Abstract
Free full text

Efficacy of DA-7218, a New Oxazolidinone Prodrug, in the Treatment of Experimental Actinomycetoma Produced by Nocardia brasiliensis
Abstract
Two recently synthesized oxazolidinones: (R)-3-(4-(2-(2-methyltetrazol-5-yl)-pyridin-5-yl)-3-fluorophenyl)-5-hydroxymethyloxazolidin-2-one (DA-7157) and its corresponding pro-drug (R)-3-(4-(2-(2-methyltetrazol-5-yl)-pyridin-5-yl)-3-fluorophenyl)-2-oxo-5-oxazolidinyl) methyl disodium phosphate (DA-7218), have shown very good activity against several Gram positive bacteria, including Nocardia and Mycobacterium. In the present work we evaluated the therapeutic in vivo effects of DA-7218 on Nocardia brasiliensis. We first determined the plasma concentration of the prodrug in BALB/c mice using several doses and then tested its activity in an in vivo experimental actinomycetoma murine model. At the end of treatment, there was a statistically significant difference between the three drug receiving groups (25, 12.5 and 5 mg/kg) and the control group (saline solution) (p=0.001), proving that DA-7218 is effective for the treatment of experimental murine actinomycetoma. This compound could be a potential option for patients affected with mycetoma by Nocardia brasiliensis.
Introduction
Oxazolidinones are newly developed antimicrobials with a target site not utilized by other antimicrobials; they inhibit protein synthesis by binding to the ribosomal P site and pleiotropically affecting fMet-tRNA binding [1,2]. Recently, a new oxazolidinone, (R)-[3-(4-(2-(2-methyltetrazol-5-yl)pyridin-5-yl)-3-fluorophenyl)-2-oxo-5-oxazolidinyl]methyl disodiumphosphate (DA-7218) has been synthesized by Dong-A Pharmaceutical Company, Yongin, S. Korea. This compound is a prodrug and after entering the bodily fluids it is quickly dephosphorylated to the active metabolite (R)-3-(4-(2-(2-methyltetrazol-5-yl)pyridin-5-yl)-3-fluorophenyl)-5-hydroxymethyloxazolidin-2-one (DA-7157). The presence of the phosphate radical in DA-7218 increases enormously its solubility (150 mg/mL) compared to that of the active compound DA-7157 (0.00259 at pH of 7.0) [3]. This facilitates its application in animals, since solubility has been observed to be an important factor in the in vivo analysis of new compounds, which can have a high in vitro antimicrobial activity, but they can not be tested in animal models because of they poorly solubility in water. Mycetoma is a chronic sub-cutaneous infection in tropical and sub-tropical countries produced by fungi and aerobic actinomycetes. In Mexico, 98% of the total cases are produced by actinobacteria and about 86% are produced by N. brasiliensis [4]. During the infection there is a swelling of the tissues with production of abscesses and scarring tissue; therefore antimicrobials should be able to penetrate deep into the tissues and maintain their antimicrobial activity even under these conditions. Several antimicrobials have been used with some success in the therapy of actinomycetoma, among them sulfonamides (DDS, sulfamethoxy-pyridazine, and sulfadoxine), streptomycin in combination with dapsone or with trimethoprim-sulfa-methoxazole (SXT), minocycline, imipenem, amoxicillin-calvulanic acid, etc. [5,6,7,8,9]. The highest cure rates (70%) have been obtained with the use of sulfamethoxazole-trimethoprim (SXT). In our dermatology clinic we have utilized the combination of SXT with amikacin to treat severe cases of mycetoma or those cases with possible spread to adjacent organs, obtaining a cure rate of about 95% in a series of 52 patients [10]. However, in some cases there is resistance or development of side effects, making necessary to look for new compounds, that are more potent and less toxic for patients. DA-7157 has been observed to be active against clinical isolates of Nocardia brasiliensis [11]. In the present work we tested the in vivo activity of the prodrug, DA-7218 to inhibit the production of experimental lesions in BALB/c mice.
Results and Discussion
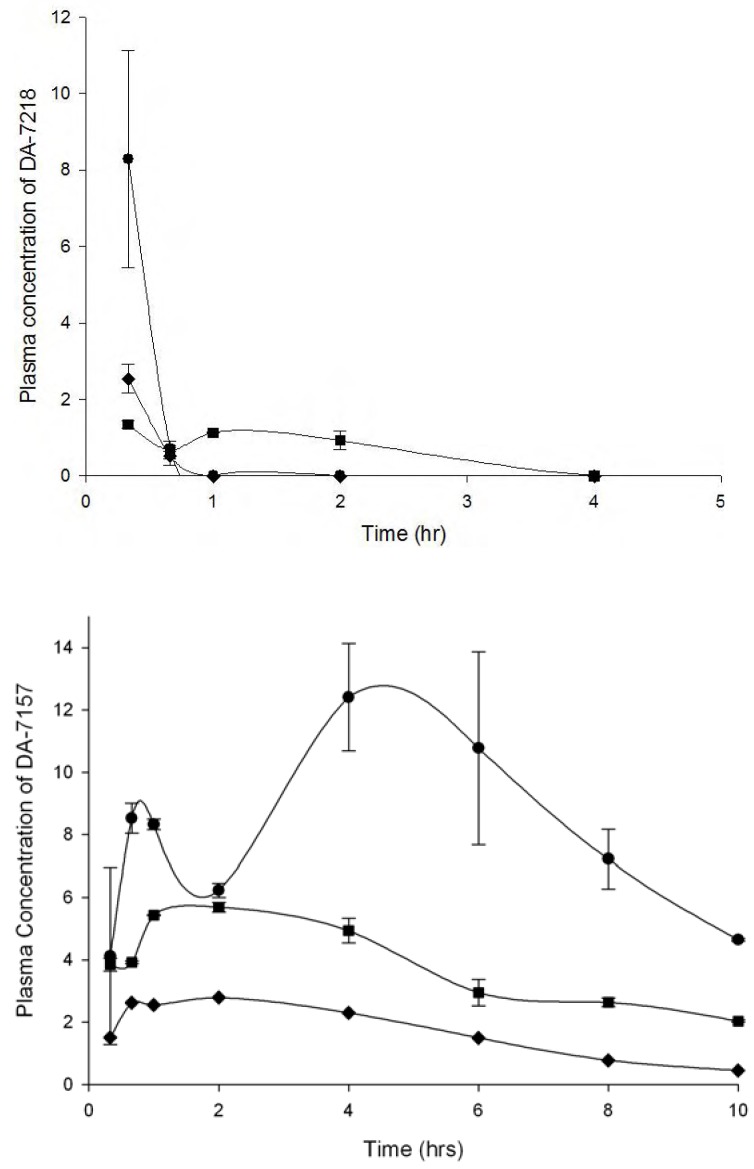
DA-7157 Plasma Levels
At 25 mg/kg, 20 min after injection of DA-7218, it was almost completely dephosphorylated to DA-7157; after that time DA-7218 practically disappeared and its metabolite DA-7157 reached its Cmax at 4 h (Figure 1). Ten hours after the initial injection, plasma levels at doses of 25 and 12.5 mg/kg, were still over the MIC90 presented by the N. brasiliensis clinical isolates (1 µg/mL). However, when using 5 mg/kg, we observed lower concentrations of DA-7157 in plasma, with levels around 1 µg/mL. Bae et al. determined the pharmacokinetics of DA-7218 in Sprague-Dawley rats using 5, 10 and 20 mg/kg administered intravenously and orally [3]. In both cases, they observed plasma levels of DA-7218 of less than 1 µg/mL after 30 min. After 8 h, at the dose of 20 mg/kg I.V., DA-7157 concentrations were about 0.5 µg/mL. By the oral route concentrations remained higher – after 12 h and at a dose of 20 mg/kg, arterial concentrations were about 7 µg/mL. In our BALB/c mice model a dose of 25 mg/kg produced a DA-7157 plasma level of about 6 µg/mL plasma levels at 10 h. The oral route in rats produces plasma levels similar to the observed in BALB/c mice using the subcutaneous route.
Experimental model
After inoculating the Nocardia there is a remarkable increase in footpad volume (Figure 2). This is possibly due to an immediate inflammatory response to the bacterial antigens, which decreases two weeks post-inoculation. From this time on the lesions became smaller but at the end of the first month, mycetoma lesions began to develop with the formation of microcolonies or granules of N. brasiliensis. The volume of the footpad increases constantly over time without spontaneous cure. We have followed the evolution of the footpad lesions for long periods of time, up to nine months; giant mycetomas corresponding to about one quarter of the weight of the mice are produced without killing the animals. These murine long term infections resemble some human cases of 20 or more years of evolution, producing extensive lesions, which are not lethal, unless they spread to important organs, such as lungs, spine, or brain [12]. The experimental infection with N. brasiliensis has been produced also in Lewis rats observing also a highly inflammatory initial reaction [13]. However, the immune system of this species is completely able to control the infection, and clear the tissues of nocardia cells, except in nude rats (rnu/rnu), were the infection can easily disseminate to the rest of the body of the animal and kill it.
Therapeutic assays
The prodrug DA-7218 was applied one week after the initial infection, for three weeks. In order to give the animals a resting period, therapy was suspended one week and then continued for three more weeks. The development of lesions was scored at the end of the first three weeks of treatment and at the end of the second cycle of three weeks. They were annotated according to the extension of the lesions from no inflammatory changes on the footpad (negative) to extensive production of abscesses and inflammation spreading beyond the metatarsal bones (4 +, Figure 3). Differences among the therapeutic groups and those animals injected with saline solution were determined by the Mood´s Median test. In Figure 4 we observe that after three weeks of therapy, there were statistically significant differences between the control group and the higher dose utilized (25 mg/kg). These statistically significant differences were observed more clearly in all the groups after the second cycle of three weeks, even with 5 mg/kg of the prodrug (p<0.001). In the dose of 25 mg/kg most of the animals presented lesions of less than 1+, and most of them were negative. These results correlate with the levels reached in plasma by DA-7157, since at 25 mg/kg levels over the MIC90 are kept for more than 10 hrs resulting in an excellent clinical response. More poor plasma levels correlated with less in vivo activity in the dose of 5 mg/kg.

BALB/c mice inoculated with N. brasiliensis HUJEG-1 showing diverse degrees of mycetomatous lesions. The lesions were scored from those without inflammatory changes (identical to the non-inoculated foot pad), to those presenting extense, abscedated lesions over the metatarsal bone.
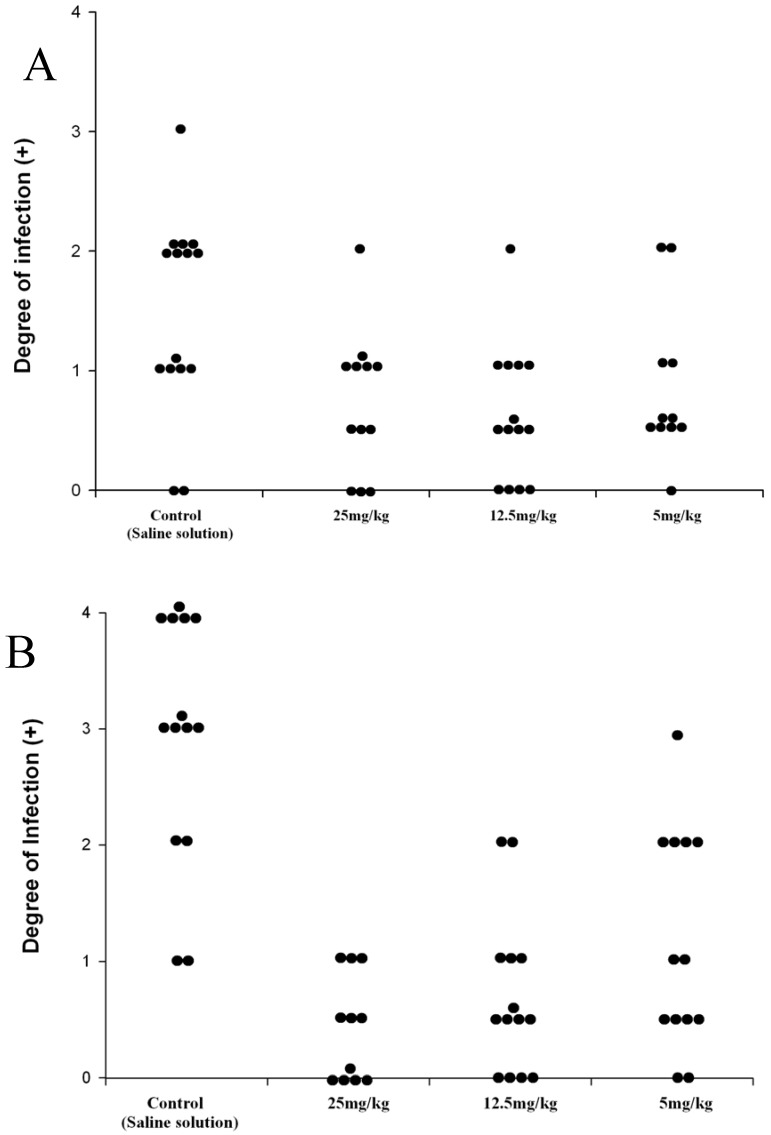
Effect of the prodrug, DA-7218 on the development of mycetomatous lesions on BALB/c mice infected with N. brasiliensis. A: Degree of infection of mice footpad after 3 weeks of therapy. B: Scores after the second cycle of three weeks of therapy. Each point represents the score of one animal.
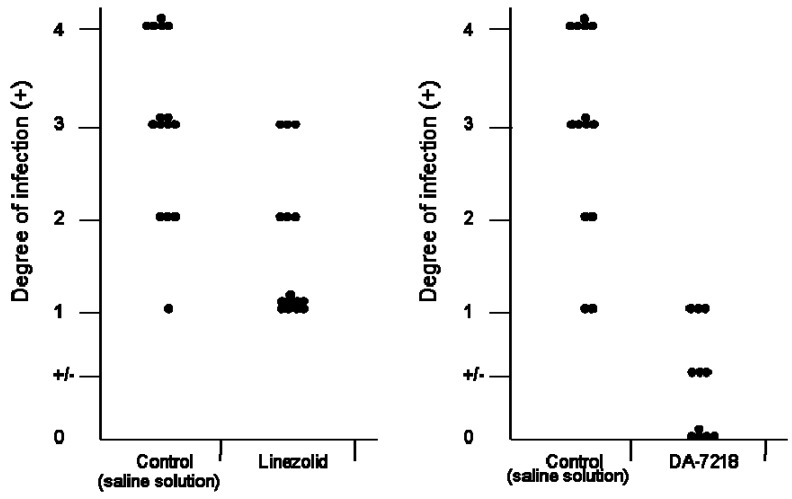
Linezolid is the only oxazolidinone available on the market since 2001; in humans at 600 mg orally it reaches a Cmax of 13.1 µg/mL and the levels are above 3.25 ± 1.02 µg/mL for 12 hrs [14]. It has been observed to be active against fastidious organism such as Nocardia and Mycobacterium tuberculosis [15]. However, the long term application of linezolid produces side effects such as ocular neuropathy, and myelosuppression [16]; therefore, although this oxazolidinone is quite active in human infections it will be necessary to have less toxic and more potent oxazolidinones.
DA-7157 has been observed to be more active than linezolid against several Gram positive microorganisms, including S. pneumoniae, methicillin resistant Staphylococcus aureus, etc [17]. Against N. brasiliensis it has been observed to have an identical MIC90 (1 µg/mL). However, when comparing the activity of linezolid with that of DA-7218 in the BALB/c mice model (Figure 5), the latter produced better effects stopping the development of animal lesions, and in contrast with linezolid, in the group treated with DA-7218 many animals have no lesions at all. If this effect is also observed in patients, less time will be needed to obtain a complete cure, however it will be necessary to determine the pharmakocinetics and toxicity level in humans before it can be used in human infections.
Experimental
Chemicals
The three oxazolidinones utilized in this study DA-7157, DA-7218 and linezolid were supplied by the Research Laboratory of Dong-A Pharmaceutical Company (Yongin, South Korea).
Microorganisms
For the animal assays we utilized Nocardia brasiliensis HUJEG-1 which has been used in previous studies [19]. The minimal inhibitory concentrations (MIC) values of this strain for linezolid, DA-7157 and DA-7218 are 0.12, 1.0 and 8 µg/mL, respectively. The strain was grown on Sabouraud dextrose agar for 7 to 10 days and a suspension in 20% skim milk of each was made. These bacterial suspensions constituted our stock cultures that were kept at –70°C in cryovials until use.
Determination of plasma levels of the antimicrobials
Female BALB/c mice, 8-12 weeks-old and weighing about 20 g were used. DA-7218 was applied intradermically in the dorsal area of mice at doses of 5, 12.5 and 25 mg/kg. Blood samples (500 µL) were collected from the retroorbital sinus of each mouse, after previous general anesthesia with ethylic ether, at 0 min, 20 min, 40 min, 1 h, 2 h, 4 h, 6 h, 8 h and 10 h. After sample collection, the plastic tubes containing the blood were centrifuged and the plasma separated and frozen at -70°C. Plasma concentrations were determined using an HPLC method, as described below.
HPLC analysis of DA-7218 and DA-7157
The concentrations of DA-7218 and DA-7157 in the above samples were analyzed by an HPLC method developed in our laboratories. An aliquot of biological sample (50 µL) was deproteinized with acetonitrile (150 µL). After vortex-mixing and centrifugation, the supernatant was filtered through 0.45 µm nylon filters (Waters, Milford, USA), and filtrates were received into 150 µL inserts (Waters). For the analysis, samples were injected directly onto the RP-18 reversed-phase HPLC column (15 cm in length, 4.6 mm i.d., particle size 5 µm; Atlantis dC18, Waters Corporation, Milford, MA, USA). The mobile phase (trifluoroacetic acid 0.1%/acetonitrile, v/v), was run in a gradient program at a flow rate of 1.0 mL∙min−1 and the column effluent was monitored using a fluorescence detector (Spectrofluorometer 474, Waters, Corporation, Milford, MA, USA), set at an excitation wavelength of 292 nm and an emission wavelength of 525 nm. The retention times of DA-7218, and DA-7157 were approximately 10.38 and 12 .07 min, respectively. The detection limits of both drug was 0.16 µg∙mL−1. The coefficients of variation were 8 and 13.8% respectively.
Therapeutic assays
N. brasiliensis HUJEG-1 was cultured on Sabouraud agar for 1 week, and then subcultured on Brain-Heart-Infusion at 37 °C in a shaker at 110 RPM for 72 hrs. The bacterial mass was then separated by centrifugation, and washed 4-times with saline solution. After grinding in a Potter-Evelham device the suspension was left to sediment and the bacterial mass was washed again with saline solution and adjusted to 20 mg (wet weight) of N. brasiliensis per 50 µL of saline solution. Eight-to-twelve week old female BALB/c mice were inoculated with 20 mg of Nocardia brasiliensis in the right hind foot-pad. Seven days later the therapeutic assay was started. Groups of 15 mice each were formed. One group was injected subcutaneously in the back with pyrogen-free saline solution (0.1 mL); the rest were treated twice daily with the prodrug DA-7218 at doses of 5, 12.5, and 25 mg/kg. Linezolid was used as control at a dose of 25 mg/kg tid. The drugs were administered subcutaneously on the back of each mouse during a three week period from Monday to Friday. After one week of rest, three more weeks of therapy were applied
Evaluation of the therapeutic assays
The lesions in the foot pad were scored depending on the extension as published before from 0 for those presenting absolutely no lesions or inflammation to 4+ for the animals presenting severe lesions involving above the metatarsal bones (Figure 3). Statistical differences among the therapeutic groups were determined using the Mood´s median test for nonparametric methods, which showed differences between treatment receiving groups and the control group. The latter had the highest infection rates. Doses of 5 and 12.5 mg/kg had equal improvement rate, and 25 mg/kg has the biggest interval rate which shows a highest significant improvement.
Acknowledgements
This research partially fulfills the requirement for the Doctor of Medicine degree (Facultad de Medicina, Universidad Autónoma de Nuevo León) of the first author (N.A. E-G).
Footnotes
Sample Availability: Requests for samples of DA-7218 or DA-7157 should be directed to Dr. Sung Hak Choi.
References
Articles from Molecules are provided here courtesy of Multidisciplinary Digital Publishing Institute (MDPI)
Full text links
Read article at publisher's site: https://doi.org/10.3390/molecules13010031
Read article for free, from open access legal sources, via Unpaywall:
https://www.mdpi.com/1420-3049/13/1/31/pdf?version=1403112468
Citations & impact
Impact metrics
Article citations
In vitro activity of tedizolid against 43 species of Nocardia species.
Sci Rep, 14(1):5342, 04 Mar 2024
Cited by: 0 articles | PMID: 38438563 | PMCID: PMC10912709
In Vivo Activity of the Benzothiazinones PBTZ169 and BTZ043 against Nocardia brasiliensis.
PLoS Negl Trop Dis, 9(10):e0004022, 16 Oct 2015
Cited by: 4 articles | PMID: 26474057 | PMCID: PMC4608729
Mycetoma medical therapy.
PLoS Negl Trop Dis, 8(10):e3218, 16 Oct 2014
Cited by: 67 articles | PMID: 25330342 | PMCID: PMC4199551
Review Free full text in Europe PMC
HPLC method for the simultaneous analysis of fluoroquinolones and oxazolidinones in plasma.
J Chromatogr Sci, 52(10):1281-1287, 03 Mar 2014
Cited by: 9 articles | PMID: 24596421
Potential role of tedizolid phosphate in the treatment of acute bacterial skin infections.
Drug Des Devel Ther, 7:243-265, 03 Apr 2013
Cited by: 12 articles | PMID: 23589680 | PMCID: PMC3622392
Review Free full text in Europe PMC
Go to all (13) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Evaluation of the Combined Therapy of DA-7218, a New Oxazolidinone, and Trimethoprim/ Sulfamethoxazole in the Treatment of Experimental Actinomycetoma by Nocardia brasiliensis.
Curr Drug Deliv, 7(3):225-229, 01 Jul 2010
Cited by: 3 articles | PMID: 20497102
In vitro activities of DA-7157 and DA-7218 against Mycobacterium tuberculosis and Nocardia brasiliensis.
Antimicrob Agents Chemother, 50(9):3170-3172, 01 Sep 2006
Cited by: 43 articles | PMID: 16940121 | PMCID: PMC1563545
In vivo therapeutic effect of gatifloxacin on BALB/c mice infected with Nocardia brasiliensis.
Antimicrob Agents Chemother, 52(4):1549-1550, 19 Feb 2008
Cited by: 7 articles | PMID: 18285484 | PMCID: PMC2292517
Tedizolid: a novel oxazolidinone for Gram-positive infections.
Clin Infect Dis, 58 Suppl 1:S1-3, 01 Jan 2014
Cited by: 21 articles | PMID: 24343826
Review