Abstract
Objective
Polyvinylidene fluoride-trifluoroethylene (PVDF-TrFE), which is a piezoelectric, biocompatible polymer, holds promise as a scaffold in combination with Schwann cells (SCs) for spinal cord repair. Piezoelectric materials can generate electrical activity in response to mechanical deformation, which could potentially stimulate spinal cord axon regeneration. Our goal in this study was to investigate PVDF-TrFE scaffolds consisting of aligned fibers in supporting SC growth and SC-supported neurite extension and myelination in vitro.Approach
Aligned fibers of PVDF-TrFE were fabricated using the electrospinning technique. SCs and dorsal root ganglion (DRG) explants were co-cultured to evaluate SC-supported neurite extension and myelination on PVDF-TrFE scaffolds.Main results
PVDF-TrFE scaffolds supported SC growth and neurite extension, which was further enhanced by coating the scaffolds with Matrigel. SCs were oriented and neurites extended along the length of the aligned fibers. SCs in co-culture with DRGs on PVDF-TrFE scaffolds promoted longer neurite extension as compared to scaffolds without SCs. In addition to promoting neurite extension, SCs also formed myelin around DRG neurites on PVDF-TrFE scaffolds.Significance
This study demonstrated PVDF-TrFE scaffolds containing aligned fibers supported SC-neurite extension and myelination. The combination of SCs and PVDF-TrFE scaffolds may be a promising tissue engineering strategy for spinal cord repair.Free full text

Aligned Fibrous PVDF-TrFE Scaffolds with Schwann Cells Support Neurite Extension and Myelination In Vitro
Associated Data
Abstract
Objective:
Polyvinylidene fluoride-trifluoroethylene (PVDF-TrFE), which is a piezoelectric, biocompatible polymer, holds promise as a scaffold in combination with Schwann cells (SCs) for spinal cord repair. Piezoelectric materials can generate electrical activity in response to mechanical deformation, which could potentially stimulate spinal cord axon regeneration. Our goal in this study was to investigate PVDF-TrFE scaffolds consisting of aligned fibers in supporting SC growth and SC-supported neurite extension and myelination in vitro.
Approach:
Aligned fibers of PVDF-TrFE were fabricated using the electrospinning technique. SCs and Dorsal Root Ganglion (DRG) explants were co-cultured to evaluate SC-supported neurite extension and myelination on PVDF-TrFE scaffolds.
Main Results:
PVDF-TrFE scaffolds supported SC growth and neurite extension, which was further enhanced by coating the scaffolds with Matrigel. SCs were oriented and neurites extended along the length of the aligned fibers. SCs in co-culture with DRGs on PVDF-TrFE scaffolds promoted longer neurite extension as compared to scaffolds without SCs. In addition to promoting neurite extension, SCs also formed myelin around DRG neurites on PVDF-TrFE scaffolds.
Significance:
This study demonstrated PVDF-TrFE scaffolds containing aligned fibers supported SC-neurite extension and myelination. The combination of SCs and PVDF-TrFE scaffolds may be a promising tissue engineering strategy for spinal cord repair.
1. Introduction
In the United States, 285,000 people suffer from spinal cord injury (SCI) and 17,500 new cases occur each year (1). SCI is a devastating condition that can have a significant impact on a person’s quality of life. After SCI, an inhibitory environment develops overtime at the lesion site that results in the failure of the axons to regenerate and in loss of function (2, 3). Many research strategies have been explored for treating SCI in the past decades (4–9). However, there is no effective treatment to date that can repair the damaged spinal cord. Tissue engineering strategies, where biomaterials are utilized in combination with cell transplantation and/or growth factor delivery to repair damaged tissues, may be a viable approach. Various types of biomaterials, including synthetic and natural materials, have been studied for axon regeneration and myelination (10, 11). Polyvinylidene fluoride-trifluoroethylene (PVDF-TrFE) is a synthetic, biocompatible polymer that has shown promise for axon regeneration and myelination (12–14). PVDF-TrFE has been explored in sensor, actuator and tissue engineering applications because of its piezoelectric properties (15–18), where it can generate an electric charge in response to mechanical deformation (19–22). Electrical stimulation (ES) has been shown to promote axon regeneration (23–25) and upregulation of gene expression for BDNF and its receptor TrkC in motor neurons, which is correlated with acceleration of axonal regeneration (25). For DRG neurons, ES also enhances axon regeneration, which is accompanied by the elevation of neuronal cAMP (26). Unlike conventional ES treatment, the use of piezoelectric materials could provide ES without electrodes and an external power source (27). PVDF-TrFE fibers can generate electrical stimulation in response to mechanical compression and flexion (19–21). Such mechanical deformations have also been observed in the spinal cord which were caused by physiological movement, such as changes in posture (28–30). Once transplanted in the spinal cord, electrical stimulation could be potentially activated by spinal cord deformation without using an external power source. In addition, electrical stimulation from piezoelectric materials can be activated by the application of ultrasound and it has been demonstrated to be an effective method for neural stimulation and cell differentiation (31–33).
Among the various cell transplantation strategies, Schwann cell (SC) transplantation therapy is one of the most promising approaches. SCs are myelinating glial cells that can secrete growth factors, including nerve growth factor (NGF), neurotrophin-3 (NT-3), brain derived neurotrophic factor (BDNF), neuregulins, fibroblast growth factor 1 and 2 (FGF-1 and FGF-2), and insulin-like growth factors 1 and 2 (IGF-1 and IGF-2) (34). SCs also deposit extracellular matrix components, including laminin, fibronectin, collagen type IV and V, heparin sulfate proteoglycan, tenascin and entactin, to promote axon regeneration after injury (34). Dedifferentiated SCs proliferate, populate the regenerating axons and eventually re-myelinate or re-ensheathe axons (35). SCs transplanted into the lesion site of an injured spinal cord promote axon regeneration, and can also form myelin sheaths around axons (5, 36, 37). Additional advantages associated with the use of SCs in spinal cord transplantation therapies include that they can be used as an autologous approach, precluding potential issues of an adverse immune response or the use of immune-suppressive treatments associated with cells from allogeneic sources (38–40). After four decades of research, SCs therapy is currently in phase I clinical trials to evaluate the safety of autologous human SC transplantation after subacute and chronic SCI (41,42) (www.clinicaltrials.gov NCT02354625).
In this study, we investigated PVDF-TrFE scaffolds consisting of aligned fibers with SCs for promoting neurite extension and myelination. Studies have shown that SC survival was limited after transplantation (43, 44) and a combination approach utilizing tissue engineering scaffolds holds great promise for improving SC survival and repair of the injured spinal cord because the scaffolds can provide structural support for SC attachment for improved engraftment, guidance for axonal growth and can be used for growth factor delivery (45, 46). In a previous study, PVDF- TrFE scaffolds have been shown to support DRG attachment and promote directional neurite extension due to the aligned fibers (13). We hypothesized that with the addition of SCs on PVDF- TrFE scaffolds, neurite extension could be further enhanced and support SC myelination. Matrigel is a natural extracellular matrix that is in liquid form at low temperature and forms a gel at body temperature. Matrigel contains multiple extracellular matrix components as well as growth factors that can promote cell adhesion and proliferation (47, 48). Matrigel has been routinely used for SC transplantation in preclinical studies (36, 45, 49, 50) and has been shown to improve SC survival (51). In a previous in vivo study, we demonstrated that SCs suspended in Matrigel and then injected into PVDF-TrFE conduits containing aligned fibers promoted greater axon regeneration over random fibers in a rat complete transection SCI model (12). Therefore, aligned fibrous PVDF- TrFE scaffolds were investigated, for the first time, in combination with SCs for supporting neurite extension and myelination in vitro. Scaffolds were investigated with or without Matrigel coating to determine its effect on SC-supported neurite extension.
2. Materials and Methods
2.1. Animals and Culture Media
Sprague-Dawley rats, purchased from Charles River Laboratories International Inc. (Wilmington, MA) and Hilltop Lab Animals Inc. (Scottdale, PA), were housed and cared for at animal facilities located at Rutgers, The State University of New Jersey, in accordance with an animal protocol approved by Rutgers University Institutional Animal Care and Use Committee (IACUC). Female Fischer rats, purchased from Envigo Inc. (Frederick, MD), were housed at Miami Project, and in accordance with an animal protocol approved by University of Miami IACUC. Green Fluorescent Protein (GFP)-SC media (GFPSCM): DMEM (Thermo Fisher Scientific, Inc., Waltham, MA), 10% FBS (GE Healthcare Life Sciences, Marlborough, MA), 0.1% Gentamicin (Thermo Fisher Scientific, Inc.), 20 μg/mL Bovine Pituitary Extract (Alfa Aesar, Tewksbury, MA), 2μΜ Forskolin (Sigma-Aldrich, Inc., St. Louis, MO) and 2.5 nM heregulin (Genentech, Inc., San Francisco, CA). Unlabeled SC media (SCM): DMEM (Coming, Inc., Tewksbury, MA), 10% FBS (Atlas Biological, Inc., Fort Collins, CO), 2mM GlutaMAX™-I (Thermo Fisher Scientific, Inc.). DRG purification media (DRGPM): Neurobasal medium (Thermo Fisher Scientific, Inc.), 2% B-27 (Thermo Fisher Scientific, Inc.), 1% penicillin-streptomycin (Thermo Fisher Scientific, Inc.), 1% D-glucose (Sigma-Aldrich, Inc.), 0.4 mM L-glutamine (Thermo Fisher Scientific, Inc.), 25 ng/ml 7S NGF (Corning, Inc.), 10 mM 5-Fluoro-2’-deoxyuridine (Sigma-Aldrich, Inc.) and 10 mM Uridine (Sigma-Aldrich, Inc.). DRG growth media (DRGGM): Neurobasal medium, 2% B-27, 1% penicillin-streptomycin, 1% D-glucose, 0.4 mM L-glutamine and 25 ng/ml 7S NGF. Standard neuronal media (SNM): Neurobasal medium, 2% B27 supplement, 1% GlutaMAX™-I, 0.08% glucose (Sigma-Aldrich, Inc.), and 50 ng/ml 2.5S NGF (Bio-Rad Laboratories, Inc., Hercules, CA). Co-culture media for neurite extension (CCMNE): DMEM (Thermo Fisher Scientific), 10% FBS, 0.1% Gentamicin and 50 ng/ml 7S NGF. Co-culture media for myelination (CCMM): MEM (Thermo Fisher Scientific, Inc.) supplemented with 10% FBS (Atlas Biological, Inc.), 0.4% glucose and 50 ng/ml 2.5S NGF.
2.2. Cell Preparation
Adult GFP SC preparation: Purified SC cultures were obtained from sural/sciatic nerves of adult female Fischer rats following previously published protocols (52). The resulting cultures were purified to greater than 95% (53). At passage 2 and at approximately 50% confluence, SCs were transduced in GFPSCM overnight with a lentiviral vector encoding enhanced GFP at a multiplicity of infection of 30. The transduction efficiency was greater than 90%. Neonatal SCs: The preparation of purified primary SCs followed the Brockes method (54) and has been described in detail and the resulting cultures were purified to greater than 99% (55). Briefly rat SCs were isolated from postnatal day 2 sciatic nerves, and contaminating fibroblasts removed by feeding the cultures with cytosine-ß-arabinofuranoside hydrochloride (Ara-C, 10 μΜ for 3 days in SCM). The remaining contaminating fibroblasts were then removed by complement-mediated killing. Purified SCs were then expanded in SCM supplemented with forskolin (2 μΜ) and recombinant EGF domain of human neuregulin-1-β1 (rhNrgl-EGFD, 5 ng/ml, R&D Systems, Inc., Minneapolis, MN). Rat SCs were used at the 4th passage in all experiments.
2.3. Scaffold Fabrication
PVDF-TrFE scaffolds were fabricated using the electrospinning technique in a closed box with a ventilation system installed. 20% (w/v) PVDF-TrFE (70/30) (400 kDa., PDI of 2.1, Solvay Solexis, Inc., Thorofare, NJ) in methyl ethyl ketone (MEK; Sigma-Aldrich, Inc.) was loaded into a syringe with an 18-gauge needle. PVDF-TrFE solution was ejected out of the syringe at 3 mL/hour using a syringe pump. A voltage of 25kV was applied to the tip of the needle and aligned fibers were collected on a grounded, fast-rotating drum at 2500 rpm. The distance between the tip of the needle and the collector was kept at 30 cm. The relative humidity in the box was controlled at 40–50%. PVDF-TrFE scaffolds were annealed to enhance the presence of the piezoelectric crystal phase (13) where PVDF-TrFE scaffolds were kept at 135°C for 96 hours followed by quenching in ice water.
2.4. Scaffolds Morphology Characterization
Fiber morphology, diameter, alignment and inter-fiber spacing were characterized using scanning electron microscopy (SEM) (LEO 1530 Gemini, Zeiss, Oberkochen, Germany). Scaffolds were sputter-coated with gold/platinum and viewed using an acceleration voltage of 4 kV and 5–6 mm working distance. Eight images were taken and all measurements (n=80) were performed using ImageJ software (1.50b) as described previously (12, 13). Fiber alignment was calculated using angular differences between fibers and a reference line as described previously (13). Inter-fiber spacing was determined by measuring the longest distance between two parallel/adjacent fibers or between the intersection of two fibers and the opposite fiber. Fiber diameter and alignment were characterized from three different batches of electrospun scaffolds.
2.5. GFP SC Culture on Scaffolds
PVDF-TrFE scaffolds were cut into 7 mm diameter disks and both sides of the disks were sterilized by ultraviolet (UV) irradiation for 15 minutes. The disks were then inserted into 96-well polypropylene plates and were either coated with diluted Matrigel solution (Corning, Inc) in phosphate-buffered saline (PBS, 1:40, ~300 ug/ml) or pre-conditioned in GFPSCM, in order to improve cellular attachment, at 4 °C overnight. Next day, the polypropylene plates with the scaffolds were transferred and kept at 37 °C for 2 hours. Matrigel solution or GFPSCM was aspirated and the scaffolds were rinsed once with PBS before seeding SCs. SCs transduced to express GFP were seeded at 15,000 cells per scaffold in GFPSCM. Cell cultures were kept at 37 °C and the medium was changed every 2–3 days. At day 1, 4 and 7, GFP SC cultures were rinsed twice with PBS and fixed using 4% paraformaldehyde in PBS. Samples were then rinsed once with PBS and stored in PBS at 4°C before imaging. Confocal images were taken using confocal fluorescence microscope at 20X and 60X (C1si, Nikon Instruments, NY).
2.6. GFP SC Growth on Scaffolds
GFP SC number was assessed using the Quant-iT PicoGreen dsDNA assay (Thermo Fisher Scientific, Inc.) at days 1, 4 and 7 in culture. GFP SC cultures were rinsed twice with deionized water and incubated in 0.1% Triton X-100 in 1X Tris-EDTA (TE) buffer for 30 minutes. Lysed cells were detached by scraping the scaffolds with pipette tips and the lysates were transferred to the wells of an opaque-walled 96-well plate. Quant-iT PicoGreen dsDNA assay reagent, diluted in 1X TE buffer (1:200), was added to each well. The fluorescent signal was detected using a plate reader (Flx800, BioTek Instrument, Inc., Winooski, VT) with excitation/emission filter set at 480/520 nm. A cell number standard curve was established by measuring the fluorescence of a series of known cell numbers to determine the cell numbers in the samples. Four samples per group per time point were examined. Experiment was repeated three times.
2.7. Rat Dorsal Root Ganglion and GFP-SC Co-culture on Scaffolds
Dorsal root ganglion (DRG) isolation was performed in accordance with an animal protocol approved by Rutgers University Institutional Animal Care and Use Committee (IACUC). PVDF- TrFE scaffolds were seeded with GFP-SCs at 15,000 cells per scaffold in GFPSCM. After four days, DRGs, isolated from E17 Sprague Dawley rat embryos, were seeded on GFP-SC containing PVDF-TrFE scaffolds. Day 4 was chosen to have adequate SC attachment throughout the scaffold prior to reaching confluency. Co-cultures were kept at 37 °C in an incubator. At day 2 after seeding DRGs, co-cultures were rinsed twice with PBS and fixed using 4% paraformaldehyde in PBS. Fixed samples were washed once with PBS and permeabilized with 0.1% Triton X-100 in blocking solution (10% goat serum in PBS) for 60 minutes. After rinsing once with PBS, polyclonal rabbit anti neurofilament 200 primary antibody (1:1000, Sigma-Aldrich, Inc.) in blocking solution was added to each well. Samples were kept at 4 °C overnight. Next day, samples were rinsed three times with PBS followed by adding goat anti-rabbit Alexa Fluor 594 secondary antibody (1:500, Thermo Fisher Scientific, Inc.) in blocking solution and incubated for 1 hour at room temperature. Samples were rinsed three times before imaging. Confocal images were taken using confocal fluorescence microscope at 10X (TE2000E, Nikon Instruments, NY). Images of each DRG were merged using Photoshop software. The length of the ten longest neurites, extending from the edge of the DRG explant, was measured for each DRG (n=3). Comparisons were made with DRG neurite extension on PVDF-TrFE scaffolds without SCs. Studies were performed three times for reproducibility.
2.8. Purified Rat Dorsal Root Ganglion Neurite Extension
To further evaluate SC supported neurite extension and myelination, a SC-DRG co-culture was utilized by using purified DRG explants. Neonatal rat SCs were used in this experiment. Lumbar DRGs were isolated from E17 Sprague Dawley rat embryos and seeded on collagen coated petri dishes for purification. DRGs were cultured in DRGPM for one week followed by culturing in DRGGM for three additional days. DRGPM was changed once and DRGGM was changed every day. The purification procedure was performed to eliminate endogenous SCs and fibroblasts. PVDF-TrFE scaffolds were cut into 15 mm diameter disks and both sides of the disks were UV- sterilized for 15 mins before inserting into the wells of a 24-well ultra-low attachment plate (Corning, Inc.). Polytetrafluoroethylene (PTFE) rings (ID: 14.3 mm; OD: 15.8 mm; 2 mm high; Zeus Inc., Orangeburg, SC) were placed on top of the scaffolds to prevent them from floating in cell culture media. PVDF-TrFE scaffolds were coated with Matrigel as described above. SCs were seeded at 3.6 × 105 cells per scaffold and incubated overnight at 37 °C. One day after seeding SCs, axotomy was performed on purified DRG explants and the DRG explants were re-plated on PVDF-TrFE scaffolds pre-seeded with or without SCs. Co-cultures were maintained at 37 °C. At day 1 and 3 after seeding DRGs, co-cultures were rinsed twice with PBS and fixed using 4% paraformaldehyde in PBS. Fixed samples were then washed once with PBS and permeabilized with 0.1% Triton X-100 in blocking solution (3% BSA in PBS) for 60 minutes. After rinsing once with PBS, mouse anti RT97 neurofilament (1:3, The Developmental Studies Hybridoma Bank, Iowa City, IA) and polyclonal rabbit anti-S100 (1:500, Thermo Fisher Scientific, Inc.) primary antibodies in blocking solution were added to each well and samples were kept at 4°C overnight. Next day, samples were rinsed three times with PBS followed by adding goat anti-rabbit Alexa Fluor 488 (1:500, Thermo Fisher Scientific, Inc.), donkey anti-mouse NorthernLights NL493(1:200, R&D Systems, Inc.) secondary antibodies in blocking solution for 1 hour. Samples were rinsed three times with PBS before imaging. Confocal images were taken using confocal fluorescence microscope at 20X (C1si, Nikon Instruments, NY). Images of each DRG were merged using Photoshop software. The length of the ten longest neurites, extending from the edge of the DRG explant, was measured for each DRG (n=3 per time point). Studies were performed three times for reproducibility.
2.9. SC Myelination
UV-sterilized PVDF-TrFE scaffolds (15mm diameter disks) were placed in wells of 24-well ultra- low attachment plates, maintained in place at the bottom of the wells by PTFE rings, and coated overnight with Matrigel (300 μg/ml in SCM) at 37°C. After removing the excess of Matrigel, 2 × 105 purified neonatal rat SCs were seeded and maintained for 48 hours in SCM with 2μΜ forskolin and 5 ng/ml rhNrg1-EGFD (proliferative conditions) to allow for the cells to populate the scaffold. SCs were then maintained for another 72 hours in SCM (non-proliferative conditions), purified DRG explants (1 explant per scaffold) were added, in myelination co-culture media CCMM. Myelination was initiated 72 hours later by the addition of ascorbic acid (Sigma Aldrich, Inc.) at 50 μg/ml to the CCMM. After 14 days, cultures were rinsed once with PBS and fixed in 1% paraformaldehyde for 20 minutes at room temperature. After washing twice with PBS, cultures were permeabilized in cold methanol (−20°C for 20 minutes) and then incubated in blocking solution (5% IgG-free BSA, 1% normal donkey-serum, 0.3% Triton X100) for 1 hour at room temperature. This was followed by an overnight incubation at 4°C with primary mouse anti-myelin basic protein (MBP; 1:500, Biolegend, Inc.) and guinea pig anti-contactin-associated protein 1 (Caspr; 1:3000, a gift from Dr. M. Bhat) antibodies in blocking solution. After washing three times with PBS, samples were incubated with secondary antibodies for 1 hour at room temperature (rhodamine-X donkey anti-mouse, 1:200 and FITC donkey anti-guinea pig, 1:200; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA). Confocal images were taken using confocal fluorescence microscope at 40x (C1si, Nikon Instruments, NY). A sample size of four was used and experiments were performed twice.
2.10. Statistics
All data were presented as mean values ± standard deviation. All statistical analyses were performed using analysis of variance (ANOVA) and Tukey pairwise comparison (p<0.05, Minitab, v17.1.0, Minitab Inc., State College, PA).PVDF-TrFE
3. Results
3.1. Scaffold morphology
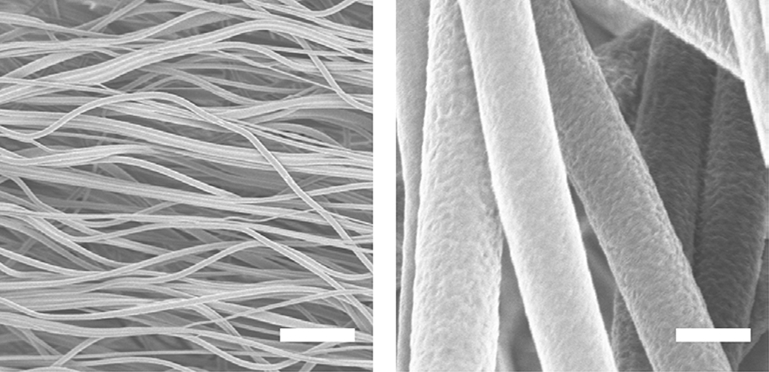
PVDF-TrFE electrospun scaffolds with aligned fibers were fabricated as shown in Figure 1. Average fiber diameter, inter-fiber spacing and alignment were 1.53 ± 0.39 μm, 12.7 ± 4.72 μm and 91.6% ± 8.8%, respectively.
3.2. Effect of Matrigel coating on SC growth and SC supported neurite extension
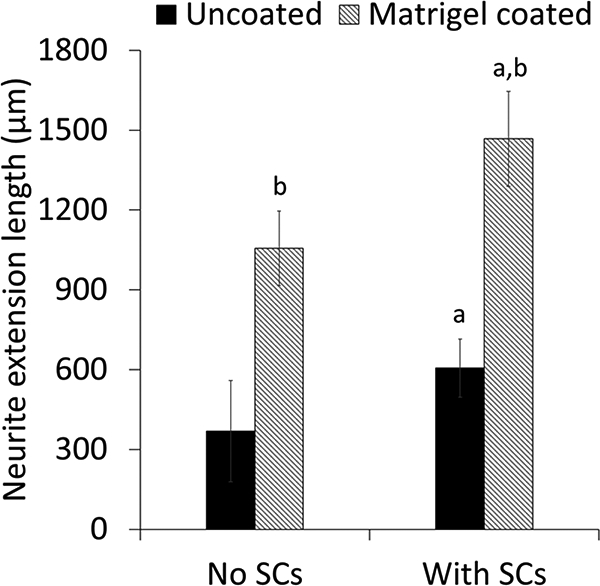
The effect of Matrigel coating on SC growth and SC supported neurite extension on the scaffolds was evaluated. SC attachment was supported on both uncoated and Matrigel-coated groups as shown in Figure 2. However, more SCs appeared to be attached to Matrigel-coated scaffolds than on uncoated scaffolds and the number of SCs on Matrigel-coated scaffolds appeared to increase with time. SCs appeared to extend long processes along PVDF-TrFE fibers. Significantly higher cell numbers were determined for the Matrigel-coated group over time and in comparison to the uncoated group (Figure 3). Cell number was maintained over time on the uncoated group (Figure 3). Scaffolds supported DRG attachment and neurite extension on both uncoated and Matrigel-oated groups as shown in Figure 4. Neurite extension for DRGs on Matrigel-coated scaffolds wasup to three-fold higher than DRGs on uncoated scaffolds. Scaffolds with SCs promoted longer neurite extension compared to without SCs (Figure 5). The longest neurite extension was determined for DRGs on Matrigel-coated scaffolds with SCs, which was approximately five-fold higher than DRGs on uncoated scaffolds.
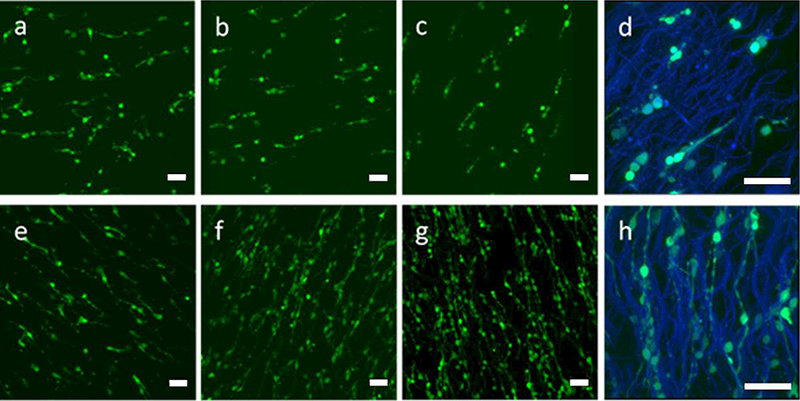
Representative confocal images of GFP SCs on PVDF-TrFE scaffolds at (a,e) day 1; (b,f) day 4; (c,d,g,h) day 7. (a-d) Uncoated scaffolds, (e-h) Matrigel-coated scaffolds. Green: GFP SCs; Blue: PVDF-TrFE scaffolds, (a-c, e-g) 20X; (d,h) 60X; Scale bar=50 pm.
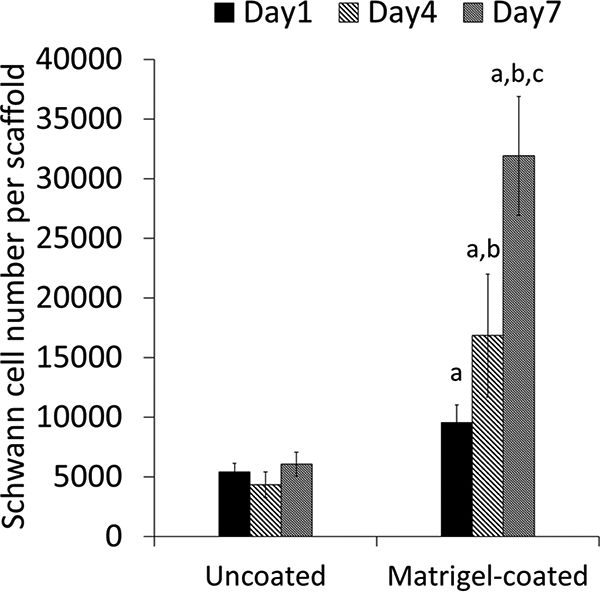
Schwann cell number on PVDF-TrFE scaffolds. ap<0.05, cell number on Matrigel-coated scaffolds is significantly higher than uncoated group. bp<0.05, cell numbers at days 4 and 7 are significantly higher than day 1 on Matrigel-coated scaffolds. cp<0.05, cell numbers at day 7 are significantly higher than day 1 and 4 on Matrigel-coated scaffolds.
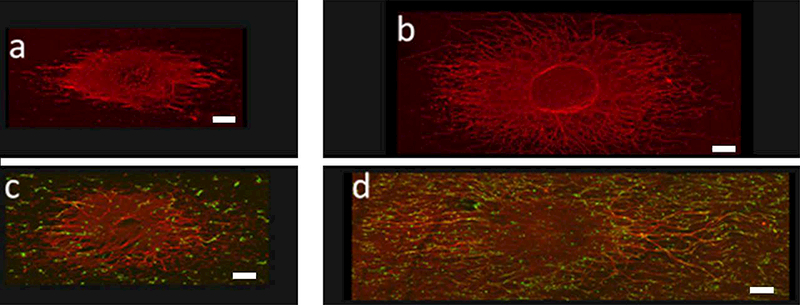
Representative confocal image of GFP SC-DRG co-culture at day 2. (a,b) DRG alone; (c,d) GFP SC-DRG co-culture. (a,c) uncoated scaffolds; (b,d) Matrigel-coated scaffolds. Green: GFP SCs; Red: neurofilament. Objective: 10X; Scale bar=200 μm.
3.3. Purified DRG Neurite Extension
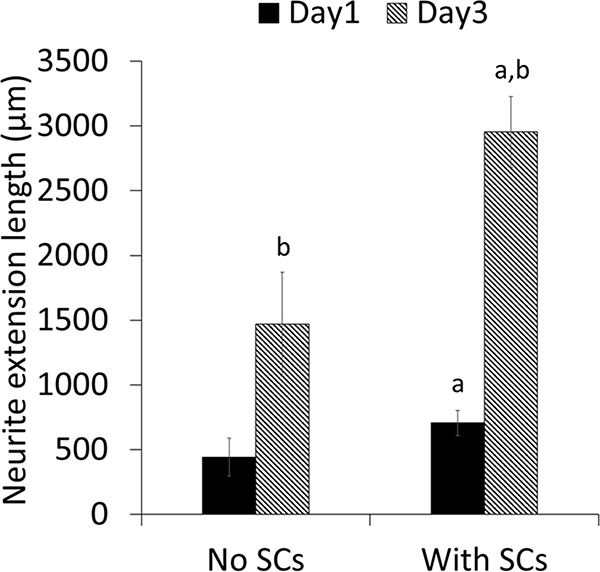
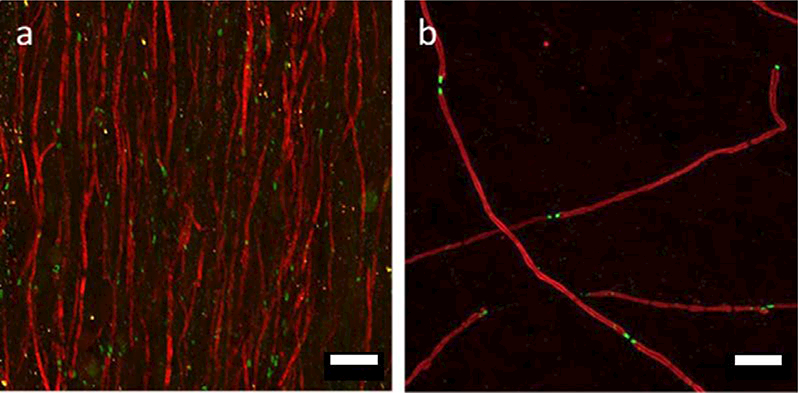
Scaffolds supported DRG attachment and neurite extension as shown in Figure 6 a&b. Neurite length significantly increased by approximately three-fold from days 1 to 3 on scaffolds without SCs (Figure 7). For scaffolds seeded with SCs, neurite length also significantly increased by approximately four-fold from days 1 to 3 and was significantly higher than scaffolds without SCs at both time points. High magnification confocal images showed that neurites are closely associated with SCs along PVDF-TrFE fibers (Figure 6 c-f).
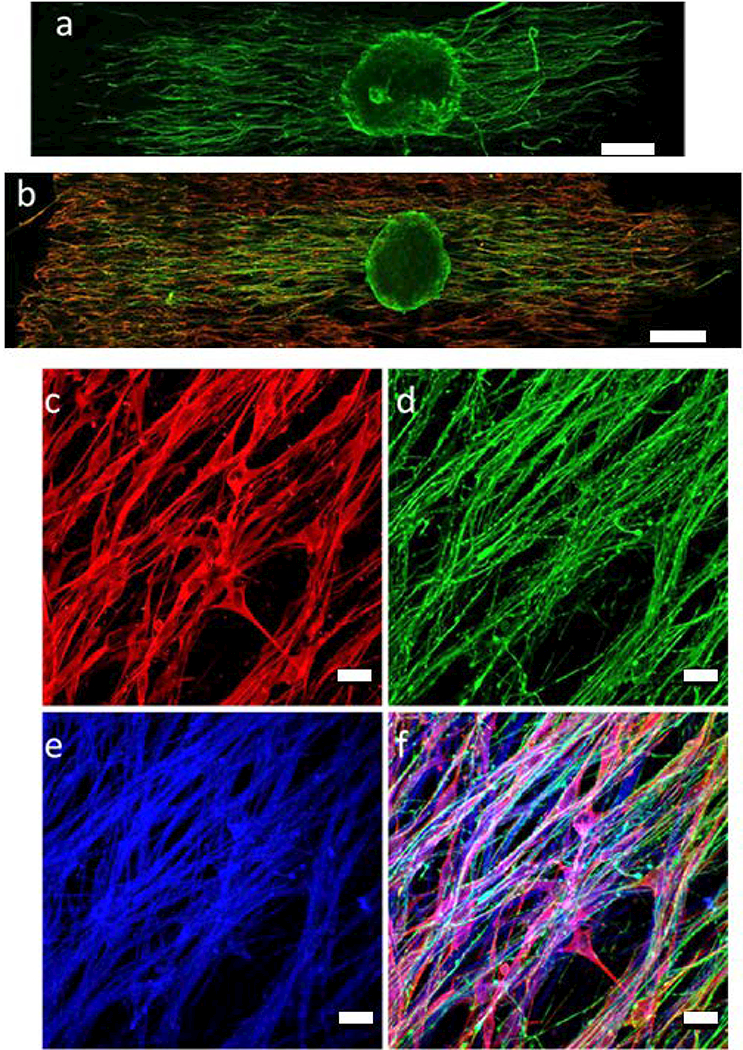
Representative confocal images of purified DRG cultures at day 1. a) DRG alone; b) SC-DRG co-culture; c) S100, SC marker; d) Neurofilament; e) PVDF-TrFE scaffolds; f) Merged image. Green: neurofilament; Red: S100; Blue: PVDF-TrFE scaffolds. (a,b) 20X; (c-f) 60X; Scale bar: (a,b=200 pm; c-f= 20 μm).
3.4. SC Myelination
In the SC myelination culture, SCs were stained positively for MBP (myelin marker). Numerous MBP-positive segments were detected (Figure 8). In contrast to the control cultures (glass coverslips), most of the myelinated segments were parallel to each other, presenting a unidirectional orientation similar to the aligned fibers in the scaffolds. The ends of most of the myelin internodes also stained positive for Caspr, a specific marker of the paranodal domains of a myelin sheath.
4. Discussion
In this study, PVDF-TrFE scaffolds consisting of aligned fibers were evaluated for SC supported neurite extension and myelination. Scaffolds were prepared using the electrospinning technique. The electrospinning process is used in tissue engineering to fabricate fibers ranging from nano to micrometer diameter dimensions, which is useful for promoting cell adhesion (56, 57). In this study, scaffolds with fiber diameters of approximately 1.5 microns promoted neurite extension and SC attachment. This fiber dimension is consistent with other studies utilizing electrospun fibers (58, 59). Although the uncoated PVDF-TrFE scaffold supported SC-neurite extension, coating with Matrigel promoted SC growth, significantly increased SC-neurite extension and supported myelination.
Many types of polymers have been studied as tissue engineering scaffolds for axon regeneration (10). In the spinal cord, nerve fiber tracts have highly aligned anatomical structure and it is important to design scaffolds that can promote directional axon regeneration so that the original anatomical structure of nerve fibers can be restored after injury. Synthetic polymers can be easily fabricated into aligned fibrous form which can provide topographic cues for guiding axon regeneration (13). One of the common issues of using synthetic polymers for tissue regeneration is that they lack ligands on the surface for cell adhesion which usually results in poor cell adhesion. Therefore, surface modifications will be used to create more bioactive scaffolds made of synthetic polymers (60–62). Matrigel was used on the PVDF-TrFE scaffolds based on our previous studies using Matrigel as a promising carrier for SCs in vivo (12). Since Matrigel is a matrix derived from mouse tumor cells, it may have limited potential for clinical translation. We also examined other commonly used ECM or ECM-derivative materials, gelatin, fibrin and hyaluronic acid as coatings on the scaffold but Matrigel outperformed these other materials in terms of promoting SC attachment and growth (Supplement Figure 1). Matrigel is an extracellular matrix consisting of approximately 60% laminin, 30% collagen IV, 8% entactin and growth factors including NGF, platelet-derived growth factor (PDGF), IGF-1, transforming growth factor - beta (TGF-β), epidermal growth factor (EGF), and FGF (manufacturer data). Matrigel has been used for SC transplantation in the spinal cord in preclinical studies because it is in liquid form at low temperature and forms a gel at body temperature, which makes it a useful matrix for cell transplantation (63). Our results showed that Matrigel coated scaffolds promoted SC attachment and growth in comparison to uncoated scaffolds. Our results were consistent with findings in other studies where Matrigel has been shown to promote SC proliferation through adhesion-dependent stimulation mediated by the interactions between Matrigel and integrins (47). In addition to promoting SC growth, the Matrigel coating promoted neurite extension. Similar findings were reported by other groups, where Matrigel and its major components, laminin and collagen Type IV, have also been shown to promote neurite extension (48, 64, 65). In one of these studies, Matrigel was used as a thick gel containing SCs and plated on top of the DRG culture on a tissue culture plate (48). Surprisingly, the addition of the SC containing Matrigel did not improve neurite extension compared to Matrigel alone. In our experiments, the PVDF-TrFE scaffolds were coated with a diluted Matrigel solution and then seeded with SCs before plating DRGs, which promoted longer neurite extension. SC-axon contact may be enhanced on the Matrigel coated fibers, which were pre-seeded with SCs, as compared to SCs suspended in Matrigel. In addition, the aligned fibers may provide additional physical cues that can promote the differentiation of SCs, which in turn may promote axon extension (66). Overall, our results demonstrate that this combination strategy utilizing PVDF-TrFE scaffolds with Matrigel coating and SCs hold great promise for axon regeneration.
To better understand SC supported neurite extension and myelination, we performed experiments using purified DRG explants. Our results showed that SCs seeded on PVDF-TrFE scaffolds promoted longer neurite extension compared to scaffolds alone. Neurites extended along PVDF-TrFE fibers and closely associated with SCs suggesting that the PVDF-TrFE fibers guide neurite extension and SC orientation. The aligned fibers of the scaffold provide physical contact cues (67) that support SC elongation and orientation, and neurite extension (13, 68–70). Similar to what occurs during PNS injury during the formation of Bungner bands, SC orientation may hold promise for providing a permissive environment for axon regeneration and myelination (71). SCs may also deposit extracellular matrix (72–77) and secrete growth factors that also promote neurite extension (34). Additional studies are needed to further examine the effects of physical guidance and piezoelectric activity on SC behavior. The detection of numerous compact myelin sheaths by MBP staining showed that the PVDF-TrFE scaffold does not have an inhibitory effect on myelination. Furthermore, the detection of Caspr, a specific marker of the paranodal domains that are characteristic of a myelin internode (78), also suggests that the formation of the structural domains that are necessary for saltatory conduction were not affected. In another study, Chew et al. studied the effects of topographic cues on SC maturation (66). Compared to SCs cultured on 2D film, electrospun fibers have been shown to promote SC maturation indicated by upregulated myelin associated genes (myelin-associated glycoprotein and myelin protein zero) and downregulated neural cell adhesion molecule 1 (a marker of immature SCs) for SCs seeded on electrospun fibers. However, Chew et al. does not evaluate SC myelin formation in their study. In this study, we have demonstrated, for the first time, that SCs form myelin on PVDF-TrFE electrospun fibers. Our findings suggest that electrospun PVDF-TrFE scaffold is a promising biomaterial to be used for SC supported myelination. Interestingly, most of the myelinated segments were parallel to each other, exhibiting a uni-directional organization that is similar to the underlying fibers of the PVDF-TrFE scaffold. This is in accordance with our previous description on the association of SCs along the fibers of the scaffold, similar to bands of Bungner-like structures. As SCs deposit their own matrix, it is possible that growing axons favored an ECM path laid out by SCs over a uniform Matrigel matrix.
5. Conclusion
To the best of our knowledge, this is the first study evaluating SC supported neurite extension and myelination on PVDF-TrFE scaffolds. Results of this study show that aligned fibrous PVDF-TrFE may be a promising scaffold for SC supported axon regeneration. The PVDF- TrFE scaffold supported SC attachment that further enhanced neurite extension. Aligned fibers for neurite extension and SC orientation may have a significant impact on directional axon regeneration and hold great promise to restore the aligned anatomical structure of damaged spinal cord tissue. In addition, PVDF-TrFE scaffolds supported SC myelination, which is critical for restoring and achieving functional recovery after SCI. Future studies will need to further optimize the SC seeding density that best promotes axon regeneration. This strategy of SCs on Matrigel coated PVDF-TrFE fibers also will be investigated in vivo for axon regeneration and myelination. Future studies also will examine the effect of the piezoelectric activity of the PVDF-TrFE scaffold on SC behavior and axon regeneration.
6. Acknowledgement
We would like to thank Yelena Pressman for providing GFP SCs and media and the RT97 antibody. We would like to thank Dr. Bryan Pfister’s lab members for assisting in the rat embryo preparation. Funding was provided by DOD W81XWH-14–1-0482 to TA and MB, NJCSCR CSCR14FEL004 to TA and SW, NSF STC Center for Engineering MechanoBiology (CMMI- 1548571) to TA and NIH/NINDS - R01 NS065218 to PM.
7. References
Full text links
Read article at publisher's site: https://doi.org/10.1088/1741-2552/aac77f
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc6125183?pdf=render
Citations & impact
Impact metrics
Article citations
Piezoelectric Scaffolds as Smart Materials for Bone Tissue Engineering.
Polymers (Basel), 16(19):2797, 02 Oct 2024
Cited by: 0 articles | PMID: 39408507 | PMCID: PMC11479154
Review Free full text in Europe PMC
Biomimetic electrospun PVDF/self-assembling peptide piezoelectric scaffolds for neural stem cell transplantation in neural tissue engineering.
RSC Adv, 14(30):21277-21291, 05 Jul 2024
Cited by: 0 articles | PMID: 38974226
Revealing an important role of piezoelectric polymers in nervous-tissue regeneration: A review.
Mater Today Bio, 25:100950, 11 Jan 2024
Cited by: 4 articles | PMID: 38318479 | PMCID: PMC10840125
Review Free full text in Europe PMC
Innervation of an Ultrasound-Mediated PVDF-TrFE Scaffold for Skin-Tissue Engineering.
Biomimetics (Basel), 9(1):2, 20 Dec 2023
Cited by: 0 articles | PMID: 38275450 | PMCID: PMC11154284
Smart and Multifunctional Materials Based on Electroactive Poly(vinylidene fluoride): Recent Advances and Opportunities in Sensors, Actuators, Energy, Environmental, and Biomedical Applications.
Chem Rev, 123(19):11392-11487, 20 Sep 2023
Cited by: 8 articles | PMID: 37729110 | PMCID: PMC10571047
Review Free full text in Europe PMC
Go to all (20) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials
- (1 citation) ClinicalTrials.gov - NCT02354625
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Enhanced noradrenergic axon regeneration into schwann cell-filled PVDF-TrFE conduits after complete spinal cord transection.
Biotechnol Bioeng, 114(2):444-456, 26 Sep 2016
Cited by: 21 articles | PMID: 27570167
Neurite extension of primary neurons on electrospun piezoelectric scaffolds.
Acta Biomater, 7(11):3877-3886, 14 Jul 2011
Cited by: 62 articles | PMID: 21810489
PVDF and P(VDF-TrFE) Electrospun Scaffolds for Nerve Graft Engineering: A Comparative Study on Piezoelectric and Structural Properties, and In Vitro Biocompatibility.
Int J Mol Sci, 22(21):11373, 21 Oct 2021
Cited by: 14 articles | PMID: 34768804 | PMCID: PMC8583857
Electrospun Polyvinylidene Fluoride-Based Fibrous Scaffolds with Piezoelectric Characteristics for Bone and Neural Tissue Engineering.
Nanomaterials (Basel), 9(7):E952, 30 Jun 2019
Cited by: 43 articles | PMID: 31261995 | PMCID: PMC6669491
Review Free full text in Europe PMC
Funding
Funders who supported this work.
Division of Civil, Mechanical and Manufacturing Innovation (1)
Grant ID: CMMI- 1548571
NINDS NIH HHS (1)
Grant ID: R01 NS065218
NJ Commission on Spinal Cord Research (1)
Grant ID: CSCR14FEL004
National Institute of Neurological Disorders and Stroke (1)
Grant ID: R01 NS065218
U.S. Department of Defense (1)
Grant ID: W81XWH-14-1-0482