Abstract
Background & aims
The existence of postinfection irritable bowel syndrome (PI-IBS) has been substantiated by epidemiology studies conducted in diverse geographic and clinical settings. However, the available evidence has not been well summarized, and there is little guidance for diagnosis and treatment of PI-IBS. The ROME Foundation has produced a working team report to summarize the available evidence on the pathophysiology of PI-IBS and provide guidance for diagnosis and treatment, based on findings reported in the literature and clinical experience.Methods
The working team conducted an evidence-based review of publication databases for articles describing the clinical features (diagnosis), pathophysiology (intestinal sensorimotor function, microbiota, immune dysregulation, barrier dysfunction, enteroendocrine pathways, and genetics), and animal models of PI-IBS. We used a Delphi-based consensus system to create guidelines for management of PI-IBS and a developed treatment algorithm based on published findings and experiences of team members.Results
PI-IBS develops in about 10% of patients with infectious enteritis. Risk factors include female sex, younger age, psychological distress during or before acute gastroenteritis, and severity of the acute episode. The pathogenesis of PI-PBS appears to involve changes in the intestinal microbiome as well as epithelial, serotonergic, and immune system factors. However, these mechanisms are incompletely understood. There are no evidence-based, effective pharmacologic strategies for treatment of PI-IBS. We provide a consensus-based treatment algorithm, based on clinical presentation and potential disease mechanisms.Conclusions
Based on a systematic review of the literature and team experience, we summarize the clinical features, pathophysiology (from animal models and human studies), and progression of PI-IBS. Based on these findings, we present an algorithm for diagnosis and treatment of PI-IBS based on team consensus. We also propose areas for future investigation.Free full text

ROME FOUNDATION WORKING TEAM REPORT ON POST-INFECTION IRRITABLE BOWEL SYNDROME
Abstract
Background & Aims
The existence of post-infection irritable bowel syndrome (PI-IBS) has been substantiated by epidemiology studies conducted in diverse geographic and clinical settings. However, the available evidence has not been well summarized and there is little guidance for diagnosis and treatment of PI-IBS. The ROME Foundation has produced a working team report was to summarize the available evidence on the pathophysiology of PI-IBS and provide guidance for diagnosis and treatment, based upon findings reported in the literature and clinical experience.
Methods
The working team conducted an evidence-based review of publication databases for articles describing the clinical features (diagnosis), pathophysiology (intestinal sensorimotor function, microbiota, immune dysregulation, barrier dysfunction, enteroendocrine pathways and genetics), and animal models of PI-IBS. We used a Delphi-based consensus system to create guidelines for management of PI-IBS and a developed treatment algorithm based on published findings and experiences of team members.
Results
PI-IBS develops in about 10% of patients with infectious enteritis. Risk factors include female sex, younger age, psychological distress during or prior to acute gastroenteritis, and severity of the acute episode. The pathogenesis of PI-PBS appears to involve changes in the intestinal microbiome as well as epithelial, serotonergic, and immune system factors. However, these mechanisms are incompletely understood. There is no evidence- based effective pharmacologic strategies for treatment of PI-IBS. We provide a consensus-based treatment algorithm, based on clinical presentation and potential disease mechanisms.
Conclusions
Based on a systematic review of the literature and team experience, we summarize the clinical features, pathophysiology (from animal models and human studies), and progression of PI-IBS. Based on these findings, we present an algorithm for diagnosis and treatment of PI-IBS based upon team consensus. We also propose areas for investigations.
INTRODUCTION
Irritable bowel syndrome (IBS) is one of the most common conditions diagnosed in gastroenterology practice. The prevalence of IBS ranges between 7–16% in western countries and is more common in females and younger individuals. Although the etiology of IBS is still obscure, its pathophysiology is dominated by a combination of both psychological factors and gastrointestinal dysfunction. Recent efforts have allowed identification of several peripheral micro-organic abnormalities. These include changes in gut microbiota, low grade mucosal inflammation, and epithelial dysfunction. Additionally, genetic polymorphisms as well as environmental factors including dietary factors and enteric infections have been shown to play a role. The identification of these factors and their interaction with the brain has opened an entirely new era in the understanding, recognition and legitimization of IBS and functional gastrointestinal disorders (FGIDs) at large. As a consequence, there has been agreement among experts to eliminate from the recently released Rome IV criteria the term “functional” and redefine these conditions as “disorders of gut-brain interactions.” 1
Acute infectious gastroenteritis represents the strongest known risk factor for IBS development; a condition known as post-infection IBS (PI-IBS). While publications from the past often alluded to the possibility that irritability of the gut could develop in the aftermath of a bacillary diarrheal episode,2, 3 the first formal description of PI-IBS was published in 1962 by Chaudhary and Truelove.4 There was a relative quiescence in this research area until the late 1990s when elegant observations were made to understand the role of peripheral and central factors in the development of IBS following intestinal infections.5–7 Several subsequent studies have described PI-IBS in a wide variety of settings summarized in a recently published meta-analysis.8 Approximately 10% of those with intestinal infection that respond to survey questionnaires endorse symptoms consistent with PI-IBS.8 These estimates have varied with the type of pathogen involved and some studies have shown estimates as high as 35–45% for PI-IBS development.9, 10 The exact burden of PI-IBS is hard to assess since there is poor recall of intestinal infections, no biomarkers have been identified. Conservative estimates suggested that PI-IBS contributes to as much as 9% of the overall number of IBS cases in the community.11
Compared to the epidemiological literature, pathophysiological mechanisms of PI-IBS have been relatively understudied. Larger outbreaks have provided numbers needed to investigate genetic associations which identified single nucleotide polymorphisms (SNPs) associated with PI-IBS, although significance level did not withstand multiple testing correction.12 PI-IBS mechanistic studies in humans have provided evidence in support of increased intestinal permeability,13–15 altered serotonin (5-HT) metabolism,16–18 increased density of lamina propria enterochromaffin (EC) cells19, 20 and T lymphocytes.15 Animal studies have postulated on the role of Campylobacter toxin21 and putative mechanisms including small intestinal bacterial overgrowth22 and loss of interstitial cells of Cajal (ICC).23 ICC are the key regulator of gastrointestinal motility through generation and propagation of electrical slow waves and mediate communication between the autonomic nervous system and smooth muscle cells.24 Animal models have used Citrobacter rodentium, Trichinella spiralis and Campylobacter as prototypic organisms to investigate host interactions at peripheral and spinal levels.25
Psychological factors like anxiety, depression, somatization and neuroticism during or in the preceding months before infection have been associated with PI-IBS development as noted in the recent meta-analysis.8 Concomitant stress has also been associated with altered neuronal plasticity at the spinal level in animal models of PI-IBS.26 A recent study showed that psychological stress may be a risk factor for enteritis itself and a unique cytokine milieu favoring Th2 immune response may exist during psychological stress.27 Except for corticosteroids28 and mesalamine,29 randomized controlled trials in PI-IBS patients have not been conducted.
The Rome Foundation commissioned a working team to help advance understanding of PI-IBS. This review is a summary of existing knowledge with an emphasis on the clinical features, diagnosis, animal studies, host response mechanisms, including microbiota, immune regulation and genetic factors, as well as and treatment of PI-IBS. The epidemiology, risk-factors, and natural history aspects were extensively reviewed in the recent meta-analysis and are only briefly summarized here.8
CLINICAL FEATURES
Diagnosis
Although, there is no validated definition of PI-IBS, this condition is characterized by new-onset, Rome criteria-positive IBS following an episode of acute gastroenteritis in individuals who did not suffer from IBS prior to the infection. The diagnostic criteria for PI-IBS proposed by the Rome Foundation Working Team (RFWT) are based on the Rome IV criteria. These criteria were not part of the original Rome IV document as they were prepared after the release of Rome IV publications (Table 1). These criteria need to be fulfilled for the last 3 months with symptom onset at least 6 months before diagnosis.30 The acute infectious gastroenteritis is ideally diagnosed by stool culture (although only occasionally obtained in community subjects), validated molecular biology analyses (e.g., polymerase chain reaction) or by the presence of ≥2 of the following: fever, vomiting, or diarrhea.31
Table 1:
Diagnostic criteria for post-infection irritable bowel syndrome (based on Rome IV)
| 1. Recurrent abdominal pain, on average, at least 1 day per week in the last 3 months, withsymptom onset at least 6 months before diagnosis, associated with ≥2 of the following: a) related to defecation b) associated with a change in frequency of stool c) associated with a change in form (appearance) of stool 2. Symptom development immediately following resolution of acute infectious gastroenteritis 3. Infectious gastroenteritis defined by positive stool culture in a symptomatic individual orpresence of ≥2 of the following acute symptoms (when stool culture not available)§: a) fever b) vomiting c) diarrhea 4. Should not meet criteria for IBS prior to onset of acute illness* |
Subtyping IBS according to bowel habit has important implications to guide management. Generally, IBS is subtyped according to bowel habit, based on the Bristol Stool Form Scale, in three categories: IBS with constipation (IBS-C), with diarrhea (IBS-D) and with mixed bowel habit (IBS-M).30 The majority of the studies report IBS-M as the most predominant pattern associated with PI-IBS.8 The next most common is the IBS-D category and the reminder of the studies (<10%) reported IBS-C.20 The IBS-D subtype has been found to remain stable over time.32 While a phenotypic switch over time may frequently occur among the different bowel habit subtypes in IBS, whether the same applies to PI-IBS remains to be investigated. According to the Rome Foundation Multi-Dimensional Clinical Profile,33 PI-IBS constitutes part of the category of clinical modifiers.
Differential diagnosis
In typical cases of PI-IBS without alarm features, physicians are encouraged to make a positive diagnosis without significant additional diagnostic assessment. A minority of cases may undergo fecal tests to exclude a chronic parasitic or protozoal infection, especially, chronic giardiasis. However, stool cultures unlikely yield positive results as long-lasting infections with Campylobacter, Shigella, Salmonella or Yersinia are uncommon. Limited testing may include a complete blood count, C-reactive protein and fecal calprotectin. However, in cases with severe or alarm symptoms like significant (>10%) weight loss, gastrointestinal bleeding or failure to respond to drugs commonly used in IBS, further investigations may be required.
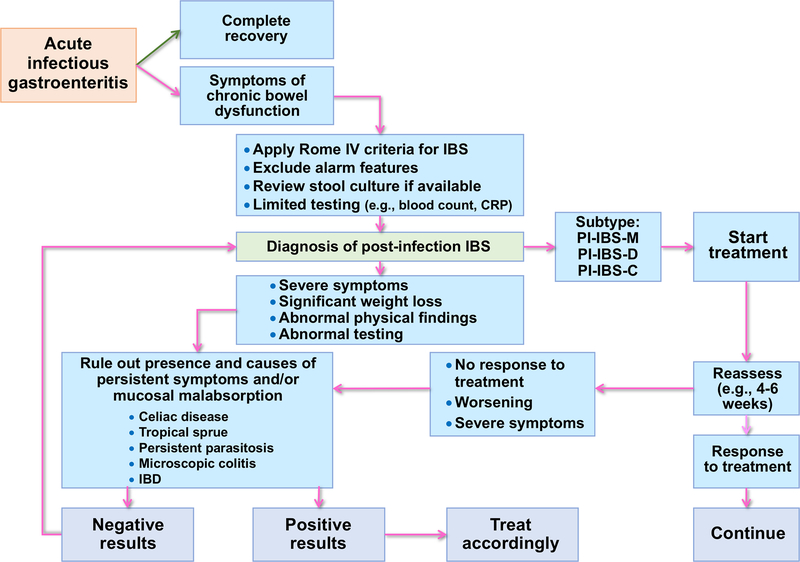
Details on differential diagnosis are provided in Supplementary material. Figure 1 provides a diagnostic algorithm for evaluation of PI-IBS.
Prevalence
The prevalence of PI-IBS among those suffering from infectious enteritis has been estimated between 4–36%.31 However, as episodes of infectious gastroenteritis occur quite frequently during lifetime (e.g., 1.4 episode per year per subject) and IBS patients may have limitations in recalling milder and remote episodes of gastroenteritis, we hypothesize that the true pathogenetic role of gastrointestinal infections in IBS is higher than currently estimated.
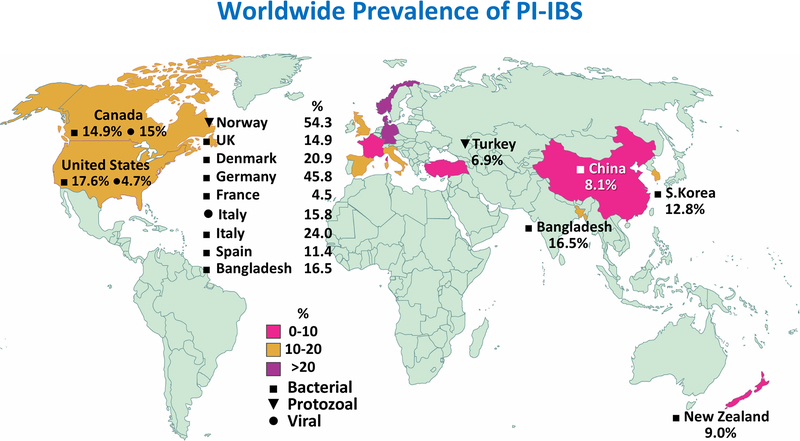
A recent systematic review of 45 studies, comprising ~21,000 individuals with enteritis, followed for 3 months to 10 years found pooled prevalence of IBS at 12 months after infectious enteritis of 10.1% (95% confidence interval [CI], 7.2−14.1).8 Figure 2 illustrates geographic variations in PI-IBS prevalence by pathogen type. Studies examining a follow up >12 months after infectious enteritis found a pooled prevalence of PI-IBS of 14.5% (95% CI, 7.7−25.5). Thirty, out of the 45 studies examined the relative risk of developing IBS compared to a cohort of uninfected patients showed a 4.2-fold risk for developing IBS over 12-month of follow-up which decreased to 2.3-fold in studies extended beyond 12 months (Supplementary Figure 1). PI-IBS has more frequently been described as a consequence of bacterial than viral infection,34 which is in contrast with the higher prevalence of viral compared to bacterial etiology of infectious diarrhea. This could be explained by the fact that mucosal damage and inflammation caused by bacterial gastroenteritis is often greater that that caused by viral agents.31 Recently, PI-IBS has also been described after Clostridium difficile infection in up to 25% of the cases.35, 36 Additionally, recently, Vibrio cholerae has been associated with PI-IBS development in 16.5% of the cases as well.37 Although most studies describing PI-IBS have been conducted in adult subjects, PI-IBS has also been described in the pediatric population with younger age representing a risk for persistence of IBS symptoms in the long term (16 years) compared to adults.38, 39
Functional dyspepsia (FD), another common FGID is characterized by postprandial fullness, early satiation, epigastric pain, and epigastric burning.40 IBS has been found to overlap with FD in up to 50% of cases41 and the occurrence of PI-FD has been also described.42 A recent systematic review reported that the prevalence of PI-FD was similar to that of PI-IBS (9%), however, the risk of developing PI-FD was lower than that of PI-IBS (2.5; 95% CI = 1.8–3.6 vs 3.5; 95% CI = 2.0–6.0).43 Moreover, the risk of overlapping PI-FD and PI-IBS was higher in children than in adults (39%; 35–90% vs 13%; 8–42%).43 In a more recent paper, the prevalence of PI-FD was 26% in the exposed individuals vs. 7% in unexposed individuals, with a relative risk of 3.9 (95% CI: 3.1–4.8) after Giardia infection.44 Among individuals fulfilling criteria for PI-IBS, 44% in the exposed group and 29% in the control group also fulfilled criteria for PI-FD.44
Risk-factors
Specific demographic, psychological, and clinical factors related to the enteritis-episode have been found to be associated with the risk of PI-IBS. A recent meta-analysis has provided pooled summary estimates for these associated factors8 and a summary on these and the natural history of PI-IBS is provided in the Supplementary material and Supplementary Figure 1.
PATHOPHYSIOLOGY
PI-IBS is a complex and likely multifactorial disorder. Studies on the pathophysiology have been performed in small group of patients and at different time points post-infection, which may contribute to incomplete information. The pathophysiology of PI-IBS is dominated by the interaction between the central and peripheral factors, the latter including the microbiota, epithelial, entero-endocrine, immunological and neuro-motor mechanisms. It is currently unknown if there are unique pathophysiological mechanisms for PI-IBS. Animal models have been instrumental to understand the mechanisms underlying gut and behavioral dysfunction after acute infection. Figure 3 provides a conceptual framework for neuro-immune interactions in PI-IBS.
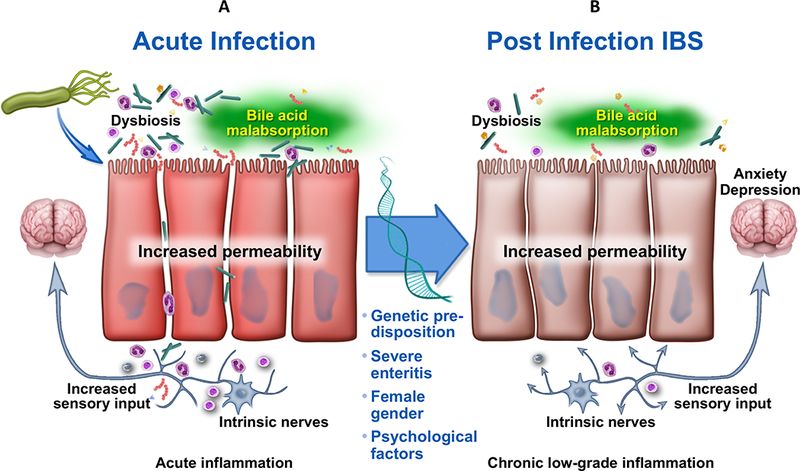
Schematic representation of putative pathophysiology underlying post-infection irritable bowel syndrome.
Gut dysmotility and visceral hypersensitivity
Although gut dysmotility and visceral hypersensitivity is considered to be of importance in the pathophysiology of IBS,30 only a few studies have assessed bowel physiology in PI-IBS.45 Gwee et al described the presence of persistent rectal hyper-reactivity and hypersensitivity 3 months following an infection and associated it with PI-IBS development.6 Further studies need to evaluate whether the described motor or sensory dysfunctions are indeed characteristics of PI-IBS.
Microbiota
The gut microbiota has a remarkable ability to resist to environmental perturbation and to preserve its structure and function.46 This is best illustrated through the preserved individual specific microbiota signature over a decade47, 48 and following broad-spectrum antibiotics.49 Ecosystem resilience occur also following recovery from an intestinal infection. However, those developing PI-IBS may have a primary inability to restore the microbial ecosystem or a secondary inability to restore gut microbiota due to host factors. Figure 4 illustrates this conceptual framework.

Putative role of infection associated shifts in microbial community post-infection IBS. A host with Clostridiales predominant microbiota type is likely to remain in eubiosis state following infection, whereas, presence of Bacteroidetes predominant community may predispose to development of long-term dysbiosis upon infection. Dysbiosis can then result in shifts in bile acid composition, cytokine and immune milieu which can affect epithelial and neuromuscular function and further perpetuate dysbiosis.
A recent study has shown that PI-IBS patients have dysbiosis. Interestingly, these microbial signatures are different from those described in IBS patients in general.50 In addition, the susceptibility to infection has been linked to microbiota composition. In poultry abattoir workers that are susceptible to infection, there were increased levels of bacteria belonging to the Bacteroidetes phylum.51 This phyla is also abundant in PI-IBS patients, but not in IBS patients that have an increased Firmicutes/Bacteroidetes ratio.52, 53,54 Conversely, travelers that develop infectious diarrhea have low levels of Bacteroidetes suggesting possible protective effect of Bacteroidetes.55 Interestingly, the incidence of PI-IBS following traveler’s diarrhea is two-fold lower than among other cases of gastroenteritis.56
Among the butyrate producing bacteria Subdoligranulum variable was found to be depleted in PI-IBS patients.54 This bacterium was found to stimulate IL-1β (pro-inflammatory cytokine) production in biopsies obtained from PI-IBS patients but not in those obtained from healthy subjects.57 This indicates a specific activity of the hosts’ immune system against a symbiotic microbe during pathological conditions, and suggests a complex and bipolar interaction between microbiota and immune responses in PI-IBS.
Intestinal permeability and immune dysregulation
A subset of PI-IBS patients has markedly increased in vivo permeability as assessed by the lactulose-mannitol excretion ratio.13–15 Longitudinal follow-up studies following bacterial enteritis reveal that increased intestinal permeability subsides over time except in those who develop PI-IBS.15 Increased intestinal permeability is considered an early event associated with low-grade immune activation. While a state of “physiological inflammation” is considered normal, several studies in patients with IBS, and PI-IBS have shown the presence of low grade intestinal immune activation.
The innate immune system including mast cells and macrophages has been reported to be altered in the intestinal mucosa of PI-IBS patients. For example, PI-IBS patients have been reported to have reduced numbers of resident CD6815 and calprotectin-positive macrophages compared to healthy subjects.58 Additionally, the number of mast cells surrounded by nerve fibers in the terminal ileum mucosa, has been shown to be increased in PI-IBS compared with healthy subjects.59 Studies have also suggested that the close interaction between nerves and mast cells correlate with abdominal bloating and pain.60, 61
Other investigations have focused on the involvement of adaptive immunity in PI-IBS. Numbers of lamina propria T lymphocytes has been demonstrated to be higher in patients with PI-IBS compared with healthy volunteers.19 Furthermore, T lymphocyte counts in both lamina propria and epithelium have been reported to be increased relative to healthy subjects.15, 58 Moreover, PI-IBS patients showed significantly increased frequency of activated/memory CD45+ T cells and decreased frequency of B cells in colonic lamina propria.62 Interestingly, it has also been reported that the frequencies of lymphocytes in the epithelial lining and lamina propria are negatively correlated with mucosal microbial diversity, suggesting an interaction between the microbiota and immune activation in PI-IBS.63 Cytolethal distending toxin B (CdtB) is produced by bacteria that cause acute gastroenteritis. Host antibodies to CdtB cross-react with vinculin. In a recent study, plasma levels of anti-CdtB and anti-vinculin antibodies were found to be significantly higher in IBS-D patients compared to IBD, healthy controls and celiac disease, suggesting that these antibodies could be utilized as relevant biomarkers in distinguishing IBS-D from other pathologic conditions in the workup of chronic diarrhea.64 Another study showed a higher prevalence of antibodies to the flagellin antigen (types A4-Fla2 and Fla-X) in IBS patients, especially those with a history of preceding gastroenteritis episode vs those without.65 However, validation of these antibodies or another biomarker has not been performed specifically in PI-IBS.
An altered cytokine expression in serum or intestinal mucosa may be seen as a result of altered activation of the immune response. Mucosal IL-1β mRNA expression has been reported to be higher in PI-IBS patients as compared to healthy subjects.59 Furthermore, an increased mucosal level of IFN-γ and a decrease level of IL-10 were reported in PI-IBS patients, suggesting involvement of the Th1 cells and Th2 cells, respectively.66 Further, the release of IL-13 (Th2 cell mediated cytokine) from mucosal biopsies was lower in PI-IBS patients compared to healthy subjects. After stimulation with the bacteria Subdoligranulum variabile or Eubacterium limosum, biopsies from PI-IBS patients resulted in higher IL-1β and lower IL-10 release as compared to biopsies from healthy subjects. This implies a possible role of altered immune response against commensal gut microbes in pathophysiology of PI-IBS.57
Entero-endocrine pathways
Enterochromaffin (EC) cells are key regulators of many gut functions, particularly, motility and sensory perception with serotonin (or 5-hydroxytrptamine; 5-HT) being a key signaling molecule. Interestingly, recent data suggest that beside host production of 5-HT, gut commensals, including spore-forming Clostridiales within the Firmicutes phylum may regulate 5-HT synthesis.67 Changes in 5-HT metabolism have been detected in patients with PI-IBS. Colonic EC cell counts were higher in patients with Campylobacter associated PI-IBS compared with healthy subjects, and EC cell counts were also positively correlated with CD3 T cell counts.15, 20 In Shigella associated PI-IBS, 5-HT containing EC cells, and Peptide YY (PYY) containing EC cells, were increased compared to healthy subjects.58 However, the role of gut hormones in PI-IBS remains controversial as another study showed that Giardia associated PI-IBS patients have lower numbers of duodenal EC cells but increased numbers of cholecystokinin (CCK) positive cells.16 It remains to be further studied if different pathogen types or different sites within the intestinal tract following same pathogen elicit variable responses to an injury.
Genetics
The Walkerton outbreak cohort examined functional gene variants in 79 genes and their association with the PI-IBS phenotype. Four variants, two in TLR9 (pattern recognition receptor), one each in IL6 (pre-inflammatory cytokine) and CDH1 (tight junction protein) were associated with PI-IBS independently of clinical risk-factors. However, these genes were not found to be significantly associated with PI-IBS after correction for the total number of SNPs.12 Another study reported association between TNFα SNP and C. jejuni PI-IBS, but these results need to be confirmed in larger studies.68
ANIMAL MODELS
Several mouse models of PI-IBS utilizing parasitic and bacterial infections mimic certain aspects of gut dysfunction and low-grade immune activation observed in patients. None of these models entirely recapitulates the full spectrum of IBS symptoms, and each of them provides certain advantages over the others. Figure 5 summarizes animal models for PI-IBS.
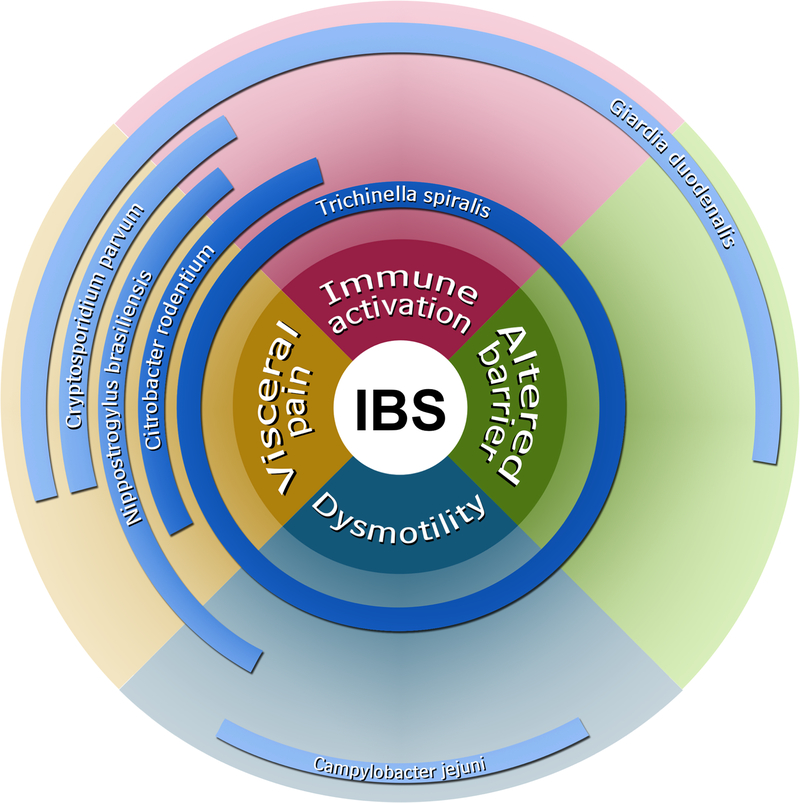
Summary of animal models for post-infection irritable bowel syndrome. Various pathogen types have ascertained unique or overlapping mechanisms associated with irritable bowel syndrome. The proximity to the center reflects the strength of association.
Trichinella spiralis model
Trichinella spiralis (Tsp) is a nematode parasite that transiently infects GI tract of rats and mice and induces chronic inflammation and gut dysfunction that is maintained after the parasite expulsion.69 The effects on the host are genetically influenced as some mouse strains, such as outbred NIH Swiss mice, display more pronounced changes in gut function than other strains.70 Tsp induces long-term neuromuscular dysfunction, characterized by muscle hypercontractility and altered release of acetylcholine, which persist for up to 42 days post-infection.71, 72 These functional changes are immune mediated and depend, among others, on T lymphocytes and M-CSF-derived macrophages.73–76 While several Th2 cytokines play an important role during the acute phase of the infection, it is the TGF-1β mediated up-regulation of cyclooxygenase 2 within the muscularis propria, which maintains the persistent neuromuscular dysfunction.74, 77 Tsp infection affects the ICC network leading to disorganized motor patters and ectopic pacemakers with occurrence of retrograde peristalsis.69, 78, 79 Visceral hyperalgesia also develops as a consequence of Tsp infection, and it can be maintained for up to 72 days, together with gut dysmotility by administration of crude Tsp antigen.69 Apart from changes within the cholinergic nerves, Tsp alters function of the sensory nerves and serotonergic system, with increased 5-HT content and release, altered expression of 5-HT(3) receptors,17 reduced serotonin reuptake transporter (SERT) expression18 and overall changes in synaptic plasticity.80 The changes with the enteric nervous system have been linked to long-lasting changes in epithelial transport, with altered secretion responses to stimulation using electrical field and secretagogues.81
Although Tsp model recapitulates most of the pathophysiological aspects seen in PI-IBS patients, its disadvantage is the fact that Tsp larvae penetrate the intestine, enter the systemic circulation and the brain. Thus at least part of the immune responses as well as accompanying gut dysfunction seen in this model, can originate from the systemic immune responses.
Nippostrogylus brasiliensis model
Nippostrogylus brasiliensis (Np) is another nematode parasite that infects rodents and induces several aspects of gut dysfunction in both rats and mice. At 30 days post-infection, Np induced changes in small intestinal migrating myoelectrical complexes that were associated with jejunal mastocytosis and enteric nerve remodeling,82 as well as altered responses to cholecystokinin stimulation.83 Np also induced changes in visceral sensitivity, including alterations in neurokinin receptors and mast cells,84, 85 but these changes did not persist at 90 days post-infection. Np infection induced long-term changes in the tetrodotoxin-resistant sodium channel in the dorsal root ganglia (DRG) neurons86 as well as altered chemosensitivity and afferent vagal signaling.87 Although this model clearly shows changes in visceral sensitivity, the effects are relatively short lasting and mainly affect small bowel.
Cryptosporidium parvum model
Cryptosporidium parvum (Cp) is an intracellular protozoan parasite affecting mainly the distal small intestine. Newborn rats infected by Cp displayed jejunal hypersensitivity to jejunal distension 120 days post-infection which was associated with increase in activated mast cells.88, 89 Treatment with octreotide, 10 days post-infection, normalized visceral hypersensitivity, immune cells numbers and changes in the structure of the enteric nervous system.89 No changes in intestinal motility or permeability in the post-infection stage were reported in this model.
Giardia duodenalis model
Giardia duodenalis (Gd) is a flagellated parasite that infects small intestine of both humans and rodents. Neonatal rats infected with Gd developed visceral hypersensitivity in both jejunum and rectum at day 50 post-infection. This was associated with changes in intestinal barrier function and increased intraepithelial lymphocytes and mast cells in the jejunum.90 Gd infection also induced short-term alterations of gut microbiota composition and long-term changes in mucosal adherence and endocytosis of bacteria, which was accompanied by up-regulation of mucosal pro-inflammatory cytokines.91 This model shows widespread changes in visceral sensitivity and microbiota-host interaction, but no effect of Gd on motility was shown.
Campylobacter jejuni model
In a rat model, Campylobacter jejuni (Cj) infection induced long-lasting changes in gut microbiota reminiscent of small intestinal bacterial overgrowth, alterations in ICC counts and changes in stool consistency,21, 23, 92 which were suggestive of intestinal dysmotility. Prophylactic treatment with rifaximin then ameliorated changes in stool consistency.93 However, this model so far has not demonstrated any direct changes in motility, permeability or visceral sensitivity.
Citrobacter rodentium model
Citrobacter rodentium (Cr) is a mouse equivalent of human enteropathogenic Escherichia coli. Mice infected with Cr and then submitted to chronic water avoidance test developed visceral hypersensitivity as assessed by measuring colonic DRG neuronal excitability and changes in multiunit afferent recordings.26 This was associated with increase in protease activity and protease inhibitors reduced neuronal excitability. Long-lasting effects of Cr infection seems to be dependent on genetic predisposition, as Th-2 predominant BALB/c mice maintained visceral hypersensitivity for longer period of time than Th-1 predominant C57Bl/6 mice.94 The advantages of this model are the use of a bacterial pathogen that closely resembles that involved in PI-IBS, and development of hyperalgesia but no changes in motility or permeability were shown so far.
TREATMENT AND CONSENSUS STATEMENTS
The RFWT performed a systematic literature search and found limited data and no existing guidelines or recommendations on specific management strategies for PI-IBS. Therefore, the RFWT adopted a Delphi process to rate quality of evidence on patient’s education regarding the condition and guidance on therapy, including the few specific treatments tested in controlled clinical trials in PI-IBS patients. Details on the Delphi process and statements assessed are provided in Supplementary material. The following statements were accepted:
Statement 1. The first step in the treatment is to educate patients about the link between intestinal infections and subsequent development of IBS.
GRADE: Strong recommendation; quality of evidence moderate. Vote: strongly agree, 64%; agree, 7%.
Statement 2. Reassurance should be provided, especially with suspected viral associated PIIBS, that symptoms are likely to improve or resolve in several patients over time.
GRADE: Moderate recommendation; quality of evidence moderate. Vote: strongly agree, 50%; agree, 21%.
Statement 3. There are no specific treatment options for PI-IBS and treatment should be guided by treatment of IBS in general (depending upon the subtype, IBS-D, IBS-M or rarely IBS-C).
GRADE: Moderate recommendation; quality of evidence moderate. Vote: strongly agree, 29%; agree, 64%.
In consideration of what reported above, an expert opinion algorithm has been proposed and reported in Figure 6 to guide the management of patients with PI-IBS.
SUMMARY
1) PI-IBS is a common condition best characterized by Rome IV symptoms occurring in around 1 in 10 subjects immediately after, and following resolution of acute infective gastroenteritis;
2) Infectious gastroenteritis to date is one of the strongest risk factors for the development of IBS. The fact that incident cases of IBS can be identified following an objective event such as infection has allowed escaping from the unfair cliché that IBS is a cryptogenic condition. The main risk factors for the development of PI-IBS include female gender, younger age, psychological factors during or prior to the acute gastroenteritis (e.g., anxiety, depression, somatization, neuroticism, negative illness beliefs) and severity of the acute episode (e.g., long duration of the acute episode);
3) Natural history studies suggest that PI-IBS symptoms decrease over time and the prognosis could be better than that of IBS, although the latter point is not substantiated by well-designed comparative studies;
4) PI-IBS provides a unique model to study the initial stages of IBS development and investigate the mechanisms that maintain altered gut physiology and symptoms when the infection has subsided, and the acute inflammatory response has weaned. The pathophysiology of PI-IBS is multifactorial (e.g., dysmotility, visceral hypersensitivity, dysbiosis, immune activation, abnormal entero-endocrine signaling, genetic factors) and to date studied only in subsets of small sample size groups of patients and at different time points after infection;
5) Animal models may lead to further identification of relevant pathophysiological factors and development of effective therapies; these include the experimental models of post-infection evoked by Trichinella spiralis, Nippostrongylus brasiliensis, Cryptosporidium parvum, Giardia duodenalis, Campylobacter jejuni and Citrobacter rodentium.
6) Although limited data exist on specific treatments guidelines for PI-IBS exist, a therapeutic algorithm and consensus has been provided in the present paper.
FUTURE CONSIDERATIONS
While PI-IBS has received increasing attention, these studies are challenging. The condition is best studied prospectively, and only large outbreaks permit to recruit substantive numbers of patients. Future mechanistic studies should focus on pathogen-specific subgroups that could lead to identification of specific pathophysiological mechanisms. Development of biomarkers that can be used in acute stages for prevention of PI-IBS development and chronically for diagnosis and specific targeting of the PI-IBS subgroup would help prevent development and personalize treatment of PI-IBS. Finally, dietary and pharmacologic clinical trials are needed specific to this subgroup based upon the unique pathophysiological characteristics. For example, enrichment with certain taxa in the fecal microbiota of IBS was associated with increased response to a low-FODMAP diet.95, 96 Also, low-grade inflammation has been shown to predict the response to mesalazine in patients with PI-IBS.97 The role of central dysfunction in modulation peripheral responses and its targeting should be examined in PI-IBS. This subset of IBS provides a unique opportunity to determine mechanisms and design treatment strategies that can be applied more broadly to IBS and other FGIDs in future.
Supplementary Material
Fig_1
Fig_legend
Materials
Acknowledgements:
We acknowledge Mr. Jerry Schoendorf for assistance with the illustrations and Ms. Lori Anderson for administrative assistance.
Funding: This work is supported by Rome Foundation, NC, USA. In addition, the authors would like to acknowledge the following funding sources: Italian Ministry of Education, University and Research; Fondazione del Monte di Bologna e Ravenna and IMA (GB) and NIDDK K23 103911 (MG).
Abbreviations:
| IBS | irritable bowel syndrome |
| PI-IBS | post-infection irritable bowel syndrome |
| FGIDs | functional gastrointestinal disorders |
| SNPs | single nucleotide polymorphisms |
| 5HT | serotonin |
| EC | enterochromaffin |
| ICC | interstitial cells of Cajal |
| PCR | polymerase chain reaction |
| IBS-C | IBS with constipation |
| IBS-D | IBS with diarrhea |
| IBS-M | IBS mixed |
| IBD | inflammatory bowel disease |
| PI-FD | post-infection functional dyspepsia |
| HADS | hospital anxiety and depression scale |
| Tsp | Trichinella spiralis |
| Np | Nippostrogylus brasiliensis |
| Cp | Cryptosporidium parvum |
| Gd | Giardia duodenalis |
| Cj | Campylobacter jejuni |
| Cr | Citrobacter rodentium |
| MCC | migrating myoelectrical complexes |
| DRG | dorsal root ganglia |
| FODMAPs | fermentable oligo-, di-, monosaccharides and polyols |
| FMT | fecal microbial transplantation |
| SERT | serotonin reuptake transporter |
Footnotes
Conflicts of interest: The authors disclose the following competing interests: GB has been a consultant or served on the advisory board or received speaker’s bureau fees and/or research support from Danone, Yakult, Ironwood, Malesci, Nestlè, Noos, Synergy, Alfa Wassermann, Almirall and Shire; MG has served on the advisory board or received research support from Takeda, DongA, Ironwood and Napo; PB has been consultant and/or served on advisory board or received research support from Nestle, Allergan, Lupin Pharma, IM Health Science and Innovate Biopharma; MC acts as consultant for Allergan and Kiowa Kirin and has been speaker for Shire and Menarini; UCG has no conflicts to disclose; LÖ has served on the advisory board of Genetic Analyses and received research support from Danone and AstraZeneca. MRS has no conflicts to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
Full text links
Read article at publisher's site: https://doi.org/10.1053/j.gastro.2018.07.011
Read article for free, from open access legal sources, via Unpaywall:
http://TechnoRep.tmf.bg.ac.rs/bitstream/123456789/4322/1/4319.pdf
Citations & impact
Impact metrics
Article citations
Long COVID and gut candidiasis: What is the existing relationship?
World J Gastroenterol, 30(37):4104-4114, 01 Oct 2024
Cited by: 0 articles | PMID: 39474404 | PMCID: PMC11514539
Review Free full text in Europe PMC
[Research progress of metabolomics in children with irritable bowel syndrome].
Zhongguo Dang Dai Er Ke Za Zhi, 26(9):989-994, 01 Sep 2024
Cited by: 0 articles | PMID: 39267517 | PMCID: PMC11404471
Review Free full text in Europe PMC
Leaky Gut Syndrome: Myths and Management.
Gastroenterol Hepatol (N Y), 20(5):264-272, 01 Aug 2024
Cited by: 1 article | PMID: 39193076 | PMCID: PMC11345991
Sex Differences in Visceral Pain and Comorbidities: Clinical Outcomes, Preclinical Models, and Cellular and Molecular Mechanisms.
Cells, 13(10):834, 14 May 2024
Cited by: 1 article | PMID: 38786056 | PMCID: PMC11119472
Review Free full text in Europe PMC
Epidemiology of Disorders of the Gut-Brain Interaction: An Appraisal of the Rome IV Criteria and Beyond.
Gut Liver, 18(4):578-592, 29 Apr 2024
Cited by: 0 articles | PMID: 38680110 | PMCID: PMC11249947
Review Free full text in Europe PMC
Go to all (88) article citations
Other citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
[Post-infectious irritable bowel syndrome].
Tunis Med, 90(3):205-213, 01 Mar 2012
Cited by: 2 articles | PMID: 22481191
Review
Postinfectious irritable bowel syndrome.
J Pediatr Gastroenterol Nutr, 48 Suppl 2:S95-7, 01 Apr 2009
Cited by: 43 articles | PMID: 19300138
Review
Post-infectious irritable bowel syndrome.
World J Gastroenterol, 15(29):3591-3596, 01 Aug 2009
Cited by: 87 articles | PMID: 19653335 | PMCID: PMC2721231
Review Free full text in Europe PMC
Emerging role of the gut microbiome in post-infectious irritable bowel syndrome: A literature review.
World J Gastroenterol, 29(21):3241-3256, 01 Jun 2023
Cited by: 12 articles | PMID: 37377581 | PMCID: PMC10292139
Review Free full text in Europe PMC
Funding
Funders who supported this work.
Fondazione del Monte di Bologna e Ravenna (1)
Grant ID: K23 103911
Institute of Management Accountants (1)
Grant ID: K23 103911
Ministero dell’Istruzione, dell’Università e della Ricerca (1)
Grant ID: K23 103911
NIDDK NIH HHS (1)
Grant ID: K23 DK103911
National Institute of Diabetes and Digestive and Kidney Diseases (1)
Grant ID: K23 103911