Abstract
Free full text

Evaluation of Combinations of 4′-Ethynyl-2-Fluoro-2′-Deoxyadenosine with Clinically Used Antiretroviral Drugs
Abstract
Drug combination studies of 4′-ethynyl-2-fluoro-2′-deoxyadenosine (EFdA) with FDA-approved drugs were evaluated by two different methods, MacSynergy II and CalcuSyn. Most of the combinations, including the combination of the two adenosine analogs EFdA and tenofovir, were essentially additive, without substantial antagonism or synergism. The combination of EFdA and rilpivirine showed apparent synergism. These studies provide information that may be useful for the design of EFdA combination regimens for initial and salvage therapy assessment.
TEXT
Combination antiretroviral therapies provide durable viral suppression and constitute the standard of care for HIV infection (http://www.aidsinfo.nih.gov/guidelines/) (1). For example, the combination of tenofovir disoproxil fumarate (TDF) and emtricitabine (FTC) (Truvada) is one of the preferred regimens for treatment of HIV-1 infection (2, 3). In vitro studies have shown that tenofovir (TFV) and FTC have synergistic antiretroviral activity (4, 5).
The investigational nucleoside reverse transcriptase inhibitor (NRTI), 4′-ethynyl-2-fluoro-2′-deoxyadenosine (EFdA) is presently under preclinical evaluation. Unlike other NRTIs used in the treatment of HIV infection, EFdA retains the 3′-hydroxyl moiety. It also contains a 2-fluoro group on the adenine base and a 4′-ethynyl group on the deoxyribose ring. Although EFdA is an adenosine analog, its activation is initiated by phosphorylation by 2′-deoxycytidine kinase (dCK), and the drug is highly resistant to degradation by adenosine deaminase (ADA) (6). EFdA shows exceptional antiretroviral activity in vitro (6–8) and in vivo (9, 10) as well as a favorable cross-resistance profile with current reverse transcriptase inhibitors (RTIs) used in the clinic.
Studies on potential interactions between EFdA and other antiretroviral drugs can provide information that could be useful in the development of combinatorial therapeutic strategies. The present study evaluates anti-HIV efficacy in combinations of EFdA with representative FDA-approved RTIs in vitro.
We first determined the antiviral potencies of five NRTIs (zidovudine [AZT], lamivudine [3TC], FTC, TDF, and EFdA) and three nonnucleoside RTIs (NNRTIs; efavirenz [EFV], etravirine [ETR], and rilpivirine [RPV]) against HIV-1NL4-3 in order to obtain an optimal range of drug concentrations for use in combination assay analyses. As previously demonstrated (6, 7), EFdA inhibited HIV-1 replication several orders of magnitude more efficiently than other currently approved NRTIs (Table 1). In the same cell-based assays, we evaluated antiretroviral activity of EFdA in combination with the FDA-approved RTIs. To obtain more comprehensive evaluations of drug combinations and to reduce analysis bias, we use two algorithms provided by the software packages MacSynergy II (version 1.0; Ann Arbor, MI) and CalcuSyn (Biosoft, Ferguson, MO), which are based on the Bliss independence model (11, 12) and the median effect principle (13), respectively. Quantitative differences in data analyses by the two algorithms used by the MacSynergy II and CalcuSyn programs are not uncommon (14). In the present work, drug interactions were considered significant only if detected by both computational approaches.
Table 1
Antiviral activity of HIV-1 inhibitors
| Compound class and name | EC50 (nM) for anti-HIV-1 activitya |
|---|---|
| NRTI | |
    AZT AZT | 180 ± 60 |
    3TC 3TC | 1,210 ± 240 |
    FTC FTC | 370 ± 70 |
    TDF TDF | 14 ± 2 |
    EFdA EFdA | 3 ± 1 |
| NNRTI | |
    EFV EFV | 1.6 ± 0.4 |
    ETR ETR | 1 ± 0.1 |
    RPV RPV | 0.4 ± 0.1 |
Most drugs tested, including the adenosine analog TDF, showed little or no drug interactions in combination with EFdA (Fig. 1 and Table 2). Data analysis with CalcuSyn suggested that the combinations of both EFdA and 3TC (EFdA-3TC) and EFdA-FTC are moderately antagonistic (Fig. 1 and Table 2). Using MacSynergy, the EFdA-3TC combination was assessed as minor antagonism, whereas the EFdA-FTC combination was considered additive; however, its value (−23.4 μM2%) was very close to minor antagonism (−25 μM2%). The observed borderline antagonism may arise from competition at the first and rate-limiting phosphorylation step as EFdA, 3TC, and FTC are all initially activated by 2′-deoxycytidine kinase (6, 15, 16). Small differences in the effect of 3TC versus FTC may arise from the longer half-life of FTC. In contrast, the combination of EFdA-RPV demonstrated apparently significant synergy, as assessed by the two different methods (41 μM2% in MacSynergy and 0.64 combination index [CI] in CalcuSyn). To confirm the synergy of HIV-1 inhibition by EFdA-RPV, we further evaluated this combination in the enzymatic assay for reverse transcriptase. Primer extension assays (7, 17) were performed with Quant-iT PicoGreen reagent (Invitrogen, Carlsbad, CA) (18). As shown in Table 2, the combination of EFdA with RPV provided synergistic effects on inhibition of reverse transcription.
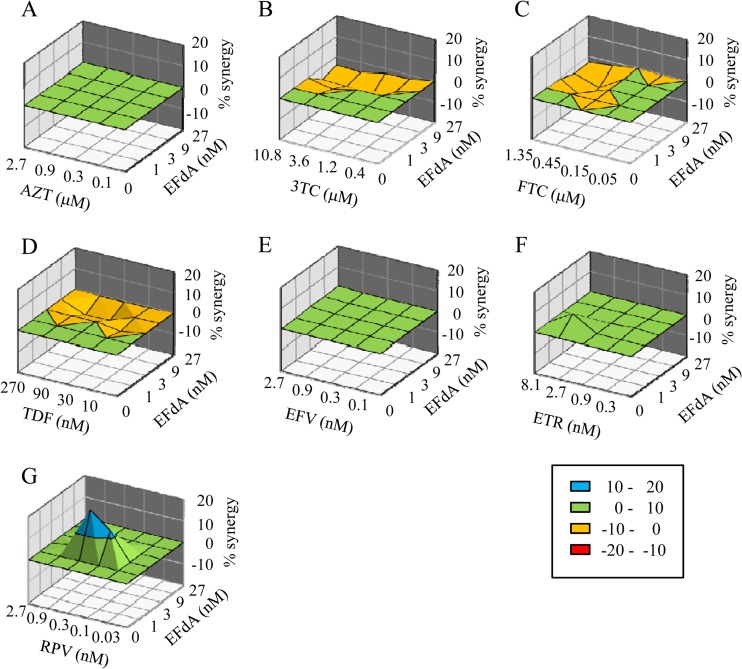
Effects of EFdA in combination with other anti-HIV-1 agents. The calculated additive surface, which represents the predicted additive interactions, is then subtracted from the experimental surface to reveal regions of greater-than-expected interactions (synergy). A resulting surface appearing as a horizontal plane at 0% inhibition above the calculated additive surface suggests that the interactions are merely additive. Peaks of statistically significant synergy (positive value) or antagonism (negative value) that deviate significantly from the expected additive drug interactions derived from 95% confidence interval data are shown in the different plots of the interaction between EFdA and other anti-HIV-1 agents in the cell-based assay, as follows: AZT (A), 3TC (B), FTC (C), TDF (D), EFV (E), ETR (F), and RPV (G). Units of μM2% are analogous to the units for area under a dose-response curve in the two-dimensional situation.
Table 2
Interactions of drug combinations for inhibition of HIV-1 virus or RT enzyme
| Drug class and EFdA combination | Target | MacSynergy analysis | CalcuSyn analysis | Proposed interactionc | ||
|---|---|---|---|---|---|---|
| Synergy/antagonism (μM2%)a | Predicted interaction | CIb | Predicted interaction | |||
| NRTI | ||||||
    AZT AZT | Virus | 0/0 | Additive | 1.18 | Additive | Neutral |
    TDF TDF | Virus | 0/−12.8 | Additive | 1.36 | Moderate antagonism | Neutral |
    3TC 3TC | Virus | 0/−39.5 | Minor antagonism | 1.23 | Moderate antagonism | Possible antagonism |
    FTC FTC | Virus | 0/−23.4 | Additive | 1.25 | Moderate antagonism | Neutral |
| NNRTI | ||||||
    EFV EFV | Virus | 1.9/0 | Additive | 0.9 | Additive | Neutral |
    ETR ETR | Virus | 7.8/0 | Additive | 1.05 | Additive | Neutral |
    RPV RPV | Virus | 41.0/−0.04 | Minor synergy | 0.64 | Synergy | Synergy |
    RPV RPV | Enzyme | 34.1/0 | Minor synergy | 0.56 | Synergy | Synergy |
We further compared the antiretroviral activity of various concentrations of EFdA alone, RPV alone, and a 1:1 molar combination of EFdA and RPV against wild-type virus and two HIV-1 mutants with reverse transcriptase (RT) mutations M184V and L100I/K103N. To evaluate the ability of the drugs to establish a barrier to subsequent infection in the absence of exogenous drug, cells were pretreated with various concentrations of each drug alone or in combination, followed by removal of exogenous drug and inoculation with HIV-1. These conditions assess intracellular persistence of drug following exogenous drug clearance, which is dependent on the intracellular half-life of the test drugs. The EFdA-RPV combination provided additive to synergistic inhibition of wild-type HIV-1 and both mutant strains (Table 3). The protective effect established by EFdA pretreatment is likely the result of EFdA resistance to degradation by adenosine deaminase (ADA) (6), consistent with a longer intracellular half-life (19). Hence, our data suggest that EFdA could be a strong candidate for use in preexposure prophylaxis, an approach in which TDF has shown efficacy in clinical studies (20, 21).
Table 3
Antiviral and prophylactic activity of EFdA, RPV, and EFdA-RPV combinations
| Activity type (pretreatment) and virus | Drug treatmenta | EC50 (nM)b | CI50c |
|---|---|---|---|
| Antiviral activity | |||
    Wild type Wild type | EFdA | 0.9 ± 0.2 | |
| RPV | 0.7 ± 0.1 | ||
| EFdA-RPV | 0.4 ± 0.2 | 0.55 | |
    M184V M184V | EFdA | 15 ± 3 | |
| RPV | 0.6 ± 0.2 | ||
| EFdA-RPV | 0.7 ± 0.1 | 0.5 | |
    L100I/K103N L100I/K103N | EFdA | 1 ± 0.5 | |
| RPV | 10 ± 2 | ||
| EFdA-RPV | 9 ± 2 | 0.45 | |
| Prophylaxis (2-h preincubation) | |||
    Wild type Wild type | EFdA | 11 ± 5 | |
| RPV | 18 ± 4 | ||
| EFdA-RPV | 5 ± 2 | 0.75 | |
    M184V M184V | EFdA | 97 ± 22 | |
| RPV | 8 ± 2 | ||
| EFdA-RPV | 10 ± 3 | 1 | |
    L100I/K103N L100I/K103N | EFdA | 12 ± 3 | |
| RPV | 175 ± 42 | ||
| EFdA-RPV | 9 ± 2 | 0.6 | |
| Prophylaxis (18-h preincubation) | |||
    Wild type Wild type | EFdA | 3 ± 1 | |
| RPV | 21 ± 4 | ||
| EFdA-RPV | 4 ± 1 | 0.9 | |
    M184V M184V | EFdA | 53 ± 13 | |
| RPV | 14 ± 4 | ||
| EFdA-RPV | 13 ± 40 | 0.6 | |
    L100I/K103N L100I/K103N | EFdA | 6 ± 1 | |
| RPV | 250 ± 25 | ||
| EFdA-RPV | 5 ± 2 | 0.75 |
Finally, we evaluated the ability of EFdA-RPV pretreatment to protect MT-2 lymphoblastoid cells from infection by a mixed virus population consisting of six HIV-1 strains: the wild type; the mutants K65R, Y181C, M184V, and D67N/K70R/T215F/K219Q (thymidine analogue mutations [TAMs]); and L100I/K103N. Cells were exposed to appropriate drugs for 16 h, and then exogenous drug was removed (Fig. 2). The breakthrough virus population harvested from cells not exposed to drug contained all six input viruses, plus some recombinant strains (Fig. 2B). Rapid virus breakthrough was evident in cells pretreated with RPV alone, and the only virus detected in the RPV-breakthrough population was the NNRTI-resistant L100I/K103N (100%). In contrast, breakthrough in cells pretreated with EFdA alone was significantly delayed. The EFdA-breakthrough population contained a mixture of the NRTI-resistant M184V mutant (54%) and TAMs (32%), plus some recombinant strains consisting of M184V plus one or more TAMs. No breakthrough was noted in cells pretreated with the EFdA-RPV combination, suggesting an enhanced protective effect of the drug combination compared to either drug alone.
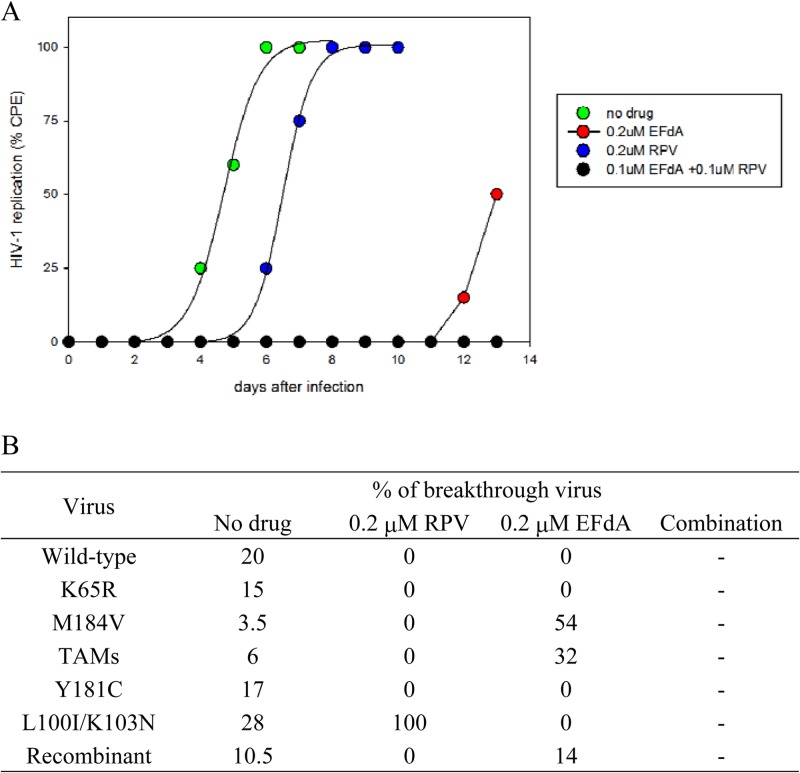
HIV-1 breakthrough in cells pretreated with EFdA, RPV, or a combination of EFdA and RPV. (A) MT-2 cells were incubated with the indicated concentrations of drug for 18 h and then extensively washed to remove exogenous drug. The washed cells were infected with a mixture of six virus strains (the wild type and five drug-resistant variants). Cells were examined daily for HIV-induced cytopathic effects. (B) Genotypic composition of breakthrough virus is shown. At least 20 clones from each breakthrough virus were sequenced. EFdA (0.1 μM) and RPV (0.1 μM) were used in combination. No virus breakthrough was noted in cells pretreated with the EFdA-RPV combination. TAMs (thymidine analog resistance mutations) included the D67N, K70R, T210F, and T219Q RT mutations. Recombinant virus possessed mutations derived from at least two of the input virus strains.
Synergistic interactions between NRTIs and NNRTIs have been previously reported in both viral (5, 22–24) and enzymatic assays (5, 25–31). NNRTIs may act synergistically with NRTIs by suppressing the phosphorolytic unblocking of NRTI-terminated primers, possibly by stabilizing the primer terminus at a posttranslocation position, where it cannot undergo phosphorolytic removal (5, 26, 27, 29). The mechanism for the apparent synergistic activity of the EFdA-RPV combination is under investigation.
In conclusion, EFdA in combination with RPV may provide a beneficial interaction against replication of drug-sensitive and certain RTI-resistant HIV-1 strains. The results of the present study indicate that EFdA may act as promising component of future antiretroviral therapies.
ACKNOWLEDGMENTS
This work was supported by a grant for the Bilateral International Collaborative R&D Program from the Korean Food and Drug Administration and the Ministry of Knowledge and Economy (S.G.S.), by National Institutes of Health (NIH) research grants AI076119-S1;, AI076119-02S1;, AI100890;, AI099284;, AI094715;, AI076119;, AI074389;, and GM103368; to S.G.S. and AI079801; to M.A.P., and by a Grant-in-Aid for the research on HIV/AIDS (H22-AIDS-001) from the Ministry of Health, Labor, and Welfare of Japan (S.O.). B.M. was the recipient of an amfAR Mathilde Krim Fellowship and a Canadian Institutes of Health Research (CIHR) Fellowship. We acknowledge the Yamasa Corporation for providing EFdA for this study.
H.M. and E.N.K. are coinventors of EFdA.
A.H. carried out the cell-based drug combination assays and wrote the manuscript. A.B.R. carried out the viral breakthrough experiments and studies with drug-resistant HIV and wrote portions of the manuscript. B.M., E.M., Y.T.O., K.A.K., and M.D.L. carried out the biochemical assays. S.G.S., M.A.P., S.O., and H.M. contributed to the design of the study, and S.G.S., L.C.R., E.N.K., and M.A.P. edited the manuscript. All authors read this paper and approved the final manuscript.
REFERENCES
Articles from Antimicrobial Agents and Chemotherapy are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/aac.00283-13
Read article for free, from open access legal sources, via Unpaywall:
https://aac.asm.org/content/aac/57/9/4554.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1128/aac.00283-13
Article citations
HIV-1 Resistance to Islatravir/Tenofovir Combination Therapy in Wild-Type or NRTI-Resistant Strains of Diverse HIV-1 Subtypes.
Viruses, 15(10):1990, 25 Sep 2023
Cited by: 1 article | PMID: 37896768 | PMCID: PMC10612037
Specific mutations in the HIV-1 G-tract of the 3'-polypurine tract cause resistance to integrase strand transfer inhibitors.
J Antimicrob Chemother, 77(3):574-577, 01 Feb 2022
Cited by: 7 articles | PMID: 34894227 | PMCID: PMC8865006
Approved HIV reverse transcriptase inhibitors in the past decade.
Acta Pharm Sin B, 12(4):1567-1590, 16 Nov 2021
Cited by: 29 articles | PMID: 35847492 | PMCID: PMC9279714
Review Free full text in Europe PMC
Avoiding Drug Resistance in HIV Reverse Transcriptase.
Chem Rev, 121(6):3271-3296, 28 Jan 2021
Cited by: 26 articles | PMID: 33507067 | PMCID: PMC8149104
Review Free full text in Europe PMC
Design, Synthesis, and Biological Evaluation of EdAP, a 4'-Ethynyl-2'-Deoxyadenosine 5'-Monophosphate Analog, as a Potent Influenza a Inhibitor.
Molecules, 24(14):E2603, 17 Jul 2019
Cited by: 1 article | PMID: 31319565 | PMCID: PMC6681032
Go to all (12) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Antiretroviral potency of 4'-ethnyl-2'-fluoro-2'-deoxyadenosine, tenofovir alafenamide and second-generation NNRTIs across diverse HIV-1 subtypes.
J Antimicrob Chemother, 73(10):2721-2728, 01 Oct 2018
Cited by: 9 articles | PMID: 30053052 | PMCID: PMC6148215
Hypersusceptibility mechanism of Tenofovir-resistant HIV to EFdA.
Retrovirology, 10:65, 24 Jun 2013
Cited by: 25 articles | PMID: 23800377 | PMCID: PMC3695782
Delayed emergence of HIV-1 variants resistant to 4'-ethynyl-2-fluoro-2'-deoxyadenosine: comparative sequential passage study with lamivudine, tenofovir, emtricitabine and BMS-986001.
Antivir Ther, 19(2):179-189, 25 Oct 2013
Cited by: 26 articles | PMID: 24162098
4'-Ethynyl-2-fluoro-2'-deoxyadenosine, MK-8591: a novel HIV-1 reverse transcriptase translocation inhibitor.
Curr Opin HIV AIDS, 13(4):294-299, 01 Jul 2018
Cited by: 47 articles | PMID: 29697468 | PMCID: PMC6449048
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NIAID NIH HHS (8)
Grant ID: R33 AI079801
Grant ID: R01 AI076119
Grant ID: R01 AI100890
Grant ID: R21 AI094715
Grant ID: R37 AI076119
Grant ID: R01 AI099284
Grant ID: R21 AI079801
Grant ID: R01 AI074389
NIGMS NIH HHS (1)
Grant ID: P50 GM103368
 a,i
a,i




