Abstract
Purpose of review
4'-Ethynyl-2-fluoro-2'-deoxyadenosine (EFdA) is a nucleoside reverse transcriptase inhibitor (NRTI) with a novel mechanism of action, unique structure, and amongst NRTIs, unparalleled anti-HIV-1 activity. We will summarize its structure and function, antiviral activity, resistance profile, and potential as an antiretroviral for use in the treatment and preexposure prophylaxis of HIV-1 infection.Recent findings
EFdA is active against wild-type (EC50 as low as 50 pmol/l) and most highly NRTI-resistant viruses. The active metabolite, EFdA-triphosphate, has been shown to have a prolonged intracellular half-life in human and rhesus (Rh) blood cells. As a result, single drug doses tested in simian immunodeficiency virus mac251-infected Rh macaques and HIV-1-infected individuals exhibited robust antiviral activity of 7-10 days duration. Preclinical studies of EFdA as preexposure prophylaxis in the Rh macaque/simian/human immunodeficiency virus low-dose intrarectal challenge model have shown complete protection when given in clinically relevant doses.Summary
EFdA is a novel antiretroviral with activity against both wild-type and NRTI-resistant viruses. As a result of the prolonged intracellular half-life of its active moiety, it is amenable to flexibility in dosing of at least daily to weekly and perhaps longer.Free full text

EFdA (4′-ethynyl-2-fluoro-2′-deoxyadenosine, MK-8591): A Novel HIV-1 Reverse Transcriptase Translocation Inhibitor
Abstract
Purpose of the review
4′-ethynyl-2-fluoro-2′-deoxyadenosine (EFdA) is a nucleoside inhibitor of reverse transcriptase (NRTI) with a novel mechanism of action, unique structure, and amongst NRTIs, unparalleled anti-HIV-1 activity. We will summarize its structure and function, antiviral activity, resistance profile, and potential as an antiretroviral for use in the treatment and pre-exposure prophylaxis (PrEP) of HIV-1 infection.
Recent findings
EFdA is active against wild type (EC50 as low as 50 pM) and most highly NRTI resistant viruses. The active metabolite, EFdA-triphosphate (TP), has been shown to have a prolonged intracellular half-life in human and rhesus blood cells. As a result, single drug doses tested in SIVmac251-infected rhesus macaques and HIV-1-infected individuals exhibited robust antiviral activity of 7 to 10 days duration. Preclinical studies of EFdA as PrEP in the rhesus macaque/SHIV low-dose intrarectal challenge model has shown complete protection when given in clinically relevant doses.
Summary
EFdA is a novel antiretroviral with activity against both wild type and NRTI-resistant viruses. As a result of the prolonged intracellular half-life of its active moiety, it is amenable to flexibility in dosing of at least daily to weekly and perhaps longer.
Introduction
A series of 4′-substituted nucleoside reverse transcriptase inhibitors (NRTIs) was synthesized and included compounds with strong antiviral properties (1, 2). One, 4′-ethynyl-2-fluoro-2′-deoxyadenosine (EFdA) (Figure 1) was demonstrated to have exceptional antiviral properties (1–4). EFdA inhibits HIV-1 replication in activated peripheral blood mononuclear cells at the picomolar range, with an EC50 in MT4 cells against HIV-1IIIb of 73 pM, 98 pm against HIV-2EHO (3) and 50 pM to HIVNL4-3 (5). When simultaneously compared to a panel of other NRTIs, the EC50 of EFdA was 3 nM compared to 180 nM for AZT, 1,210 nM for 3TC, 370 nM for FTC, 14 nM for tenofovir (TFV) (6). Moreover, EFdA retains high levels of activity against multidrug-resistant HIV strains and clinical isolates, and with impressive selectivity indices due to favorable cytotoxicity (2, 3). These notable properties are attributed to its distinctive structural characteristics and mechanism of action. EFdA represents the first nucleoside reverse transcriptase translocation inhibitor (NRTTI). It is amenable to weekly dosing and has demonstrated activity in preventing SHIV infection in rhesus macaques. For these reasons, it is a promising new antiretroviral in preclinical and clinical development.
EFdA: Structure and Function
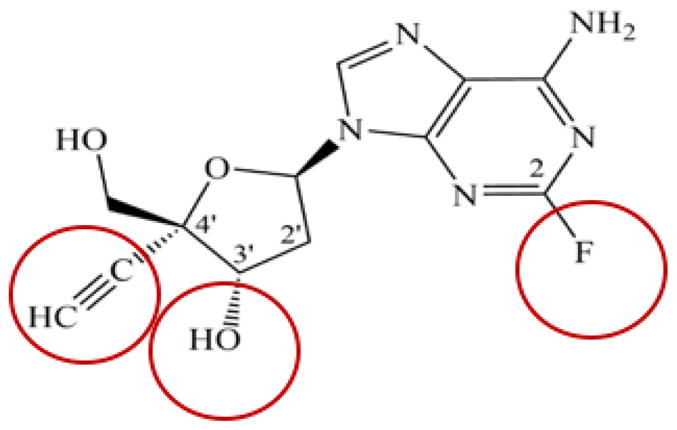
EFdA has three structural attributes that make it unique among NRTIs: a) it possesses a 3′-OH group, which is typically absent in anti-HIV chain terminating NRTIs, and thus, resembles the natural substrates, more so than other NRTIs; b) it has a 4′-ethynyl group (4′-E) on the pseudo-sugar ring, and c) it has a 2-fluoro (2-F) on the adenine base ring. These features are circled in Figure 1.
The structural characteristics of EFdA contribute to its high potency by affecting multiple factors, including a) activation by cellular kinases following cellular uptake; b) metabolism and degradation to inactive species; c) binding interactions with HIV RT; and d) mechanism of action.
Studies have shown that the critical first phosphorylation step to EFdA-monophosphate, is primarily accomplished by 2′-deoxycytidine kinase (dCK) (3). The apparently efficient activation of EFdA may be affected by its similarity to deoxynucleoside triphosphates (dNTPs), which are the natural substrates of cellular kinases. Complete conversion of EFdA to its active metabolite EFdA-triphosphate (EFdA-TP) used by RT, is reported to be efficient (7).
Unlike other adenosine-based nucleosides, EFdA’s fluorinated adenosine ring is remarkably resistant to oxidation by adenosine deamination (3, 8). This unusual stability is primarily due to the presence of the 2-F substitution (1, 8), which alters the electronic distribution in EFdA’s adenine ring, which decreases susceptibility to hydrolysis, resulting in a more than 100-fold increase in overall potency (3). In addition to the key role of the 2-F group in EFdA’s stability, the 4′-E group also contributes to the decreased degradation of EFdA, likely by sterically decreasing its binding at the catalytic site of adenosine deaminase (8). The stability to degradation imparted by the 2-F and 4′-E substitutions is likely the key factor leading to its suitability as a long-acting antiviral (9, 10).
Crystallographic studies of various DNA polymerization reaction intermediates of RT inhibition by EFdA-TP elucidated the structural basis of its strong binding affinity with RT (11). Specifically, the structure of HIV-1 RT in complex with its DNA substrate and an incoming EFdA-TP inhibitor molecule shows that the potency of EFdA stems from hydrophobic interactions of its 4′-E at a previously unexploited conserved hydrophobic pocket in the polymerase active site (Figure 2). The presence of a 3′-OH in EFdA-TP also contributes towards tighter binding through additional polar interactions with the β-phosphate and conserved peptide backbone atoms. All of these interactions are unique among NRTIs used in antiretroviral therapy.
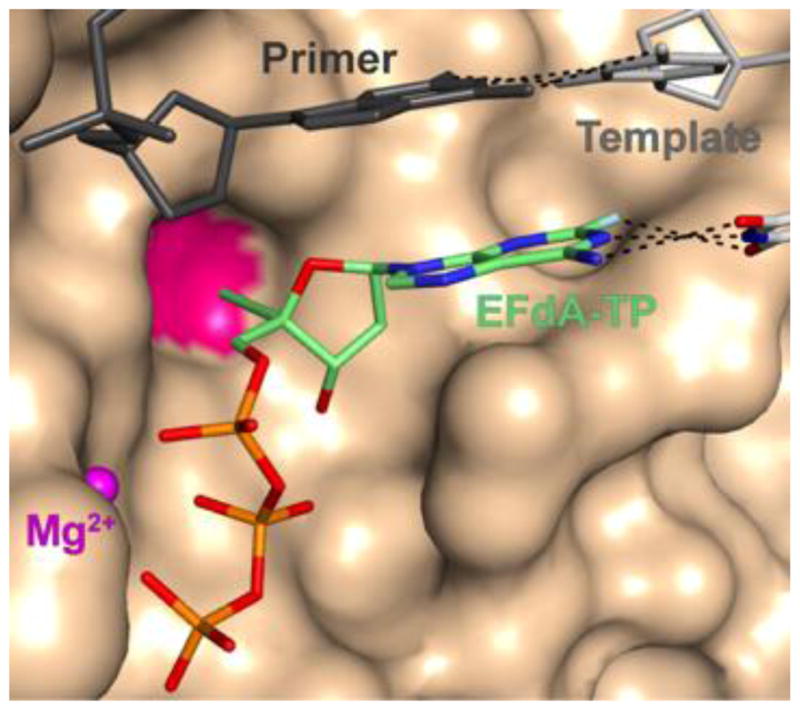
Interactions of EFdA-triphosphate (EFdA-TP) at the dNTP binding site of RT shown as Van der Waals surface. The 4′-E group of EFdA-TP interacts with RT residues at a conserved hydrophobic pocket (red) contributing to strong inhibitor binding prior to catalysis. After catalysis and incorporation of EFdA-MP to the primer (primer·EFdA-MP; not shown) the 4′-E of EFdA-MP may decrease RT translocation, a step required for vacating the active site for binding of the next incoming dNTP and further DNA synthesis. This decrease in translocation would manifest itself as immediate chain termination (Figures 2 and and33 was prepared using PDB ID 5J2M crystal structure and PyMOL).
The distinctive structural characteristics of EFdA also determine its biochemical mechanism of action, which is the most distinguishing feature of this inhibitor. Unlike other NRTIs, EFdA blocks RT by multiple mechanisms. RT uses EFdA-TP and other NRTI-TP in the same way it uses its dNTP canonical substrates, for DNA synthesis. However, traditional NRTIs lack a 3′-OH group, which is required for DNA polymerization, and thus act as immediate (obligate) chain terminators after they are incorporated into the nascent DNA chain by RT. Surprisingly, even though EFdA retains a 3′-OH group, it often acts as an immediate chain terminator, preventing addition of further nucleotides. This is because of the strong favorable interactions of the inhibitor’s 4′-E at the dNTP binding site (Figure 2), even after its incorporation into the primer (5), resulting in decreased translocation of the extended primer that hampers binding and incorporation of subsequent nucleotides. Therefore, EFdA is the first inhibitor of reverse transcriptase to be identified as an NRTTI, a nucleoside reverse transcriptase translocation inhibitor. Interestingly, depending on the nucleic acid substrate sequence, EFdA-TP may occasionally function as a delayed chain terminator, allowing incorporation of additional dNTP before blocking DNA synthesis. In this case, subsequent primer extension is prevented by steric clashes between the 4′E in the EFdA-containing primer and residues of the upstream “primer grip” region of RT (Figure 3), that likely result in dissociation of the primer and suppression of further DNA synthesis. An additional inhibition mechanism involves efficient misincorporation of EFdA by RT, leading to mismatched primers that cannot be extended or removed by excision.
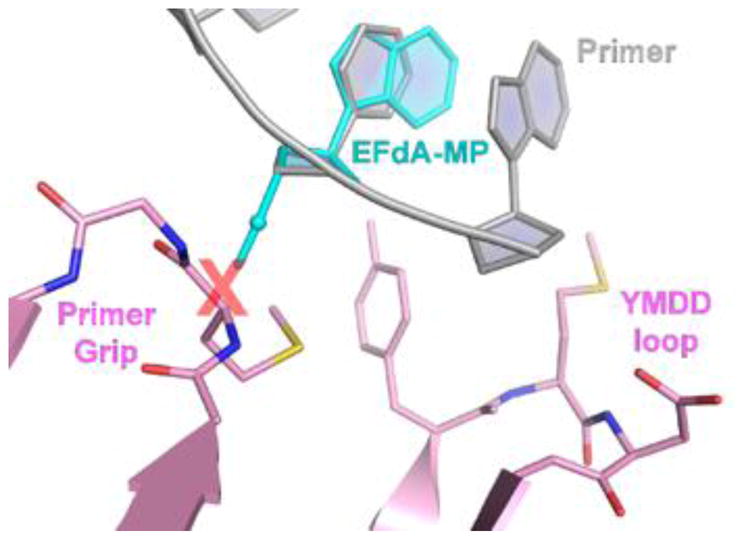
Structural basis of RT inhibition by EFdA acting as a delayed chain terminator. In some cases, after incorporation of EFdA-monophosphate (EFdA-MP, in cyan) into the primer strand (gray sticks), an additional nucleotide can be added. Upon translocation of the extended primer·EFdA-MP·dN-MP substrate, the 4′-E would be expected to sterically clash (red X) with residues of the upstream “primer grip” region of RT, leading to dissociation of the nucleic acid and suppression of further DNA synthesis (Molecular model prepared by superposition of EFdA-MP with the equivalent nucleotide that is present in PDB ID 5J2M crystal structure, as described in (11).
As mentioned above, EFdA-TP has high affinity for the RT binding site (12), and the context of template sequence can affect the relative contribution of each inhibition mechanism, and also the relative binding affinity of EFdA-TP (13). Preferential binding of EFdA-TP at various sites may also explain the reported surprising lack of antagonism between adenosine analogs EFdA and TFV (6, 13).
Resistance to EFdA
Mild resistance to 4′-E antivirals was reported in in vitro selection studies that used EdA, the non-fluorinated analog of EFdA. After 58 passages an EdA-resistant virus emerged carrying the I142V/T165R/M184V substitutions (3). The first appearing mutation, M184V, imparted a 7.5-fold reduction in EFdA susceptibility, whereas subsequent addition of the I142V and T165R mutations resulted in the highest level of reduced susceptibility to EFdA, 22-fold. Of note, neither I142V nor T165R was associated with reduced susceptibility to EFdA, confirming that M184V is indeed the main determinant of reduced susceptibility to EFdA. The ability of M184V/I to confirm only low resistance to EFdA was also confirmed by other investigators (14). Finally, it was also shown that a mixture of 11 highly multi-drug-resistant clinical HIV-1 isolates developed resistance far more rapidly against other NRTIs including 3TC, FTC, and TDF than against EFdA, and that EFdA remained very active against TDF- and EFdA-selected variants (15).
In general, NRTI resistance can be mediated by two mechanisms- a) excision by pyrophosphorolysis, a common underlying mechanism of resistance to drugs such as AZT, d4T, and ABC (16) or b) mutation(s) at the dNTP binding site resulting in discrimination against the incorporation of the NRTI-TP, as is the case for 3TC and FTC (17). While EFdA can be excised (5) it can be re-incorporated very efficiently, and as a result the excision reaction does not significantly affect EFdA susceptibility. However, the active site M184V mutation that confers high level resistance to both 3TC and FTC due to substantial steric hindrance-based interactions for both 3TC-TP and FTC-TP incorporation results in relatively low steric-based interactions for EFdA-TP incorporation resulting in low level, approximately 2 to 10-fold reduced susceptibility. (3). Importantly, in a small study with SIV-infected macaques, it was shown that EFdA was fully effective in maintaining suppression of M184V virus throughout the drug treatment period (18). Recently, it was shown that addition of the clinically relevant NNRTI-associated E138K mutation, which has been shown to compensate for fitness loss due to M184V/I and could theoretically increase resistance to EFdA, did not significantly affect the antiviral activity of EFdA (14). Moreover, it is not surprising that EFdA retains activity against a wide range of NRTI-resistant mutants – both clones generated by site directed mutagenesis as well as clinical isolates, including the very highly resistant to AZT M41L/T69-insertion/T215Y mutants (3). A panel of clinical isolates highly resistant to other NRTIs, including 3TC, were shown to be either susceptible or only mildly resistant to EFdA (3).
It has been shown that HIV-1 variants containing K65R, a resistance mutation selected for by TFV is hypersusceptible to EFdA (19). Viruses containing K65R are 2.1-fold less susceptible to TFV, but 2.5-fold more susceptible to EFdA when compared to wild type HIV-1. The mechanism is thought due to reduced excision of chain terminating EFdA-monophosphate (MP). This interaction would strongly support the use of EFdA with tenofovir disoproxil fumarate (TDF) or tenofovir alafenamide (TAF) in combination antiretroviral therapy regimens or for treatment of patients that fail TFV-based therapies.
To date, there are no data on EFdA resistance in HIV-1 infected individuals treated with EFdA. However, 2 rhesus macaques were treated with 5 mg/kg 3TC monotherapy for 14 days to select for the emergence of M184V viral variants. They were treated with 2 doses of EFdA at 30 mg/kg 7 days apart and a 2 log10 suppression of viremia was observed, demonstrating the in vivo activity of EFdA against viruses that are highly resistant to both 3TC and FTC (9).
EFdA for the treatment of HIV-1 infection
There is a paucity of clinical trial data as EFdA remains in early clinical development. However, the main toxicity of NRTIs is related to the interaction between NRTIs and mitochondrial DNA polymerase g (pol g) (20–22) When NRTI-monophosphates are incorporated into mitochondrial DNA by pol g, an associated myopathy, lipodystrophy, lactic acidosis or liver failure may emerge. EFdA-TP was incorporated into mitochondrial DNA polymerase g 760-fold more slowly and with 6-fold less affinity than dATP (23). This would suggest a low potential for mitochondrial toxicity during EFdA dosing. That said, the drug, currently in clinical development by Merck Laboratories, and referred to as MK-8591 is in early phase 2 clinical development and a full safety profile will require identification.
EFdA efficiently suppresses HIV viremia in humanized mice and macaques (18, 24–26) and has favorable pharmacokinetic properties (26, 27). It can be efficiently formulated by itself or in combination with other antivirals as a vaginal microbicide film to potentially prevent HIV-1 sexual transmission (28, 29). In terms of use in humans, the drug has been given to limited numbers of HIV-1 infected individuals. After documenting prolonged intracellular levels of the active metabolite, EFdA-TP, in both human and rhesus cells, Friedman and colleagues administered a single 10mg dose of EFdA to 6 HIV-1 infected individuals and monitored both antiviral activity and pharmacokinetics (30). The study subjects were all male, had mean plasma HIV-1 RNA levels of 137,400 copies/mL (range: 10,200 to 470,000) and mean CD4+ T cell counts of 582 cells/mm3 (range: 365–646). At day 7 the mean viral load reduction was 1.67 log10 (range: −1.97 to 1.31) and by day 10 fell further by 1.78 log10. After 168 hours (7 days) the plasma levels of EFdA were below detection however intracellular levels of the active metabolite, EFdA-TP, were 1.01 pmol/106 peripheral blood mononuclear cells (PBMC) (range: 0.77–1.4) and above the target concentration of 0.53 pmol/106 PBMC. The drug was well tolerated with 6 reports of headache among the 15 adverse events reported.
More recently a dose ranging study of EFdA was performed in 30 HIV-1 infected individuals. In this open-label Phase 1B study, participants were given a single administration of one of 5 doses of EFdA; 0.5 mg, 1 mg, 2 mg, 10 mg, and 30 mg. Adverse events, antiviral activity, and pharmacokinetics and pharmacodynamics were determined (10).
Twenty-seven of 30 treated individuals experienced a total of 60 adverse events. No relationship between dose and number or intensity of adverse events was seen. There were no serious adverse events reported and all adverse events were mild to moderate. Headache, upper respiratory infection, diarrhea and vomiting were the most common adverse events reported with headache, diarrhea and eczema the most common adverse events that were assigned as related to EFdA therapy. There were 9 reports of headache, 2 of diarrhea and 2 of eczema. There were no significant laboratory, electrocardiographic or vital sign changes reported.
The area under the curve of EFdA concentrations over 168 hours was dose proportional. Intracellular levels of EFdA-TP were 0.116, 0.164, 0.188, .0.983, and 4.83 pmol/106 cells in the 0.5 mg, 1 mg, 2mg, 10 mg, and 30 mg dosing groups respectively.
There was a clear association between dose and reduction in plasma viral load (R2=0.228, p=0.005). However, robust antiviral activity was demonstrated at all doses. Viral load reductions were −1.18, −1.28, −1.32, −1.64, and −1.57 in the 0.5 mg, 1 mg, 2mg, 10 mg, and 30 mg dosing groups respectively. In summary, EFdA given as one dose was well tolerated. The active triphosphate displayed an intracellular half-life of 78.5 to 128 hours, and robust antiviral activity was clearly documented in doses as low as 0.5 mg. A phase 2b study of a combination regimen of MK-8591, the investigational NNRTI doravirine, and lamivudine currently is enrolling (DRIVE2SIMPLIFY, NCT03272347).
EFdA for the prevention of HIV-1 infection
Given the long intracellular half-life of EFdA-TP, apparent excellent penetration of drug in tissue (31), and the potency and resistance profile of EFdA, it is an attractive candidate for use as pre-exposure prophylaxis (PrEP). EFdA has been tested in the SHIV/rhesus macaque model to assess its ability to prevent SHIV infection after low dose intrarectal challenge. Once weekly oral doses of 3.9 mg/kg or greater in SIV-infected rhesus macaques (RM) resulted in robust antiviral activity with reductions of 1.8 log10 copies/ml in plasma levels of SIV RNA. 2 groups of 8 male RM were given either 5 mL/kg of 10% Tween80 with (treated) or without (placebo) 3.9 mg/kg EFdA by oral gavage on day 0, day 7 and weekly thereafter for a maximum of 14 doses or until SHIV infection was confirmed. All animals were challenged intrarectally with 50 TCID50 of SHIVC109P3 (32), a viral stock derived from the third passage in RM of the molecular clone SHIVC109F.PB4, containing an HIV Env derived from a newly HIV-infected Zambian individual. Challenges occurred on day 6 and weekly thereafter for a maximum of 12 challenges or until infection was confirmed. Prior to weekly challenge, blood was drawn to determine infection status and drug levels. Infection was confirmed by real-time RT PCR amplification of viral gag sequences in plasma. Proviral DNA was measured by PCR and virus-specific antibody responses were assessed. Intracellular levels of MK-8591-triphosphate (TP) were measured. All placebo animals became infected after 1–4 challenges (median 1, mean 2). All treated animals remained uninfected after 12 challenges and were followed through week 24 without evidence of infection as determined by the absence of plasma viremia, proviral DNA and seroconversion. EFdA-treated macaques had a 41.5-fold lower risk of infection (95% C.I 7.3, 237.9) compared with placebo macaques (p<0.0001, log-rank test).(33) The mean trough concentration of the active EFdA-TP at the time of challenge was 0.81 pmol/106 PBMC and compares favorably with levels achieved by a weekly oral dose of 10mg in HIV-1-infected humans (30). The remaining 8 animals have been treated with 6 weekly oral doses of 1.3, 0.43, and 0.1 mg/kg EFdA prior to and after 4 weekly IR challenges of 50TCID50 SHIV109CP3 (34). All animals were protected at the 2 higher doses whereas 6 of 8 remained protected at the 0.1mg/kg dosing level. Estimated levels of EFdA-TP at the time of challenge at this lowest dosing levels is 24 fmol/106 PBMC, levels that are theoretically achievable in humans at weekly doses of less than 250 mg weekly or 10 mg daily, consistent with EFdA utility in extended duration prophylaxis against HIV infection. Current development plans for EFdA as prevention include expanding the safety data base in healthy uninfected individuals, identifying a target concentration of MK-8591-TP for prevention, and exploring novel delivery methods such as implantable devices capable of delivering therapeutic levels of drug over a period of months.
Conclusions
EFdA is a novel NRTTI with potent antiviral activity against wild type and drug resistant HIV-1 variants. Its unique pharmacokinetic profile allows for weekly dosing. The drug is being developed for both HIV-1 treatment and prevention for use as PrEP.
Future directions include larger clinical trials to assess safety, tolerability, and antiviral activity in cART as well as its potential in HIV-1 uninfected individuals as PrEP.
Acknowledgments
Funding: This work was supported by funding received from the National Institutes of Health: R01AI076119, R01AI121315, R01GM118012, U54GM103368.
Footnotes
Acknowledgements: none
Conflicts of interest: M.M. is a paid consultant to Merck Research Laboratories. His research program receives grant support from Merck Laboratories. Merck, Sharp, and Dohme has also provided him with honoraria for serving as a plenary speaker at MSD organized symposia. He also receives grant support from Gilead Sciences and GlaxoSmithKline/ViiV. He is also a member of the Gilead Speakers Bureau.
S.G.S. has served as a paid consultant to Merck Research Laboratories.
References
Full text links
Read article at publisher's site: https://doi.org/10.1097/coh.0000000000000467
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc6449048?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1097/coh.0000000000000467
Article citations
Intracellular islatravir-triphosphate half-life supports extended dosing intervals.
Antimicrob Agents Chemother, 68(9):e0045824, 06 Aug 2024
Cited by: 0 articles | PMID: 39105584 | PMCID: PMC11382622
4'-Ethynyl-2'-Deoxycytidine (EdC) Preferentially Targets Lymphoma and Leukemia Subtypes by Inducing Replicative Stress.
Mol Cancer Ther, 23(5):683-699, 01 May 2024
Cited by: 0 articles | PMID: 38064712 | PMCID: PMC11286238
Synthesis of Fluorinated Nucleosides/Nucleotides and Their Antiviral Properties.
Molecules, 29(10):2390, 19 May 2024
Cited by: 0 articles | PMID: 38792251 | PMCID: PMC11124531
Review Free full text in Europe PMC
Polymer Delivery Systems for Long-Acting Antiretroviral Drugs.
Pharmaceutics, 16(2):183, 28 Jan 2024
Cited by: 1 article | PMID: 38399244 | PMCID: PMC10892262
Review Free full text in Europe PMC
Pharmacokinetic Modeling to Guide Preclinical Development of an Islatravir-Eluting Reservoir-Style Biodegradable Implant for Long-Acting HIV PrEP.
Pharmaceutics, 16(2):201, 30 Jan 2024
Cited by: 0 articles | PMID: 38399255 | PMCID: PMC10893066
Go to all (46) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Protein structures in PDBe
-
(2 citations)
PDBe - 5J2MView structure
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The High Genetic Barrier of EFdA/MK-8591 Stems from Strong Interactions with the Active Site of Drug-Resistant HIV-1 Reverse Transcriptase.
Cell Chem Biol, 25(10):1268-1278.e3, 30 Aug 2018
Cited by: 16 articles | PMID: 30174310 | PMCID: PMC6261781
MK-8591 (4'-Ethynyl-2-Fluoro-2'-Deoxyadenosine) Exhibits Potent Activity against HIV-2 Isolates and Drug-Resistant HIV-2 Mutants in Culture.
Antimicrob Agents Chemother, 61(8):e00744-17, 25 Jul 2017
Cited by: 15 articles | PMID: 28559249 | PMCID: PMC5527656
Probing the molecular mechanism of action of the HIV-1 reverse transcriptase inhibitor 4'-ethynyl-2-fluoro-2'-deoxyadenosine (EFdA) using pre-steady-state kinetics.
Antiviral Res, 106:1-4, 12 Mar 2014
Cited by: 13 articles | PMID: 24632447 | PMCID: PMC4020981
Islatravir for the treatment and prevention of infection with the human immunodeficiency virus type 1.
Curr Opin HIV AIDS, 15(1):27-32, 01 Jan 2020
Cited by: 25 articles | PMID: 31658118
Review
Funding
Funders who supported this work.
NIAID NIH HHS (3)
Grant ID: R01 AI076119
Grant ID: R01 AI121315
Grant ID: R37 AI076119
NIGMS NIH HHS (2)
Grant ID: U54 GM103368
Grant ID: R01 GM118012