for several categories of recipients, but especially for massively transfused trauma patients with severe hemorrhage. To highlight the clinical importance of such treatment, it is worth noting that the most recent data from the Center for Disease Control indicate that trauma is the leading cause of death under the age of 46 and results in more life years lost than both cancer and cardiovascular disease combined.[28] Acute mortality in trauma is dependent on three main factors: hypothermia, acidosis, and coagulopathy. Late death by trauma ensues hours to week after the injury and is principally caused by multiple organ failure or acute lung injury, which account for approximately 20% of total mortality.[28] Surgical procedures that divert the mesenteric lymph in trauma/hemorrhagic shock patients have been shown to attenuate acute lung injury.[29] We recently documented significant alterations of the human and rat plasma metabolome in response to trauma and hemorrhage.[17] Here we sought to investigate shock-induced metabolic alterations to mesenteric lymph and BALF, which both undergo significant exchange with plasma. Plasma, lymph, and BALF were isolated from rats, either hemorrhaged to a mean arterial pressure (MAP) of 30 mmHg (MAP 30) by cannulation of the mesenteric artery (Shock group – Figure 3B), or that had undergone laparotomy without hemorrhage (Sham group). The three-minute method was used to observe that the metabolic phenotypes of all three biofluids were affected by severe hemorrhage, which altered fluid compositions with respect to amino acids, nucleosides, and lactate, the latter of which is a key driver of post-shock acidosis and a marker of poor prognosis in the trauma population (Figure 3C). Extended vectorial versions of the heat maps in Figure 3 – also including metabolite names - are provided in Supplementary Figure 3, while measurement data are provided in Supplementary Table 4. The accumulation of the TCA cycle intermediate succinate in post-shock plasma has been previously associated with coagulopathy/fibrinolysis and inflammatory responses due to its potential role in the activation of neutrophils, which in turn contribute to the etiology of acute lung injury.[17,30] Here we show that mesenteric lymph diversion does not correct the shock-induced accumulation of succinate, and instead promotes its accumulation in the mesenteric lymph and the BALF (Figure 3D).
Application 2: In vivo steady-state isotope tracing in the lungs of rats undergoing trauma/hemorrhagic shock
BALF only offers an indirect measurement of the lung metabolome. We thus sought to determine the actual extent of succinate accumulation within the lung that represents a potential marker of inflammatory lung injury, and also to mechanistically identify the metabolic substrate that fuels the production of this pro-inflammatory dicarboxylic acid [31] in the lung. Therefore, we injected rats with a bolus of 13C515N2-glutamine right after laparotomy alone (Sham), or with hemorrhage to MAP 30 (Shock) (Figure 4A). After a 45-minute incubation period to allow for tissue uptake and metabolism of heavy glutamine, lungs were harvested from sacrificed animals for the detection of steady state isotopes in glutamine catabolites (Figure 4B). Based on our preliminary results indicating that less than 10% of succinate produced de novo after shock is derived from 13C6-glucose,[32] we anticipated that a larger portion of shock-dependent accumulation of succinate would be produced via glutaminolysis. Indeed, heavy-labeled glutamine and TCA cycle intermediates, including uniformly labeled 13C4-succinate, were elevated by 3- to 10-fold in Shock lungs compared to Sham (Figure 4C). This accumulation resulted in ~15% enrichment of the heavy succinate isotopologue in the Shock group and a < 2% incorporation of glutamine-derived heavy carbon atoms into succinate in the Sham group as early as 30 min after bolus injection. These results indicate that glutaminolysis contributes significantly to succinate accumulation in response to hemorrhagic shock and suggests that enzymes central to this pathway, such as glutaminase, may represent potential therapeutic targets to improve patient resuscitation strategies in trauma.

(A) Eight rats underwent laparotomy to induce trauma with and without induction of hemorrhage after 30 minutes to mean arterial pressure = 30 mmHG (MAP 30). At the 30 minute time point, a bolus of 13C515N2-glutamine was injected into circulation and allowed to metabolize for 45 minutes, at which point the rat lungs were isolated and analyzed by the three-minute method. (B) A scheme for heavy atom incorporation into TCA cycle intermediates is shown. Accumulation of succinate in particular is known to activate neutrophils and lead to induction acute lung injury (ALI). (C) The metabolism of 13C5,15N2-glutamine to fuel the TCA cycle in the lung is shown to be shock-dependent.
Application 3: Ex vivo quantitative tracing experiments of stored RBCs as a tool to probe the mechanisms of the storage lesion
Based on the suitability of the three-minute method to perform (i) high-throughput tracing experiments and (ii) absolute quantification of compounds using the heavy isotope-labeled internal standards, we sought to combine these two approaches to achieve absolute quantification of heavy isotopologues produced de novo during a tracing experiment by using orthogonally labeled stable isotope compounds for quantitation. To test the feasibility of this approach, four leukocyte and platelet-filtered packed RBC units were stored under routine blood bank conditions for 42 days in presence of 11 mM 13C1,2,3-glucose (Figure 5A). Samples were extracted using the metabolite extraction solution supplemented with a commercially-available standard mixture of 18 13C15N-amino acids (see details in the methods section). The presence of 15N atoms in these internal standard compounds provided masses that were unique to those of endogenous unlabeled or solely 13C-labeled isotopologues that were synthesized by RBC through the metabolism of 13C1,2,3-glucose. Absolute levels of amino acids could thus be determined by comparing the peak areas of endogenous light/13C-only labeled amino acids to those of the fully-labeled 13C15N heavy isotopologues added at a known concentration. An example for L-alanine is described in Figure 5B, which shows the formula to quantify total levels of alanine in addition to the de novo synthesized 13C3-alanine isotopologue that is derived from pyruvate transamination (scheme presented in Figure 5C). Using this approach, we were able to quantify not just the total levels of alanine in RBCs and the additive solution supernatant (Figure 5D), but also the absolute levels of de novo synthesized 13C3-alanine over the 42-day storage period (Figure 5E).
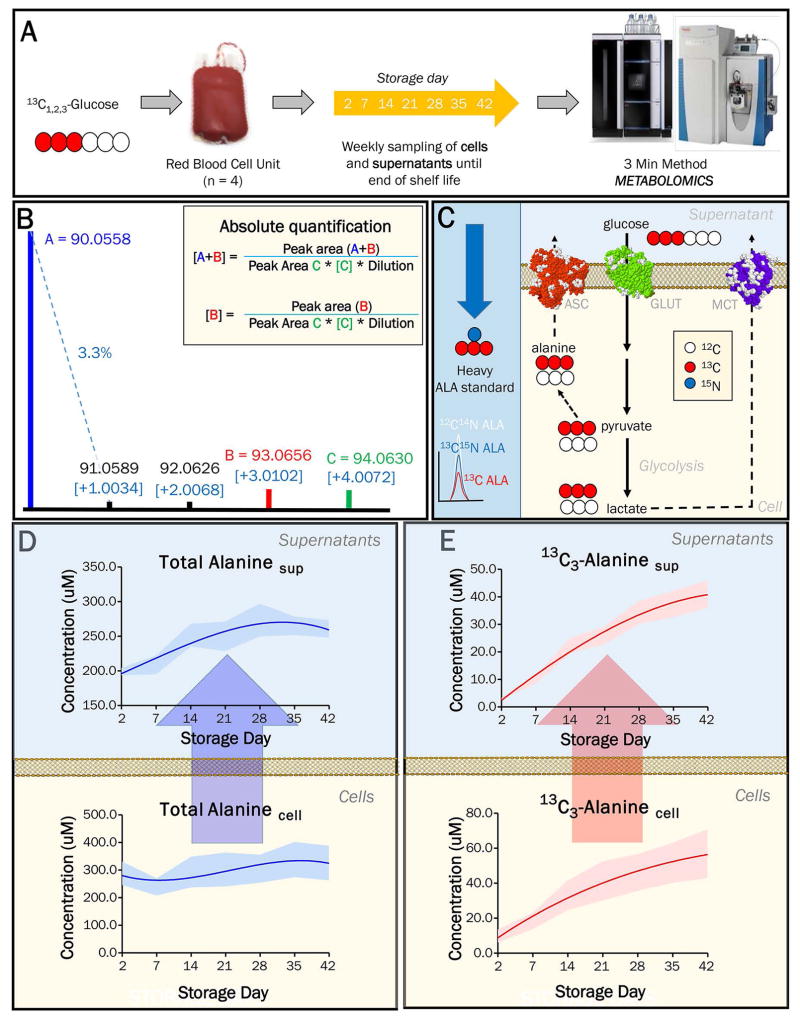
(A) Red blood cells were stored for 42 days in the presence of 13C1,2,3-Glucose and were sampled on a weekly basis. (B) The predicted isotopologue distribution of 13C315N2-Alanine, which was included as an internal standard in the metabolite extraction solution. Formulas to calculate the absolute quantity of alanine are depicted in the inset, and in (C) a schematic showing the metabolism of 13C1,2,3-Glucose into alanine and the expected chromatographic overlap of the various alanine isotopologues. (D) Absolute quantities of total alanine in the cells and in the additive solution superntant throughout storage are shown. (E) Absolute quantities of de novo synthesized alanine during storage are shown.
Conclusions
The development of high-throughput methods for metabolomics applications [3] is required for large-scale studies and routine clinical practice.[4] Here we describe a three-minute method that combines the advantages of rapid flow-injection TOF-MS with the selectivity of convential chromatography-based metabolomics to cover pathways of central carbon and nitrogen metabolism.
By exploiting the recent technical advancements in UHPLC and fast scanning high-resolution MS technologies, this method maintains comparable analytical performance with regards to sensitivity, selectivity, reproducibility, and robustness at the chromatographic and MS level. While this approach is not applicable for the measurement of hydrophobic compounds such as lipids and long-chain acyl carnitines, it is capable of rapidly profiling key metabolic pathways such as glycolysis, PPP, TCA cycle, urea cycle, polyamines, amino acid and nucleotide metabolism in hundreds of samples per day. Furthermore, the robustness of this approach makes it useful for the analysis of a wide range of biological matrices relevant to basic science and clinical routine practice, including biofluids, cell and tissue extracts. We describe representative applications of clinical relevance to the fields of intensive care and transfusion medicine, though the method described here can be applied to other areas including cancer, immunology and cardiovascular research. The applications presented here highlight the capabilities of this method for the rapid and high-throughput profiling of metabolism, relative and absolute quantitation of target metabolites, and tracing experiments both in vitro and in vivo. By combining the use of heavy labeled compounds for absolute quantitation and tracing experiments, we further highlight the utility of this method to accurately quantify de novo synthesized metabolites through central carbon and nitrogen pathways upon incubation with appropriate heavy labeled substrates. The proposed method herein significantly reduces instrument time and consumable cost (i.e. columns and solvents for UHPLC phases). At the same time, it provides critical robustness and sensitivity of relative and absolute quantitation in steady state and tracing experiments, and enables high-throughput cost-effective routine screening of central metabolic pathways for biomedical research and clinical applications, such as in the field of personalized medicine. Taken together, this method can be used to elucidate the intricacies of altered metabolism for both small-scale and highly-powered clinical studies in a time-efficient and cost-effective manner.
Supplementary Material
Supp Fig S1
Supplementary Figure 1:Heat maps of RBC metabolic changes during storage in the blood bank, as gleaned through the three minute method or gradient-based C18 and HILIC methods.
Supp Fig S2
Supplementary Figure 2:Biomarkers of the RBC metabolic storage lesion (as identified by Paglia et al.[21]) and their relative values over storage (normalized to day 0 controls), as gleaned through the three minute method or gradient-based C18 and HILIC methods.
Supp Fig S3
Supplementary Figure 3:Extended vectorial heat map of biofluid analyses in trauma/hemorrhagic shock rats.
Supp TableS1
Supplementary Table 1:IROA standard analysis report with the three minute and HILIC methods.
Supp TableS2
Supplementary Table 2:Raw data for the calculation of calibration curves for representative metabolites of glycolysis, TCA cycle and glutaminolysis/glutathione homeostasis.
Supp TableS3
Supplementary Table 3:Metabolite levels in stored RBCs as gleaned through the three minute method and gradient-based C18 and HILIC methods, and characterization of all the tested metabolites in technical mixes.
Supp TableS4
Supplementary Table 4:Metabolite levels in plasma, mesenteric lymph and BALF from rats undergoing trauma and hemorrhage, with and without mesenteric lymph diversion.
Acknowledgments
The Authors are grateful to Drs. Anthony Bacon, Annie Slaughter, Anirban Banerjee for the rat models of trauma/hemorrhagic shock; Dr. Tatsuro Yoshida for the red cell storage tracing experiments, as detailed in the paper. The authors would also like to thank Dr. Robert Hodges for reviewing the manuscript. Research reported in this publication was supported in part by funds from the National Blood Foundation Early career grant 2016 (AD), University of Colorado Comprehensive Cancer Center Core Support (P30 CA046934-17), the National Institute of General Medical Sciences of the National Institutes of Health under Award Numbers P50GM049222 and T32GM008315, and Grant #P50 GM049222 from NIGMS, NIH (CS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest: All the authors disclose no conflict of interests in relation to the contents of the manuscript.
References
Full text links
Read article at publisher's site: https://doi.org/10.1002/rcm.7834
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc5364945?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1002/rcm.7834
Article citations
Clinical and immune responses to neoadjuvant fulvestrant with or without enzalutamide in ER+/Her2- breast cancer.
NPJ Breast Cancer, 10(1):88, 06 Oct 2024
Cited by: 0 articles | PMID: 39368973 | PMCID: PMC11455938
Blocking Tryptophan Catabolism Reduces Triple-Negative Breast Cancer Invasive Capacity.
Cancer Res Commun, 4(10):2699-2713, 01 Oct 2024
Cited by: 0 articles | PMID: 39311710 | PMCID: PMC11484926
Cysteamine dioxygenase (ADO) governs cancer cell mitochondrial redox homeostasis through proline metabolism.
Sci Adv, 10(40):eadq0355, 02 Oct 2024
Cited by: 0 articles | PMID: 39356760 | PMCID: PMC11446280
3,3-Dimethyl-1-Butanol and its Metabolite 3,3-Dimethylbutyrate Ameliorate Collagen-induced Arthritis Independent of Choline Trimethylamine Lyase Activity.
Inflammation, 17 Aug 2024
Cited by: 0 articles | PMID: 39153148
Non-canonical metabolic and molecular effects of calorie restriction are revealed by varying temporal conditions.
Cell Rep, 43(9):114663, 20 Aug 2024
Cited by: 0 articles | PMID: 39167490 | PMCID: PMC11427179
Go to all (154) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Translational Metabolomics of Head Injury: Exploring Dysfunctional Cerebral Metabolism with Ex Vivo NMR Spectroscopy-Based Metabolite Quantification
CRC Press/Taylor & Francis, Boca Raton (FL), 14 Aug 2015
Cited by: 0 articles | PMID: 26269925
ReviewBooks & documents Free full text in Europe PMC
A High-Throughput Targeted Metabolomics Workflow for the Detection of 200 Polar Metabolites in Central Carbon Metabolism.
Methods Mol Biol, 1859:263-274, 01 Jan 2019
Cited by: 7 articles | PMID: 30421235
Characterization of rapid extraction protocols for high-throughput metabolomics.
Rapid Commun Mass Spectrom, 31(17):1445-1452, 01 Sep 2017
Cited by: 6 articles | PMID: 28586533 | PMCID: PMC5547002
Rapid UHPLC-MS metabolite profiling and phenotypic assays reveal genotypic impacts of nitrogen supplementation in oats.
Metabolomics, 15(3):42, 12 Mar 2019
Cited by: 8 articles | PMID: 30868357 | PMCID: PMC6476850
Funding
Funders who supported this work.
NCI NIH HHS (1)
Grant ID: P30 CA046934
NIGMS NIH HHS (2)
Grant ID: P50 GM049222
Grant ID: T32 GM008315
National Blood Foundation (1)
Grant ID: Early Career Grant 2016 cycle





