Abstract
Free full text

Estrogen receptor interaction with estrogen response elements
Abstract
The estrogen receptor (ER) is a ligand-activated enhancer protein that is a member of the steroid/nuclear receptor superfamily. Two genes encode mammalian ER: ERα and ERβ. ER binds to specific DNA sequences called estrogen response elements (EREs) with high affinity and transactivates gene expression in response to estradiol (E2). The purpose of this review is to summarize how natural and synthetic variations in the ERE sequence impact the affinity of ER–ERE binding and E2-induced transcriptional activity. Surprisingly, although the consensus ERE sequence was delineated in 1989, there are only seven natural EREs for which both ERα binding affinity and transcriptional activation have been examined. Even less information is available regarding how variations in ERE sequence impact ERβ binding and transcriptional activity. Review of data from our own laboratory and those in the literature indicate that ERα binding affinity does not relate linearly with E2-induced transcriptional activation. We suggest that the reasons for this discord include cellular amounts of coactivators and adaptor proteins that play roles both in ER binding and transcriptional activation; phosphorylation of ER and other proteins involved in transcriptional activation; and sequence-specific and protein-induced alterations in chromatin architecture.
INTRODUCTION
The estrogen receptor (ER) is a ligand-activated enhancer protein that is a member of the steroid/nuclear receptor superfamily that includes 60 different ‘classical’ members of the nuclear hormone receptor family; by comparison the fly proteome has 19 and the worm proteome has 220 (1). Nuclear receptors share a highly conserved structure and common mechanisms affecting gene transcription (2). Mammalian ER is encoded by two genes: alpha and beta (ERα and ERβ) that function both as signal transducers and transcription factors to modulate expression of target genes (3). Here the term ER will refer to both ERα and ERβ whereas ERα and ERβ indicate that particular subtype. In response to ligand binding, ER undergoes conformational changes, termed ‘activation’, accompanied by dissociation of hsp90, hsp70 and other proteins (reviewed in 4), forming a ligand-occupied ER dimer (5).
Stimulation of target gene expression in response to 17β-estradiol (E2), or other agonists, is thought to be mediated by two mechanisms: (i) ‘direct binding’ where E2-liganded ER (E2–ER) binds directly to a specific sequence called an estrogen response element (ERE) and interacts directly with coactivator proteins and components of the RNA polymerase II transcription initiation complex resulting in enhanced transcription (6); and (ii) ‘tethering’ where ER interacts with another DNA-bound transcription factor in a way that stabilizes the DNA binding of that transcription factor and/or recruits coactivators to the complex. In mechanism (ii) ER does not bind DNA. Examples of the tethering mechanism of ER transactivation include ERα interaction with Sp1 in conferring estrogen responsiveness on uteroglobin (7), RARα (8), insulin-like growth factor-binding protein-4 (9), transforming growth factor α (10), bcl-2 (11) and the LDL receptor (12) genes; ERα interaction with USF-1 and USF-2 in the cathepsin D promoter (13); and ERα and ERβ interaction with AP-1 (14–16).
The focus of this review is how differences in ERE sequence impact ER binding affinity and transcriptional activation. While the effect of single nucleotide changes in each position of the glucocorticoid response element (GRE) on glucocorticoid receptor (GR) and progesterone receptor (PR) activity has been examined and reviewed (17–20), such detailed analysis is not complete for ERα–ERE interaction (21) and there is limited information regarding the effect of ERE sequence on ERβ activity (22–26).
ERα and ERβ are Class I nuclear receptors (NR) along with other the steroid receptors, e.g. glucocorticoid, mineralocorticoid, progesterone and androgen receptors (GR, MR, PR and AR, respectively) that bind to DNA as homodimers. ER differs from the other steroid receptors that bind to derivatives of a common response element [i.e. the consensus GRE: 5′-GGTACAnnnTGTTCT-3′, where n is any nucleotide (20,27)] in that ER binds to the ERE: 5′-GGTCAnnnTGACC-3′ (28). GR binds with highest affinity to 5′-GG T/G ACA G/T G G/A GGTACAnnnTGTTCT-3′; AR binds with highest affinity to 5′-GGTAC A/G CGGTGTTCT-5′; and PR binds 5′-G/A G G/T AC A/G TGGTGTTCT-3′, where the slash indicates approximately equal preference for either nucleotide (20).
Class I NR differ from the class II NR [e.g. retinoic acid receptor (RAR), retinoid X receptor (RXR), vitamin D receptor (VDR), thyroid receptor (TR) and peroxisome proliferator activated receptor (PPAR)] that bind to their response elements, i.e. various spacings of 5′-AGGTCA-3′, as heterodimers with RXR (29). Additionally, the NR superfamily includes ‘orphan receptors’, denoted as such because their endogenous ligands, if necessary, are either unknown, e.g. chicken ovalbumin upstream promoter transcription factor (COUP-TF), or have recently been identified, e.g. the pregnane X receptor/steroid X receptor (PXR/SXR) that binds steroids and xenobiotics (30). The evolutionary relationship among the steroid/nuclear receptors has been deduced by the high conservation in their DNA binding domains (DBDs) and in their less-conserved ligand binding domains (LBDs) and indicates that this large group of proteins arose from a common ancestral molecule (31). This common origin accounts for the similarities in mechanisms of DNA binding and transcriptional activation among NR superfamily members.
STRUCTURAL DOMAINS OF ERα AND ERβ
ERα and ERβ have six domains named A–F from N- to C-terminus, encoded by 8–9 exons (32). The three major functional domains of the ER are: (i) an N-terminus (domains A and B) that modulates transcription in a gene- and cell-specific manner through Activation Function-1 (AF-1); (ii) a highly conserved central DBD, consisting of the C domain, comprised of two functionally distinct zinc fingers through which ER interacts directly with the DNA helix; and (iii) the LBD (domain E) that contains Activation Function-2 (AF-2). In ERα, the F domain plays a role in distinguishing estrogen agonists versus antagonists, perhaps through interaction with cell-specific factors (33).
There is little conservation in amino acid sequence in the N-terminal regions of ERα and ERβ (34). Indeed, the activity of AF-1 in ERβ is negligible compared with that of ERα (26). The most conserved region between ERα and ERβ is the DBD featuring two cys–cys zinc fingers (CI and CII) with which the receptor interacts with the major groove and phosphate backbone of DNA, respectively (34). The specificity of the DBD in targeting ER for gene regulation was demonstrated by domain-swapping experiments in which the DBD of ERα was switched with that of the GR. The chimeric receptor, containing AF-1 and AF-2 of ERα and the DBD of GR, bound to GREs but up-regulated transcription in response to E2 (35), thus demonstrating the specificity of the DBD in target gene regulation.
ER INTERACTION WITH EREs
ERα and ERβ bind with high affinity to EREs (Tables (Tables11 and SS1).1). The ERE was first identified by aligning sequences with shared homologies in the 5′ flanking regions of the estrogen-regulated vitellogenin genes A1, A2, B1 and B2 from Xenopus laevis and chicken and the chicken apo-VLDLII gene (36). Four short blocks of sequence homology were identified at equivalent positions in the vitellogenin genes of both Xenopus and chicken. A short sequence with 2-fold rotational symmetry, i.e. the perfect palindrome: 5′-GGTCAnnnTGACC-3′ (n, any nucleotide), located at similar positions upstream of the five vitellogenin genes was also present as two copies close to the 5′ end of the chicken apo-VLDLII gene (36). The derived minimal consensus ERE sequence is a 13 bp palindromic inverted repeat (IR): 5′-GGTCAnnnTGACC-3′ (37), and differs in only 2 bp in each half-site from the GRE (38). This ERE sequence was shown to act on a heterologous promoter in an orientation- and distance-independent manner, thus fitting the definition of an enhancer element, as understood at that time (37). Extension of the length of the ERE palindrome by an additional nucleotide in each arm of the IR, e.g. 5′-AGGTCAnnnTGACCT-3′, forming a 15 bp palindromic IR, and the sequence of the nucleotides immediately flanking the ERE are important in determining the affinity with which ERα binds the ERE (21,39–46).
Table 1.
| Name | Sequence | ERα binding
(Kd in nM) | ERβ binding
(Kd in nM) | Activation by 10 nM E2 (unless otherwise
indicated) in the given cell type |
| Xenopus vitellogenin A2 (vitERE) | 5′-GTCAGGTCACAGTGACCTGATCAAAGTTAATGTAACCTCA-3′ (19 bp ERE) | 0.2 (136); 0.39 (137); 0.31 (138); 1.2 (139); 2 (140); 1.8 (141); 1.0 (142); 2 (143); 0.8–1 (74); 10 (74); DBD alone, 1 (51) | 8 (143); ERβ binds but Kd ND (144) | 25-fold in MCF-7 (28)9.3-fold in MCF-7 transfected with ERα (136)16.7-fold in T-47D (145)5.2- and 5-fold in COS-1; 6- and 3-fold in HeLa transfected with ERα and ERβ, respectively (143)6.3-fold in CHO (142)5-fold in HeLa with 1 nM E2 (146)19-fold in HepG2 expressing ERα with 100 nM E2 (104)1.4-fold in rat calvarial osteoblast cells with 100 nM E2 (145)2.5-, 3.3- and 5-fold with ERα and 3.9, 2.6 and 3.6-fold with ERβ in CEF, HeLa and COS-1, respectively (143)30-fold in HeLa transfected with HEO ERα vector (147)17.8-fold in MCF-7 (148)4.8-fold in MCF-7 (22) |
| Chicken vitellogenin A2 | 5′-GTCCAAAGTCAGGTCACAGTGACCTGATCAAAGTT-3′ (19 bp ERE) | Ka = 80–100 µM (149) | 5-fold induction with 100 nM E2 in P19 EC (150)6-fold in HepG2 transfected with ERα with 1 µM moxestrol (151)12-fold in T-47D (152) | |
| Xenopus vitellogenin B1 | 5′-GATCTGAGTAAGTCACTGTGACCCAACCCAAGTTATGATGACC-3′ | ERα binds with ~4.3-fold lower affinity than EREc (153) | 14.3-fold in MCF-7 with 200 nM E2 (154)3-fold induction with Xenopus ERα in Xenopus fibroblast cells (153) | |
| Xenopus vitellogenin B1 (ERE2) | 5′-GATCTGAGTAAGTCACTGTGACCTGTAAT-3′ (15 bp imperfect ERE) | 28.46 (142); DBD alone 10 (51) | 2-fold in CHO (142)No induction in Xenopus fibroblast cells (153)1.5-fold induction (102) | |
| Xenopus estrogen receptor | +480: 5′-GGTCAnnnTGACG-3′ | 10× less binding versus consensus ERE (155) | 4–5-fold (155) | |
| Chicken apo very low density lipoprotein II (apoVLDL II) | –221: 5′-GGGCTCAGTGACC-3′; then 44 bp and –178: 5′-GGTCAGACTGACC (ERE1) | ERα binds quantitatively differently to each ERE (156) | ||
| Chicken ovalbumin | –47/–43: 5′-TGGGTCA-3′ which is half ERE and an AP-1 binding site (157) | ND (158); ERα binds half-sites as a dimer with 50–100-fold lower affinity than consensus ERE (119) | ||
| Human angiotensinogen | 63-CCTGGGAACAGCTCCATCCCCACCCCTCAGCTATAAATAGGGCATCGTGACCCGGCCAGGGGA-1 | Two-tandem copies increased reporter expression (159) | ||
| Human bcl-2 | Two functional EREs: ERE-E-3 +195: 5′-GGTCGCCAGGACC-3′; ERE-E-4 + 276: 5′-GGTCCCCATGACC-3′ | ND (160) | Each gives 2.5-fold induction in MCF-7Together E-3 and E-4 give a 4-fold induction (160) | |
| Human BRCA1 | +2023: 5′-TGGTCAGGCTGGTCTGGAACTCCTGACCTG -3′ | ND | ND | 10 nM E2 induces 1.5-fold increase in MCF-7 (105) |
| Human calbindin-D9k | 5′-GATCCAGGTTAGTGTGATTTG-3′ | No binding (161) | ||
| Human cathepsin D | (–270 to –249) 5′-GGGCCGGGCTGACCCCGCGGG-3′ (called the E2 site) | 3 (136) 136 nM at 200 mM KCl (108) | Lower apparent affinity of ERβ versus ERα (144) | 0.6-fold in MCF-7 transfected with ERα (136) |
| Human choline acetyltransferase | 5′-GATCCAGGAGGCCACGATGACATGCTC-3′ | ND (144) | Lower apparent affinity of ERβ versus ERα (144) | |
| Human complement C3 | –236: 5′-GTGTTCACCAGGTGGCCCTGACCCTGGGAG-AGTCCA-3′; +25: 5′-TGTCCCTCTGTCCCTCTGACCCTGCACTGTCC-CAGCAACCATG(start)-3′; for EMSA: 5′-CACCAGGTGGCCCTGACC-3′ (162) | ERα bound, but not all supershifted with ERα antibody, Kd ND (162) | 5.6-fold in HepG2, 4-fold in HeLa and 10-fold in T47D transfected with ERα (162)2-fold in MCF-7 (22)The –240 ERE is functional, but the +33 ERE is not (162) | |
| Human cytochrome c oxidase subunit VIIa-related protein (COX7RP) | + 443 (intron): 5′-TCACTGCAGGGGTCAAGGTGACCCCCGGGGTCA-3′ | ERα binding identical to vitERE (163) | 6-fold induction with 100 nM E2 in MCF-7 (163) | |
| Human ERβ | –1510: 5′-TGGTCAGGCTGGTC(N9)TGACC-3′ | ND (106) | ND (106) | |
| Human estrogen responsive finger protein (efp) | 3′ non-coding region: 5′-TTCAGGGTCATGGTGACCCTGAT-3′ | ND (164–166) | ||
| Human Ha-ras Exon1 | +1713: 5′-GCGCTGACCATCCAGCTGATCCAGAACC-3′ | ND (167) | 3.7-fold increase in MCF-7 (167) | |
| Human hepatic α2u globulin | –606: 5′-GATCCAAAAGAGGGTCATTTCCTGTGACTGGAG-3′ | ND (168) | Negatively regulated by E2 (168–171) | |
| Human lactoferrin | –374: 5′-AAGAAGATAGCAGGTCAAGGCGATCTGTAAAGACCCTCTGCTCT-3′ | 6.5-fold in RL-95-2 cells (172)7-fold in HEC-1B transfected with ERα (57) | ||
| Human progesterone receptor (hPR) | Form B is initiated at +744: +540: 5′-ATGGAGGCCAAGGGCAGGAGCTGACCAGCGCCGCCCT-3′ Form A is initiated at + 1236: +1148: 5′-TCCTGCGAGGTCACCAGCTCTTGGT-3′ (173) | ‘Weak but detectable’ (174) | Induction equal to vitERE in COS-7 transfected with ERα (164) | |
| +565: AGGAGCTGACCAGCGCCGCCCTCCCCCGCCCCCGACC-3′ | Foot-prints (175) | 1.7-fold in CHO transfected with ERα (175) | ||
| Human quinone reductase | –476: 5′-AATTAAATCGCAGTCACAGTGACTCAGCAGAATCTGAGCCTAGG-3′ | ND (176) | ERβ binds, Kd ND (176) | E2 does not induce4-OHT activates 2- and 4-fold induction in HEC-1 transfected with ERα and ERβ, respectively (176) |
| Human pS2 | 5′-CTTCCCCCTGCAAGGTCAGCGTGGCCACCCCGTGAGCCACT-3′ | 0.40 (E2) and 1.14 (no ligand) (138); 22.1 (142) | ERβ binds but Kd ND (144) | 4.5-fold in HeLa transfected with HEO ERα vector (147)2.5-fold in CHO (142) |
| Human VEGF | –1560: 5′-AATCAGACTGACTGGCCTCAGAGCC-3′ | ND (177) | Two tandem copies give a 4.2-fold increase with 100 nM E2 in Ishikawa cells transfected with ERα (177) | |
| Human genome Alu ERE | 5′-TGGTCAGGCTGGTCTCAAACTCCTGACCTCGTGATCTCA-3′ | 100 nM E2 activates 8-fold induction in HepG2 (178) | ||
| Rat calbindin-D9k | 5′-GATCCAGGTCAGGGTGATCTG-3′ | ND (161,179) | ||
| Rat creatine kinase B | –569: 5′-GGGAAGGTCAGAACACCCTGGGTGCTTCCCC-3′ | ND (180) | 7-fold in HeLa (180)8-fold induction in ECC-1 (181) | |
| Rat hsp70-related gene | 5′-GGTCACTCCGACC-3′ | Not estrogen responsive (182,183) | ||
| Rat luteinizing hormone B | 5′-TCACATGGACACCATCTGTCCCGATCGGCTCCAAGGTTACATTGACCAC-3′ | ND (184) | 2.5-fold in GH3 cells (184) | |
| Rat c-jun | +1021 (exon): 5′-CTGAAGCAGAGCATGACCTTGAACTGAAGCAGAGCATGACCTTGAA-3′ | 10–20 (148) | ERβ binds; Kd ND (144) | 2.7-fold in H301 cells (148) |
| Rat c-jun (JUN5) | 5′-GATCCTGAAGCAGAGCATGACCTTGAA-3′ | No direct binding, but competed for ERα–vitERE binding (185) | 4-fold induction in a yeast reporter assay (185) | |
| Rat oxytocin | (–115 to –85) 5′-AGTGTGGAACAGTTTGACCCAAGAGACCTGCTGTGACCA-3′ C-3′ (imperfect 13 bp ERE)(–147 to –177): 5′-GATCCAGGCGGTGACCTTGACCCCAGC-3′ | 8-fold with 100 nM E2 in P19 EC cells (150) | ||
| Rat prolactin | –1713: 5′-TCCAGGTCACCAGCTGCTTCAGATGATC-3′ | 70 (188) | No effect of E2 (189) | |
| –1573: 5′-GATCCTTGTCACTATGTCCTAGAGTGGATC-3′ (186,187) | 602 (188) | |||
| –1547: 5′-AGCTATAGATCATGAGGTCATAACGATTTATG-3′ | ND (59,188) | |||
| –1786: 5′-AGCTAGAACCAGGTCATCTGTCAGTCCAAATG-3′ –1573: 5′-AGCTGCTTTGGGGTCAGAAGAGGCAGGCAGAG-3′ | ||||
| Rat vasopressin | –4324: 5′-TGCTTCTGCAGGGCCAGCCTGACCGTGTGT-3′ | ND (190) | ND (190) | E2–ERα induces 1.6-fold; E2–ERβ induces 1.3-fold500 nM 4-OHT- ERα induces 2.9-fold; 500 nM ICI 182,780-ERα induces 3.4-fold (190) |
| Rat VEGF5′ | VEGF5′ between TATA box and +1: +: 5′-GATCGACAGGGCAAAGTGACTGACCT-3′ | ERα < ERβ binding (191) | Two tandem copies in the forward orientation inhibit ERα activity by >50%, but show a 2-fold induction if cloned in reverse orientation. Two tandem copies in the forward orientation are inactive with ERβ, but show 2-fold activation with ERβ if cloned in reverse orientation; however ICI 182,780 doesn’t block E2–ERβ activity. This is the first report of an orientation-dependent effect on ERα transcription (191). | |
| Rat VEGF3′ | In 3′UTR: 5′-GATCTGCAAGAGCACCCTGCCCTCTGG-3′ | Binding of ERα and ERβ is approximately equal (191) | Two tandem copies give 3- and 1.5-fold increase with ERα and ERβ, respectively, in transfected HeLa (191) | |
| Mouse c-fos | –278: 5′-GCGGAAGGTCTAGGAGACCCCCTAG-3′ | ND | ERβ binds; Kd ND (144) | 2–5-fold (192) |
| 3′ to gene: 5′-TTTATCCAGGTCACCACAGCCCAGGCCATG-3′ | 1–10 (148) | 4.5-fold (148) | ||
| Mouse oviduct-specific glyoprotein | –115: 5′-GTCAGCGGTCATTGTGATCTTGAATCATTGTTTCT-3′ | ND (193) | 2.5-fold in MCF-7 cells treated with 100 nM E2 (193) | |
| Rabbit uteroglobin | –275: 5′-GCAGGTGGCCAGGTCACCATGCCCTCGGGGGGCAGGCACC-3′ | ND (194); 3–4-fold < vitERE (195) | 7-fold in Ishikawa (195)Role for Sp1 (7) | |
| Guinea pig estrogen sulfotransferase gene 2 | –2442: 5′-AGGTCATCCAACCA-3′ –982: 5′-AGGTCATGTTGTTC-3′ | ND (196) |
The species and gene name are indicated. The underlined nucleotides constitute the consensus ERE half-site sequence and nucleotides in bold type are altered from the consensus ERE palindrome.
ND, not determined.
Specific contacts between the ER dimer and the sugar–phosphate backbone of the ERE are important in sequence recognition and high affinity binding (47). Each ER monomer is bound to DNA in the major groove with the ER dimer located predominantly on one face of the DNA helix (47). Three specific amino acids within the ‘P box’ of zinc finger CI interact in the major groove in a sequence-specific manner (48). The fourth base pair of the ERE half site (AGGTCA) provides a positive contact for the P-box, whereas the third base pair (AGGTCA) provides binding energy (49–51). The CII zinc finger is involved in half-site-ERE spacing recognition and ER dimerization (52). Phosphate methylation interference assays showed that ERα forms the strongest interaction with the underlined nucleotides: 5′-GGTCAGCGTGACC-3′ (47) whereas ethylation and thymine interference assays indicate ERα contacts the underlined nucleotides in the chicken vitellogenin II ERE: 5′-CTGGTCACGCTGACCGG-3′ (53). Thus, the technique used to analyze ER–DNA contact gives differences in nucleotide recognition by ERα.
There has been controversy over whether ER can bind to an ERE half-site as a monomer. We reported that ERα binds EREs with a stoichiometry of two molecules of E2-bound ERα per ERE, indicating that ERα binds EREs as a homodimer (39–41,54,55). Thus, the stoichiometry of ER–ERE binding is 2:1. However, another group reported that 1 mol of ERα is bound to 1 mol of ERE, rather than the expected stoichiometry of 2 ER/ERE as would be predicted if ERα binds an ERE as a homodimer (56). The authors postulated that active ER is a monomer or heterodimer, but not a homodimer (56). However, recent studies of ERα interaction with the lactoferrin promoter which contains an SF-1 response element (SFRE) 26 bp upstream of an imperfect ERE (sequence in Table Table1)1) indicate that one ERα dimer binds the SFRE (57). The authors postulated that one ERα monomer binds the core element and the other monomer anchors on the surrounding sequence for stabilization (57). Similarly, ERα bound as a homodimer to an SFRE (58), to ERE half-site regions within the rat prolactin gene promoter (59), and to the imperfect ERE in the pS2 gene (60).
ROLE OF PHOSPHORYLATION IN ER–ERE BINDING
All the steroid receptors, including ERα (61), are phosphorylated after binding their respective ligands (reviewed in 62). In addition, ERα and ERβ can be phosphorylated and activated in the absence of ligand binding (63–68). Phosphorylation of ERα increases ERα–ERE binding in vitro (61,62,69), although the effects of phosphorylation on the affinity of ER–ERE binding have not been determined.
EFFECT OF HIGH MOBILITY GROUP (HMG) PROTEINS 1 AND 2 ON ER–DNA BINDING
HMG domain proteins are architectural proteins involved in chromatin function (70). HMG-1 and HMG-2 have been shown to stabilize ERα–ERE binding by decreasing the rate of ERα–ERE dissociation (71–74). HMG-1 increased the affinity of baculovirus-expressed recombinant human (rh) ERα binding from 10 to 0.25 nM as detected by electrophoretic mobility shift assay (EMSA) (74). HMG-1 also facilitates the binding of PR to PREs (75). HMG-1 and HMG-2 are thought to facilitate ER–ERE binding by inducing structural changes in the target DNA that enhance ER–ERE binding. HMG-1 also enhanced transcriptional activation by ERα in transfected HeLa cells and enhanced the agonist activity of 4-OHT in MDA-MB-231 cells transfected with rhERα (74). Together these results indicate that HMG-1 and HMG-2 play roles in stabilizing ER–ERE binding and in transcriptional activation, perhaps through mediating assembly of nucleoprotein complexes (76) and chromatin decondensation (reviewed in 77).
EFFECT OF LIGAND ON ER–ERE BINDING
The reported effect of ligand on ER–ERE binding affinity varies depending on the source and purity of ER and the method used to quantitate binding affinity. Ligand binding is required for maximal ERα–ERE binding in vivo, but not in vitro (78). However, ligand stabilizes ER–ERE binding (79). Additionally, although unliganded ER binds EREs in vitro, ligand binding affects the migration of the ER–ERE complex in EMSA experiments, indicating a role for ligand in altering ER conformation, as anticipated from crystal structure studies (80–83).
Recent anisotropic measurements using purified, baculovirus-expressed recombinant human ERα and a 35 bp ERE oligomer (called F-ERE in Table S1) showed no effect of ligand, i.e. unoccupied or occupied with E2, ICI 182,780 or 4-OHT, on ERα–ERE interaction in a buffer containing 200 mM KCl. These results, with those of other investigators, indicate that the effect of ligand on ER transactivation occurs at a step distal to ERE binding, e.g. promoting or inhibiting coactivator recruitment (reviewed in 6,84).
EFFECT OF NATURAL VARIATIONS IN ERE SEQUENCE ON ER BINDING AND TRANSCRIPTIONAL ACTIVATION
Most estrogen-regulated genes contain imperfect, non-palindromic EREs (21,45). Table Table11 lists examples of 38 estrogen-responsive genes whose promoters or 3′UTRs contain functional EREs. This list also reports the affinity with which ERα and ERβ interact with these EREs and the fold-induction of E2-stimulated reporter gene activity. These summary data indicate that ERα binds the Xenopus vitellogenin A2 ERE with higher affinity than ERβ and that the ERα–ERβ heterodimer binds with an affinity similar to that of ERα rather than ERβ. Overall, ERα binds the Xenopus vitellogenin A2 ERE with higher affinity than any other natural ERE. Further, the data indicate that the more nucleotide changes there are from the consensus within a half-site of the ERE palindrome, the lower the ERα binding affinity and the lower the transcriptional activity. We conclude that EREs in which nucleotides are altered in each arm of the palindrome show lower transcriptional activity than those containing alterations in only one half of the ERE palindrome. Experiments using synthetic and natural EREs confirm this conclusion (21,41,85–88). Additionally, these data indicate that the amount of transcriptional activation detected from the same ERE varies between cell types, indicating that cell-specific factors, e.g. the type and amount of coactivators, regulate ER transcriptional activation. In general, ERα shows higher transcriptional activity than ERβ (89).
One of the most widely studied estrogen-responsive genes is the PR and measurements of PR are used as a prognostic indicator in breast tumor samples. While long thought to be a primary estrogen-response gene, recent experiments suggest that PR may be indirectly activated by ER (90). Evidence for this suggestion comes from the observation that in Rat1 cells stably transfected with human ERα containing a point mutation (Gly400 to Val400) (91), the time course of PR gene transcription did not parallel E2 binding to ERα (90). Additionally, ERα levels were decreased to 15% by 3 h and undetectable by 24 h, although PR gene transcription rate gradually increased over the 24 h of E2 treatment (90). Recent in vivo DNase I footprinting experiments indicate that ERα interacts with an ERE half-site located 4 bp 5′ to the first of two adjacent Sp1 binding sites in the promoter for PR-A (sequence in Table Table1),1), and that ERα increases Sp-1–DNA binding (92).
Flanking sequences impact ER–ERE binding (40,41,43–46,93) and transcriptional activation in vivo (24,25,85,86,94–96). A survey of genes whose transcription is highly upregulated by E2, e.g. the vitellogenins (Xenopus and chicken) and chicken apo-VLDLII, revealed that these genes contain an ERE in which the region flanking the ERE, but not overlapping the ERE, is AT-rich (44). For example, the most commonly used ERE palindrome from the Xenopus vitellogenin A2 gene has a 19 bp perfectly palindromic ERE and 14 of the next 20 nt immediately 5′ flanking the ERE are either A or T (70% AT-rich) (36). While the mechanisms by which AT-rich DNA enhances transcriptional activity are unknown, the presence of AT-rich DNA flanking the ERE enhances ERα binding affinity (24,39–41,43–46,55,85,95,97). One possible mechanism by which AT-rich DNA may affect ER activity is by altering DNA conformation. Regions of DNA enriched for AT nucleotides are more easily deformed compared to random DNA (98). Moreover, ERα binding to an ERE results in a bend of the DNA toward the major groove (99,100) and AT-rich regions would enhance deformation. DNA bending is thought to facilitate interactions between components of the transcription complex bound to different sites (101).
EFFECT OF SYNTHETIC MUTATIONS IN ERE SEQUENCE ON ER BINDING AND TRANSCRIPTIONAL ACTIVATION
Early studies showed that mutations in each arm of the ERE palindrome decreased the efficiency of E2-dependent synergy between imperfect EREs (102). Screening of large libraries of degenerate oligonucleotides in a yeast-based screen was used to identify ERα-responsive sequences (103,104). Sequencing revealed that the majority of the identified sequences contained at least a 4/5 nucleotide match to a palindromic ERE half-site. Some contained half-sites arranged as direct repeats (DR) while some contained an ERE half-site plus an AT-rich sequence (104). A consensus septamer: 5′-GGTCAMV-3′, where M is A or C and V is not T, was identified. Yeast-based screening of genomic DNA from MCF-7 cells identified a novel ERE that is a variant Alu sequence containing an imperfect ERE palindrome plus a perfect 3′ERE half-site located 9 bp 3′ to the 3′ERE half-site in the ERE palindrome (see Alu ERE in Table S1) (103,104). Similar Alu ERE variants have been identified in the human BRCA1 gene (105) and ERβ gene promoter (106). The affinity of ERα binding to the yeast-screen identified EREs was not determined. Select EREs identified in the yeast screen were cloned into a luciferase reporter as 1, 2, 3, 4, 5 or 6 tandem copies. Whereas one copy of the Xenopus vitellogenin ERE gave a 29-fold induction in luciferase activity in response to 100 nM E2, the synthetic EREs resulted in 2.2–13-fold induction, indicating lower ERα binding and transactivation (104). These results were the first hint that ER–ERE binding does not always result in a corresponding level of transcriptional activation.
Over the past 13 years we have investigated the effect of altering individual nucleotides within each arm or within both arms of the ERE palindrome on ERα binding affinity by gel filtration chromatography (39,107), a microtiter plate assay in which the ERα–ERE reaction was captured by histones fixed to the wells (40–42,44–46,54), DNase I footprinting (97) and EMSA (21,24,25). We also evaluated the effect of insertion or deletion of nucleotides from the 3 bp spacer. Table S1 shows the ERE sequences and results from these experiments. In summary, our data show that ERα does not bind to ERE half-sites in which the palindrome is separated by 2, 4 or 5 bp. We have demonstrated that the length of the ERE palindrome is critical for high affinity ER–ERE binding. We observed that there is a 10-fold higher Kd for ERα binding to EREc13 versus EREc15 (Table (Table1)1) (P.C.Kulakosky, S.C.Jernigan, M.A.McCarty and C.M.Klinge, manuscript submitted), indicating that the minimal ERE should be considered to be EREc15 and not EREc13 as earlier reported (37). In contrast to our expectations, further extension of the ERE palindrome by either 1 or 2 bp, generating EREc17 and EREc19 (Table S1), did not further increase affinity for either ERα or ERβ. It is noteworthy that the Xenopus vitellogenin A2 ERE palindrome is 19 bp in length (Table (Table1).1). Our data indicate that the ERE sequence providing the highest affinity for E2–ERα binding is 5′-C(A/G)GGTCAnnnTGACC(T/C)G-3′ (21; P.C.Kulakosky, S.C.Jernigan, M.A.McCarty and C.M.Klinge, manuscript submitted). These data are in agreement with data demonstrating the importance of the equivalent –7/+7 position in the GRE for dimeric GR binding (17) and in the PR response element (PRE) for PR binding (19). Other experiments demonstrated that the nucleotide composition of the 3 bp spacer as well as the –7 position in the GRE, PRE and AR response element (ARE) differentially impact the affinity of GR, PR and AR binding, thus yielding receptor-selective binding sites (20).
Recently, other investigators have employed fluorescence anisotropy to examine effects of variations in ERE sequence on ERα–ERE binding kinetics (108). The synthetic ERE variants used in these studies contained two symmetric nucleotide changes in each arm of the ERE palindrome (see F-ERE mut sequences A–F in Table S1). The authors concluded that each of the base pairs in the palindromic ERE contributes significantly to ERα binding affinity (108).
ER BINDING TO AN ERE HALF-SITE
While in theory one might anticipate that ER could bind to a single ERE half-site as a monomer, this probably does not occur in vivo because ERα readily forms stable dimers (109–113). Using EMSA, a microtiter plate ER–ERE binding assay and gel affinity chromatography we did not detect ERα binding to a single half-site ERE (41,44–46,93,97). Similarly, others have not observed ERα binding to a single ERE half-site (114) nor did ERα footprint a single ERE half-site in the rainbow trout ER gene promoter (115). Recent studies using baculovirus-expressed recombinant mouse ERα showed that one ERα dimer binds to two half-site oligomers in EMSA with an affinity at least 20-fold lower than ER–ERE binding (59). Other studies suggest that ERα binds to a single ERE half-site closely spaced with Sp1 binding sites in the presence of Sp1-DNA binding in the promoters of certain estrogen-regulated genes, e.g. hsp27 (116), TGFalpha (10), vitellogenin A1 io promoter (117) and PR (92). In conclusion, the data indicate that neither an ERα monomer nor dimeric ERα alone bind a single ERE half-site, but that dimeric ERα can bind an ERE half-site when stabilized by protein–protein interactions with Sp1 bound to its GC-rich response element nearby in the promoter.
ER BINDING TO DIRECT REPEAT (DR), INDIRECT REPEAT AND EVERTED REPEAT (EVR) SEQUENCES
DNA binding experiments have demonstrated that ERα binds DR of the ERE half-site 5′-AGGTCA-3′, as well as ERE palindromes (118–120). A study of 5′-AGGTCA-3′ DR spacing, i.e. DR1 (where 1 refers to the number of nucleotides separating half sites), DR2, DR3, DR4, DR5, DR10, DR15, DR20, DR25, DR35, DR50, DR100, DR150 and DR 200 showed that E2 stimulated transcription from all constructs in which the DR were separated by >10 bp in transiently transfected COS-1 cells (120). Although not noted by the authors, the spacer of constructs DR15 and greater contained an imperfect half-site 5′-CGGTCT-3′, the significance of which is unknown. At best, E2-induced transcription ~6-fold from DR15 and DR20 compared to 19-fold from a perfectly palindromic ERE (120). DR separated by 35, 50, 100, 150 or 200 bp showed decreased E2-induced transcription (120). Another study reported that ERα bound specifically to DR6, but 8–15-fold less retarded ER–DNA complex was formed on DR6 than on the ERE palindrome (121). In competition binding experiments, DR6 and a single ERE half-site competed for ERα ~6–10-fold less efficiently than the 13 bp palindromic ERE. A more recent study showed that neither ERα nor ERβ bound to DR1 or DR4, irrespective of the presence or absence of RXR (122). Thus, specific rules defining ER–DR binding, the affinity of such interaction, and the functional consequences of ER–DR binding, i.e. transcriptional responsiveness, remain to be clarified.
To that end, we recently determined the affinity of ERα and ERβ binding to synthetic DR5, DR11, DR16, DR21 and a DR16 construct in which the spacer region was AT-rich (called DR16AT) (88). ERβ consistently bound DRs with a higher affinity than ERα. Using the parameters of spacer length and the ratio of the length of the longest continuous AT-rich region within the spacer to the spacer length, we defined an equation by which the affinity of ERα (equation 1) and ERβ (equation 2) binding to DRs can be estimated:
LN (Kd) = [(0.55 × BPsubst) – (1.82 × HS) +3.11] ± 1.29 1
LN (Kd) = [(0.50 × BPsubst) – (1.48 × HS) +3.41] ± 1.17 2
where LN (Kd) is the natural logarithm of Kd, 1.29 and 1.17 are the standard errors of the predicted LN (Kd), HS is the number of half EREs (where half ERE is 5′-AGGTCA-3′); and BPsubst is the number of (AT)→(GC) substitutions in the ERE sequence (88). The number of half EREs and the number of (AT)→(GC) base pair substitutions within the 15 bp candidate ERE sequence are statistically independent predictors of the affinity of ER–ERE interaction as described in these two equations (88).
When the ERα DBD is expressed as a single molecule in which the two DBD monomers are joined by a peptide linker, the linker dimerized-ERα DBD bound to an EVR separated by 15 bp, i.e. 5′-n11-TGACCT-n15-AGGTCA-n11-3′ with a Kd of 100 nM, the consensus ERE with a Kd of 38 nM, and the pS2 imperfect ERE with a Kd of 110 nM (123). However, the linker dimerized ERα did not bind to a DR15 sequence, i.e. 5′-n11-AGGTCA-n15-AGGTCA-n11-3′ (123). These data indicate that the orientation of the half-sites determines the binding of the linker dimerized-ERα.
In contrast to these reports showing ER binding to EVR and DR sequences, ERα did not bind IR sequences, regardless of the number of base pairs separating the half-site, other than IR3 that is the same as a palindromic, consensus ERE (121). Similarly, ERα did not bind to IR5, even in the presence of 3′ flanking AT-rich nucleotides that increase ERα–ERE binding (39).
EFFECT OF MULTIPLE TANDEM ERES ON ER BINDING AND TRANSCRIPTIONAL ACTIVATION
Early studies showed synergism, i.e. more than additive induction of reporter gene expression, for ERα bound to closely adjacent EREs and that the distance between the response elements was important in determining the amount of reporter gene induction (124). Transcriptional synergism from multiple EREs has been reported for ERα (24,94,95,125,126). For both ERα and ERβ, we detected synergistic activation of reporter gene transcription from three tandem copies of EREc38 (sequence in Table S1), but not two copies of EREc38 (24,94). Synergy was independent of the distance of these EREs from the TATA box. These data correspond with the cooperative binding and higher affinity binding ERα to three or four tandem copies of EREc38 versus one or two tandem copies of EREc38 (24,39,41,55). Although the exact mechanism for ERα cooperative binding and transcriptional synergism is unknown, both the LBD and A/B domains are required (127). AF-1 is not required for transcriptional synergy from three or four tandem copies of EREc38, since both ERα and ERβ have similar fold-synergy, even though the absolute transcriptional activation by ERβ is lower than ERα (24).
Synergism also occurs for natural genes containing two EREs. The Xenopus vitellogenin B1 and B2 genes each contain two EREs, called the B1 estrogen responsive unit (ERU), that have low estrogen responsiveness alone, but act synergistically to achieve high estrogen inducibility (128). Analysis of ERα binding to the B1 ERU revealed cooperative interaction of ERα dimers with the two adjacent imperfect EREs which most likely explains the synergistic stimulation observed in vivo (129). ERα bound cooperatively to the vitellogenin B1 ERE (52), substantiating a role for cooperative ERα binding in transcriptional synergy.
The ‘rules’ of ERE spacing and synergistic transcriptional activation by ER are not yet defined because the available data do not indicate a correlation between ERE spacing and transcriptional activation. For example, comparison of the transcriptional activation of reporter gene activity in transiently transfected MCF-7 cells showed that two consensus EREs placed 6 or 19 bp apart were equally active (130). More experiments of this nature are needed to define how spacing between EREs impacts ER binding affinity and transactivation.
Transcriptional synergy from two or four tandem EREs has been reported to be cell-specific, i.e. functional synergism was detected in CHO cells transfected with hERα, but not in XL-10, HepG2 or CTC-2 cells (125). This indicates a role for cellular factors, perhaps coactivators, in ER synergism at multiple EREs. ERα bound cooperatively to an ERE consisting of two overlapping EREs separated by 5 bp (center-to-center, i.e. ‘overERE’ in Table S1) and synergistically activated reporter gene expression in transiently transfected HepG2 and MCF-7 cells (127). We reported cooperative ERα binding to three or four, but not two tandem copies of a 38 bp consensus ERE, EREc38 in Table S1 (39–41,46,55). More recently we reported that three or four tandem copies of EREc38 synergistically activated reporter gene expression in transfected MCF-7, COS-1 and CHO-K1 cells transfected with ERα or ERβ (22,78,79). Although E2 treatment of CHO-K1 cells resulted in significantly lower induction of luciferase activity by ERβ than by ERα, there was no difference in the fold-synergy induced by ERα or ERβ (24). Synergy depends on the ligand bound to ERα, implicating the LBD as well as the DBD in transcriptional synergy (24,85,94,95). Indeed our observation that ERβ synergistically transactivates gene expression from multiple tandem EREs despite the fact that the N-terminal AF-1 domain of ERβ is non-functional (131), indicates that AF-1 is not involved in functional synergy.
Transcriptional synergy may result from several possible mechanisms. These include cooperative recruitment of a coactivator(s), action at distinct rate-limiting steps in transcription initiation, cooperative ER–DNA binding (132), and/or direct protein–protein interactions between ERα dimers. Also among the possible mechanisms for transcriptional synergism, ER may cause changes in DNA topology that are transmitted to another ER bound nearby. ERα bends DNA (100,133). Thus, one may speculate that the distinct local topologies induced by binding of one ERα dimer have differential allosteric effects on ERα conformation and activity at adjacent sites. There are no reports as to whether ERβ bends DNA. We and others have demonstrated that the stereoalignment of EREs on the DNA helix and their spacing influence synergistic responses to E2 (18,39–41,45,46,94,134). In yeast cells, changes in chromatin structure, protection of the EREs and hypermethylation in the flanking regions demonstrated that DNA binding of the ER per se promotes local changes in chromatin conformation in the absence of induced transcription (78), supporting a role for changes in DNA topology in transcriptional synergy. Further experiments are required to examine these potential mechanisms for transcriptional synergy.
QUANTITATIVE COMPARISON OF ER–ERE BINDING AFFINITY AND TRANSCRIPTIONAL ACTIVATION
Few investigators have examined the relationship of ERα–ERE binding affinity and transcriptional activation. The data in Table Table11 reveal that these parameters have been determined for only seven natural estrogen-responsive genes. Figure Figure11 compares the transcriptional activity and affinity (Kd) of E2–ERα for Xenopus and chicken vitellogenin A1, Xenopus vitellogenin B1 ERE2, human cathepsin D, rat cJun, human pS2 and mouse cFos 3′ERE. There is a good correlation between ERE binding affinity and transcriptional activation for these EREs, especially since, as indicated in Table Table1,1, these data are from various laboratories using different experimental techniques. For ERβ, we measured binding affinity and transcriptional activation for the Xenopus vitellogenin A1, human pS2, human Fos and human PR EREs and observed a correlation between Kd and reporter gene activation with EREs binding ERβ with a Kd < 80 nM (88).
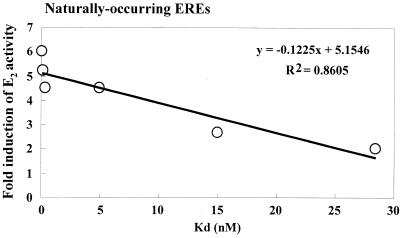
Comparison of ERα binding affinity and transcriptional activation. E2–ERα binding affinity and transcriptional activation from natural EREs: Xenopus and chicken vitellogenin A1, Xenopus vitellogenin B1 ERE2, rat cJun, human pS2 and mouse cFos 3′ERE EREs (data in Table Table1)1) were plotted in Excel and the linear R2 value is indicated.
For synthetic EREs (Table S1), both ERα–ERE binding affinity and transcriptional activation have been determined for seven EREs. Comparison of the transcriptional activity and affinity (Kd) of E2–ERα or E2–ERβ for EREc13, EREc15, EREc19, EREc17,4, EREc17,6, EREc17,11 and EREc38 (sequences in Table S1) indicated no significant correlation between ER–ERE binding affinity and E2-induced transcriptional activation. The limited data available indicate the need for further experiments to clarify the relationship between ER binding affinity and transcriptional activation.
CONCLUSIONS AND PROPOSED GUIDELINES FOR ER–ERE BINDING AND TRANSCRIPTIONAL ACTIVATION
A limitation of our understanding of the effect of ERE sequence on ER binding and transcriptional activation stems from the limitations of the assay methods used, e.g. measurements of ER binding to ‘naked’ DNA and transient transfection in mammalian cells. Since EREs are usually located in gene promoters containing multiple response elements for different transcription factors, the next logical step will be to examine ER interaction and transactivation from different gene promoters.
Table Table22 presents a summary of nucleotide changes that have been studied in the consensus ERE and how these changes affect ER binding and/or transcriptional activation. Positions +2, +3 and +6 are identical for all vertebrate steroid hormone receptors; positions +4 and +5 differ and form the basis for discrimination between a GRE/PRE/ARE and an ERE. In earlier work, we proposed that ERα binding requires that at least 10 of the 12 nt located between 2 and 7 nt from the center of the ERE IR, i.e. from –7 to +7 in Table Table2,2, must be of the consensus sequence (21). Others reported that ERα cannot accommodate a T in position –3 or +3 (49). Others proposed that position +6/–6 is important exclusively for the GRE/PRE family whereas position –3/+3 of an ERE can accommodate a C, T or G in one half-site within the palindrome or even a C in both halves, but a symmetric change to T prohibits ERα binding (49).
Table 2.
| Base Positions | |||||||||||||||||
| 5′ | 3′ | ||||||||||||||||
| C | A | G | G | T | C | A | G | A | G | T | G | A | C | C | T | G | Consensus ERE |
| –8 | –7 | –6 | –5 | –4 | –3 | –2 | –1 | 0 | +1 | +2 | +3 | +4 | +5 | +6 | +7 | +8 | |
| Substitution | | | | | | | | | | | | | | | | | ERα Kd/fold
induction |
| Consensus ERE above | 0.25 nM (21); 0.07 nM (88)/3.7-fold (88) | ||||||||||||||||
| A | T | C | G | A | 6.9 nM (21) | ||||||||||||
| A | T | n | C | n | G | A | No binding (21) | ||||||||||
| A | T | n | n | G | G | A | No binding (21) | ||||||||||
| A | T | n | n | n | G | G | A | No binding (21) | |||||||||
| A | T | n | n | n | n | T | G | A | No binding (21) | ||||||||
| T | n | n | n | G | A | n | Binding (21) | ||||||||||
| T | G | n | n | G | G | A | Binding (21) | ||||||||||
| T | n | 0.54 nM (21) | |||||||||||||||
| T | n | n | n | G | n | T | n | 20 nM (21) | |||||||||
| T | n | n | G | A | n | 20 nM (21) | |||||||||||
| G | n | T | n | n | 20 nM (21) | ||||||||||||
| C | T | C | T | C | C | A | G | No binding (182,183) | |||||||||
| G | n | n | G | n | G | C | n | n | n | n | T | G | ND/3.5-fold in yeast (103) | ||||
| G | G | n | n | n | n | n | G | C | C | G | n | T | n | n | C | C | ND/9.8-fold in yeast (103) |
| A | C | n | n | n | A | n | C | n | n | n | C | G | T | T | C | ND/11.5-fold in yeast (103) | |
| A | n | n | C | C | n | n | n | T | No binding (197) | ||||||||
| G | C | n | n | G | 0.15–0.25 nM (56,198) | ||||||||||||
| A | T | 185 nM (138) | |||||||||||||||
| C | C | C | No binding (199) | ||||||||||||||
| A | G | 1.39 nM (39) | |||||||||||||||
| T | 1.58 nM (39) |
The effect of single or multiple base substitutions on ERα binding affinity and induction of reporter gene activity in transfected cells is indicated. Where no nucleotide or n is indicated, the nucleotide is identical to the consensus ERE. Nucleotides in italics correspond to changes in the center 3 bp spacer.
ND, not determined.
The data in Table Table22 suggest additional guidelines for ER–ERE binding: two nucleotide changes, one in each arm of the palindrome at whatever position, even if the change results in a palindrome, inhibit ERα binding, resulting in reduced ER binding affinity. Further, one or two nucleotide changes in one half-site decrease ERα binding affinity even in the presence of a perfect ERE half-site in the imperfect palindrome.
Review of data from our own laboratory and those in the literature indicate that ERα binding affinity does not always relate linearly with E2-induced transcriptional activation. While we detected a correlation between ER binding affinity and fold-induction of reporter gene activity with natural EREs (Fig. (Fig.1),1), no correlation was detected for synthetic mutant EREs. We suggest that the reasons for this discord are manifold and include the distance between the response element and the transcription start site (18); cellular amounts and roles for other transcription factors, coactivators and adaptor proteins both in ER binding and transcriptional activation (6); phosphorylation of ER and other proteins involved in transcriptional activation (62,135); and sequence-specific and protein-induced alterations in chromatin architecture (78). Clearly, additional experiments are needed to fully dissect the molecular mechanisms by which the transcriptional apparatus mitigates sequence-specific differences in ER–ERE binding affinities. In that regard, we speculate that different sets of coactivator proteins may be recruited to the unique ERE-containing enhancesome sequences in estrogen-regulated genes.
SUPPLEMENTARY MATERIAL
Table S1 is available as Supplementary Material at NAR Online.
References
Articles from Nucleic Acids Research are provided here courtesy of Oxford University Press
Full text links
Read article at publisher's site: https://doi.org/10.1093/nar/29.14.2905
Read article for free, from open access legal sources, via Unpaywall:
https://academic.oup.com/nar/article-pdf/29/14/2905/9905958/292905.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1093/nar/29.14.2905
Article citations
Molecular, Histological, and Functional Changes in Acta1-MCM;FLExDUX4/+ Mice.
Int J Mol Sci, 25(21):11377, 23 Oct 2024
Cited by: 0 articles | PMID: 39518930 | PMCID: PMC11545788
miR-10a/b-5p-NCOR2 Regulates Insulin-Resistant Diabetes in Female Mice.
Int J Mol Sci, 25(18):10147, 21 Sep 2024
Cited by: 0 articles | PMID: 39337631 | PMCID: PMC11432729
The Dual Faces of Oestrogen: The Impact of Exogenous Oestrogen on the Physiological and Pathophysiological Functions of Tissues and Organs.
Int J Mol Sci, 25(15):8167, 26 Jul 2024
Cited by: 0 articles | PMID: 39125736 | PMCID: PMC11311417
Review Free full text in Europe PMC
The Influence of Sex Steroid Hormone Fluctuations on Capsaicin-Induced Pain and TRPV1 Expression.
Int J Mol Sci, 25(15):8040, 24 Jul 2024
Cited by: 0 articles | PMID: 39125611 | PMCID: PMC11312332
Unraveling the Dynamics of Estrogen and Progesterone Signaling in the Endometrium: An Overview.
Cells, 13(15):1236, 23 Jul 2024
Cited by: 1 article | PMID: 39120268 | PMCID: PMC11312103
Review Free full text in Europe PMC
Go to all (562) article citations
Other citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Estrogen response element-dependent regulation of transcriptional activation of estrogen receptors alpha and beta by coactivators and corepressors.
J Mol Endocrinol, 33(2):387-410, 01 Oct 2004
Cited by: 107 articles | PMID: 15525597
Estrogen receptor beta isoforms exhibit differences in ligand-activated transcriptional activity in an estrogen response element sequence-dependent manner.
Endocrinology, 145(1):149-160, 18 Sep 2003
Cited by: 30 articles | PMID: 14500565
A mathematical approach to predict the affinity of estrogen receptors alpha and beta binding to DNA.
Mol Cell Endocrinol, 182(1):109-119, 01 Aug 2001
Cited by: 10 articles | PMID: 11500244
Anatomy of the estrogen response element.
Trends Endocrinol Metab, 15(2):73-78, 01 Mar 2004
Cited by: 154 articles | PMID: 15036253
Review





