Abstract
Purpose
The PRIMA study (ClinicalTrials.gov identifier: NCT00140582) established that 2 years of rituximab maintenance after first-line immunochemotherapy significantly improved progression-free survival (PFS) in patients with follicular lymphoma compared with observation. Here, we report the final PFS and overall survival (OS) results from the PRIMA study after 9 years of follow-up and provide a final overview of safety.Methods
Patients (> 18 years of age) with previously untreated high-tumor-burden follicular lymphoma were nonrandomly assigned to receive one of three immunochemotherapy induction regimens. Responding patients were randomly assigned (stratified by induction regimen, response to induction treatment, treatment center, and geographic region) 1:1 to receive 2 years of rituximab maintenance (375 mg/m2, once every 8 weeks), starting 8 weeks after the last induction treatment, or observation (no additional treatment). All patients in the extended follow-up provided their written informed consent (data cutoff: December 31, 2016).Results
In total, 1,018 patients completed induction treatment and were randomly assigned to rituximab maintenance (n = 505) or observation (n = 513). Consent for the extended follow-up was provided by 607 patients (59.6%) of 1,018 (rituximab maintenance, n = 309; observation, n = 298). After data cutoff, median PFS was 10.5 years in the rituximab maintenance arm compared with 4.1 years in the observation arm (hazard ratio, 0.61; 95% CI, 0.52 to 0.73; P < .001). No OS difference was seen in patients randomly assigned to rituximab maintenance or observation (hazard ratio, 1.04; 95% CI, 0.77 to 1.40; P = .7948); 10-year OS estimates were approximately 80% in both study arms. No new safety signals were observed.Conclusion
Rituximab maintenance after induction immunochemotherapy provides a significant long-term PFS, but not OS, benefit over observation.Free full text

Sustained Progression-Free Survival Benefit of Rituximab Maintenance in Patients With Follicular Lymphoma: Long-Term Results of the PRIMA Study
Abstract
PURPOSE
The PRIMA study (ClinicalTrials.gov identifier: NCT00140582) established that 2 years of rituximab maintenance after first-line immunochemotherapy significantly improved progression-free survival (PFS) in patients with follicular lymphoma compared with observation. Here, we report the final PFS and overall survival (OS) results from the PRIMA study after 9 years of follow-up and provide a final overview of safety.
METHODS
Patients (> 18 years of age) with previously untreated high–tumor-burden follicular lymphoma were nonrandomly assigned to receive one of three immunochemotherapy induction regimens. Responding patients were randomly assigned (stratified by induction regimen, response to induction treatment, treatment center, and geographic region) 1:1 to receive 2 years of rituximab maintenance (375 mg/m2, once every 8 weeks), starting 8 weeks after the last induction treatment, or observation (no additional treatment). All patients in the extended follow-up provided their written informed consent (data cutoff: December 31, 2016).
RESULTS
In total, 1,018 patients completed induction treatment and were randomly assigned to rituximab maintenance (n = 505) or observation (n = 513). Consent for the extended follow-up was provided by 607 patients (59.6%) of 1,018 (rituximab maintenance, n = 309; observation, n = 298). After data cutoff, median PFS was 10.5 years in the rituximab maintenance arm compared with 4.1 years in the observation arm (hazard ratio, 0.61; 95% CI, 0.52 to 0.73; P < .001). No OS difference was seen in patients randomly assigned to rituximab maintenance or observation (hazard ratio, 1.04; 95% CI, 0.77 to 1.40; P = .7948); 10-year OS estimates were approximately 80% in both study arms. No new safety signals were observed.
CONCLUSION
Rituximab maintenance after induction immunochemotherapy provides a significant long-term PFS, but not OS, benefit over observation.
INTRODUCTION
Follicular lymphoma (FL) is the second most common lymphoma subtype in the United States and Western Europe, accounting for approximately 25% of all non-Hodgkin lymphoma cases and 70% of indolent lymphomas.1-3 Although the prognosis of patients with FL has significantly improved since the introduction of rituximab to first-line (1L) and salvage therapies,4-10 advanced-stage FL is believed to remain incurable in most patients because of inevitable relapses; however, strides have been made to prolong the duration of remission without exposure to additional cytotoxic treatment.
Previous studies have demonstrated a significant clinical benefit for rituximab maintenance in patients with relapsed disease after induction with chemotherapy with or without rituximab9-11 or single-agent rituximab,12,13 and in patients undergoing autologous stem-cell transplantation.14 Rituximab maintenance after chemotherapy15 or single-agent rituximab16 has also been studied in patients with previously untreated FL, with favorable results; however, neither of these induction regimens is considered optimal for patients with advanced-stage disease.
The pivotal PRIMA study (ClinicalTrials.gov identifier: NCT00140582) was the first phase III trial, to our knowledge, to evaluate the potential benefit of 2 years of rituximab maintenance in patients with high–tumor-burden FL responding to 1L rituximab-containing immunochemotherapy.17 After a median follow-up of 3 years, rituximab maintenance significantly prolonged progression-free survival (PFS) compared with observation; risk of disease progression was reduced by 45% (hazard ratio [HR], 0.55; 95% CI, 0.44 to 0.68; P < .001), and 3-year PFS rates were 74.9% and 57.6%, respectively. This PFS benefit was achieved regardless of the induction regimen, response to induction treatment, or patient age. Time to next antilymphoma treatment (TTNLT) and time to next chemotherapy treatment (TTNCT) were also significantly prolonged with rituximab maintenance, but no overall survival (OS) benefit was seen. An updated 6-year follow-up of the PRIMA study confirmed these results.18 Rituximab maintenance is now widely recommended for patients with FL responding to 1L rituximab-based immunochemotherapy.19 We present the final PFS and OS results from the PRIMA study after 9 years of follow-up and a final overview of safety.
METHODS
Study Design, Patients, and Treatments
PRIMA was an open-label, international, multicenter, randomized phase III trial in patients with previously untreated, high–tumor-burden FL. The study comprised two phases: induction and maintenance or observation (undertaken between December 2004 and April 2007, in 223 centers in 25 countries). Patients eligible for induction therapy were older than 18 years with untreated FL (histologic grade 1, 2, or 3a), diagnosed by a lymph node biopsy performed within 4 months of study registration. Inclusion and exclusion criteria are described in full elsewhere.17
During the induction phase, patients received rituximab in combination with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP; six cycles); cyclophosphamide, vincristine, and prednisone (CVP; eight cycles); or fludarabine, cyclophosphamide, and mitoxantrone (FCM; six cycles).17 Each center selected their preferred regimen for all patients enrolled at that center. Rituximab (375 mg/m2) was administered intravenously on day 1 of each chemotherapy course. CHOP- and FCM-treated patients received two additional rituximab infusions to ensure equivalent exposure during the induction phase.
Response was assessed 2 to 4 weeks after last induction treatment. Patients achieving a complete response (CR), an unconfirmed complete response (CRu), or a partial response (PR) were eligible for the next study phase. Eligible patients must have received at least four cycles of rituximab plus CHOP , six cycles of rituximab plus CVP, or four cycles of rituximab plus FCM. At least six infusions of rituximab were required for each treatment regimen, without a delay of more than 2 weeks between each cycle.
Responding patients were randomly assigned 1:1 to receive rituximab maintenance (375 mg/m2, once every 8 weeks), starting 8 weeks after last induction treatment, or observation (no additional treatment). All randomly assigned patients received rituximab maintenance or underwent observation for 2 years or until disease progression, whichever occurred first. The random assignment procedure has been reported previously.17 Patients who completed this phase were initially followed for 3 years (data cutoff: January 31, 2011, per initial protocol) or 5 years (data cutoff: January 31, 2013, per protocol amendment). Patients in this extended follow-up study consented in writing to approximately 2 more years of follow-up (data cutoff: December 31, 2016).
PRIMA was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. The study protocol and amendments were approved by local and national ethics committees, according to the laws of each country. Patients provided written informed consent.
Assessments
Response was evaluated according to the 1999 International Working Group response criteria for non-Hodgkin lymphoma.20 During the 2-year rituximab maintenance or observation phase, patients were assessed by clinical examination every 8 weeks and had a computed tomography scan every 6 months. If bone marrow involvement was initially documented, a biopsy was required at the end-of-treatment assessment to confirm CR. Patients completing the rituximab maintenance or observation phase underwent a final restaging assessment within 28 days of the last rituximab dose (or within a corresponding timeframe for those randomly assigned to observation). For patients with no disease progression, follow-up assessments were scheduled every 3 months for the first 2 years, then every 6 months for an additional 3 years, and then annually in patients consenting to the extended follow-up. Patients with disease progression were followed annually for the initiation of new treatment and OS for 5 years, or until data cutoff in patients consenting to the extended follow-up.
Efficacy and Safety Analyses
The primary end point was investigator-assessed PFS. Secondary end points included TTNLT, TTNCT, OS, and transformation rate at relapse. Safety outcome measures included adverse events (AEs), serious AEs, grade 3 or higher AEs, and deaths. Grading of AEs was according to National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0.
Statistical Analysis
PFS was defined as the time from random assignment to progression, relapse, or death from any cause. Responding patients and patients lost to follow-up were censored at their last tumor assessment date. OS was determined from the date of random assignment to the date of death regardless of cause. Survival end points were estimated by Kaplan-Meier methodology and compared using a two-sided log-rank test stratified by induction regimen and induction response. Histologic transformation rates at first relapse were compared using a χ2 test.
RESULTS
Patients
Overall, 1,018 patients completed induction treatment and were randomly assigned to rituximab maintenance (n = 505) or observation (n = 513); these patients were the primary population for efficacy analyses. Nine patients (rituximab maintenance, n = 4; observation, n = 5) withdrew before the first maintenance treatment cycle or observation visit and were excluded from the safety analyses. Consent for the extended follow-up was provided by 607 patients (59.6%) of 1,018 (rituximab maintenance, n = 309; observation, n = 298). An overview of the trial profile is provided in Figure 1.
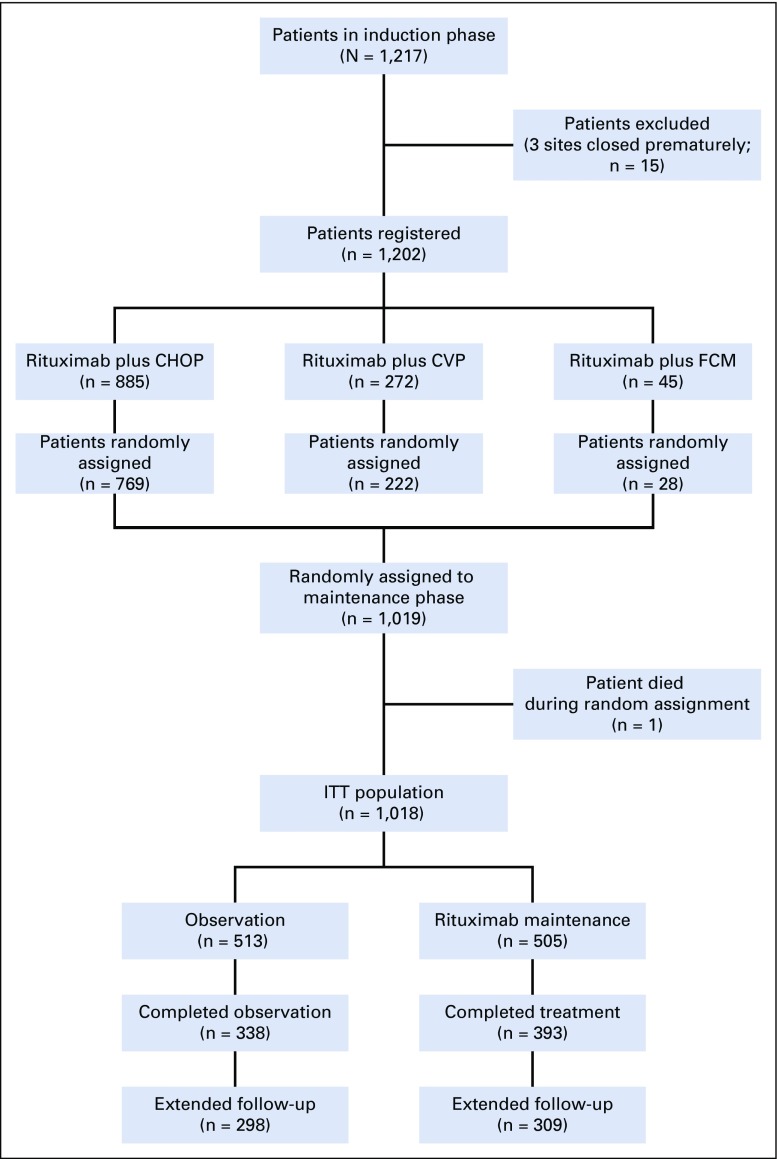
CONSORT diagram. Full details of the trial profile before follow-up have been published previously.17 CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; CVP, cyclophosphamide, vincristine, and prednisone; FCM, fludarabine, cyclophosphamide, and mitoxantrone; ITT, intent-to-treat.
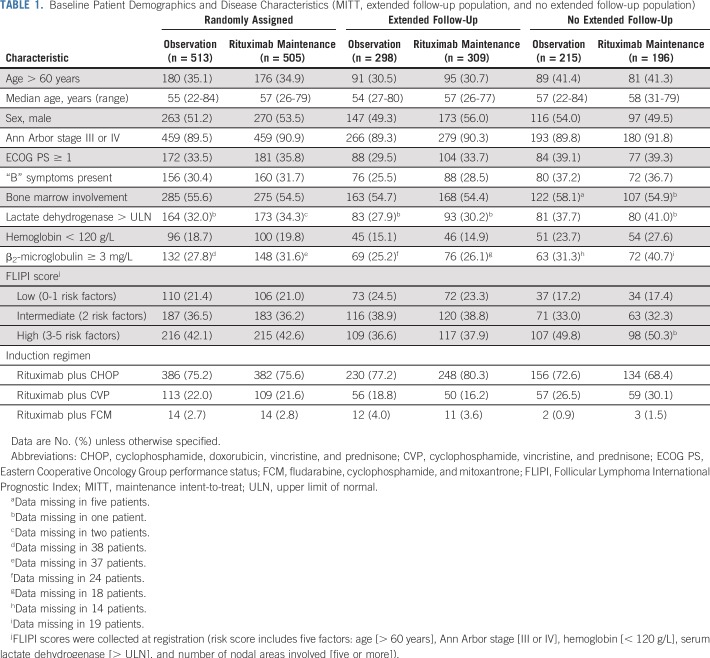
Median duration of follow-up was 9.0 years (range, 0.0 to 11.5 years) from random assignment and was well balanced between arms (rituximab maintenance, 9.1 years; observation, 9.0 years). Patient demographics and disease characteristics at random assignment are listed in Table 1. Patients not included in the extended follow-up exhibited adverse prognostic factors more frequently than those in the extended follow-up, mainly because of the automatic exclusion of patients who died before the current analysis [data not shown]).
TABLE 1.
Baseline Patient Demographics and Disease Characteristics (MITT, extended follow-up population, and no extended follow-up population)
Efficacy
Rituximab maintenance after rituximab-containing induction immunochemotherapy continued to provide a significant long-term PFS benefit compared with observation (Table 2; Fig 2A). At the final data cutoff, median PFS was 10.5 years in patients randomly assigned to rituximab maintenance versus 4.1 years in patients randomly assigned to observation (HR, 0.61; 95% CI, 0.52 to 0.73; P < .001). Ten-year PFS estimates were 51.1% in the rituximab maintenance arm and 35.0% in the observation arm. Evaluation of PFS in prespecified patient subgroups, categorized by age, sex, FLIPI score, induction chemotherapy, and response to induction, showed a consistent benefit of rituximab maintenance over observation (Fig 3). Patients in CR, CRu, or PR at end of induction consistently benefited from rituximab maintenance (Data Supplement). PFS by FLIPI risk factor category in the two treatment arms is shown in the Data Supplement.
TABLE 2.
Overview of Key Efficacy Results After 6 and 9 Years of Follow-Up From Random Assignment

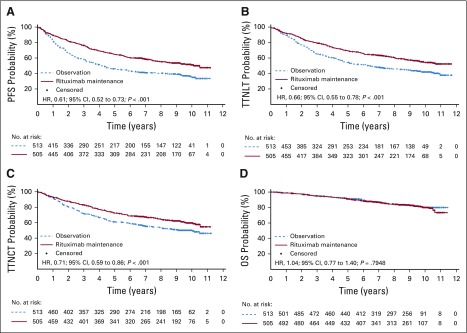
Kaplan-Meier estimates of (A) progression-free survival (PFS), (B) time to next antilymphoma treatment (TTNLT), (C) time to next chemotherapy treatment (TTNCT), and (D) overall survival (OS) from random assignment. HR, hazard ratio.
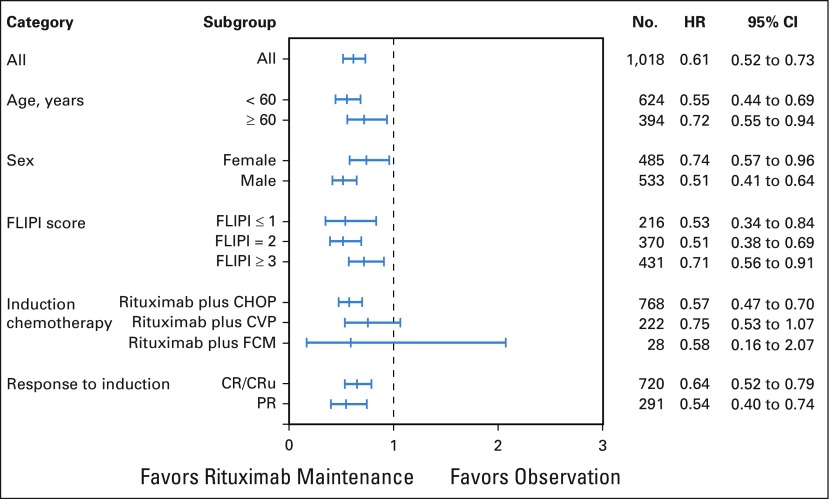
Risk of progression according to prespecified subgroups. CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; CR, complete response; CVP, cyclophosphamide, vincristine, and prednisone; FCM, fludarabine, cyclophosphamide, and mitoxantrone; FLIPI, Follicular Lymphoma International Prognostic Index; HR, hazard ratio; PR, partial response; CRu, unconfirmed complete response.
Rituximab maintenance also provided a significant benefit over observation in terms of time to next treatment; median TTNLT was not reached in the rituximab maintenance arm versus 6.1 years in the observation arm (HR, 0.66; 95% CI, 0.55 to 0.78; P < .001; Table 2; Fig 2B). At the final data cutoff, 212 patients (42.0%) of 505 in the rituximab maintenance arm and 284 patients (55.4%) of 513 in the observation arm had either started a new antilymphoma treatment or died before receiving it. Ten-year TTNLT estimates were 53.4% in the rituximab maintenance arm and 41.2% in the observation arm.
Median TTNCT was not reached in the rituximab maintenance arm versus 9.3 years in the observation arm (HR, 0.71; 95% CI, 0.59 to 0.86; P < .001; Table 2; Fig 2C); at data cutoff, 188 patients (37.2%) of 505 in the rituximab maintenance arm and 244 patients (47.6%) of 513 in the observation arm had either started a new chemotherapy treatment or had died before receiving it.
The above-mentioned beneficial effects of rituximab maintenance did not translate into an OS benefit (Table 2; Fig 2D), with 10-year OS rate estimates of approximately 80% (observation, 79.9%; rituximab maintenance, 80.1%) in both study arms; median OS was not reached in either arm (HR, 1.04; 95% CI, 0.77 to 1.40; P = .7948). OS according to FLIPI risk factor categories in patients randomly assigned to rituximab maintenance or observation is shown in the Data Supplement. OS after progression or relapse (ie, time from first progression until death) was shorter in the maintenance arm versus the observation arm, explaining equivalent OS in both arms (Data Supplement).
A total of 503 patients had documented disease progression. The rate of progression with disease transformation was low, but similar in both study arms (Data Supplement). No difference in time to transformation was observed (Data Supplement).
Second-Line Treatment
Of 503 patients who experienced disease progression, 453 received documented second-line (2L) therapy. The most common subsequent chemotherapy regimens were rituximab with a platinum-based regimen (27.2%), fludarabine-based regimen (12.1%), or bendamustine (8.6%; Data Supplement). Significantly more patients in the observation arm than in the rituximab maintenance arm received a rituximab-containing therapy at relapse or progression (81.5% v 73.2%, respectively; P = .04). Slightly more patients in the observation arm received radioimmunotherapy (24.4% v 16.9%, respectively; P = .06). One hundred twenty patients (26.5%) underwent high-dose therapy followed by autologous stem-cell transplantation with no difference between the two arms (29.3% v 22.4%, respectively; P = .13). Response to 2L regimen was similar between 1L treatment arms, with overall response and CR rates of 78.2% and 47.3%, respectively (rituximab maintenance) versus 80.4% and 46.4%, respectively (observation). However, the rate of CR/CRu for patients who experienced early progression within 18 months of random assignment (corresponding to 24 months after induction) was inferior in the maintenance arm compared with the observation arm (39.3% v 56.3%; P = 0.029), thus demonstrating that the patients who experienced disease progression during maintenance were those with a more aggressive disease (Data Supplement).
Safety
Since random assignment, 285 patients (56.9%) of 501 in the rituximab maintenance arm and 194 patients (38.2%) of 508 in the observation arm have experienced at least one AE (Data Supplement). Rituximab maintenance was associated with a higher rate of grade 3 to 4 AEs (24.4% v 16.9%) and serious AEs (21.2% v 13.4%) compared with observation; the higher rate of grade 3 to 4 AEs was driven largely by higher rates of cytopenias (5.2% v 1.6%) and infections (4.4% v 1.0%). The most common grade 3 to 4 AEs were neoplasms benign, malignant, and unspecified (including cysts and polyps), with a similar incidence between study arms (approximately 4% in both arms). Grade 5 (fatal) AEs occurred in eight patients (1.6%) of 501 and three patients (0.6%) of 508 randomly assigned to rituximab maintenance and observation, respectively (Data Supplement).
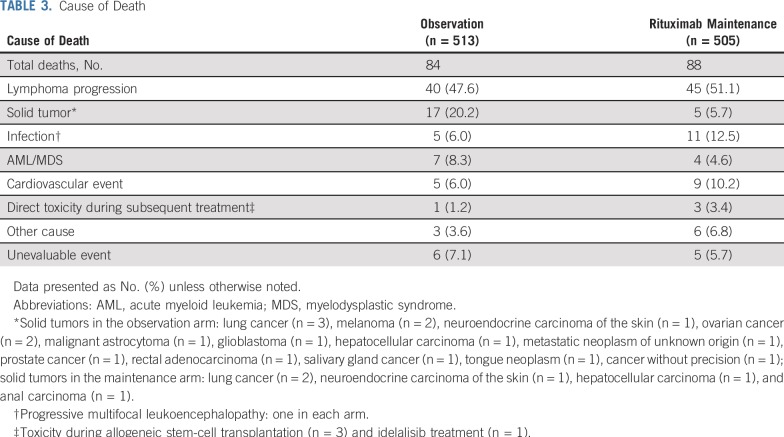
A total of 88 patients (17.4%) have died in the rituximab maintenance arm since random assignment versus 84 (16.4%) in the observation arm (Table 3). The most frequent causes of death were progressive disease (rituximab maintenance, 51.1%; observation, 47.6%) and solid tumors (rituximab maintenance, 5.7%; observation, 20.2%).
TABLE 3.
Cause of Death
DISCUSSION
The primary analysis from PRIMA demonstrated prolonged PFS with rituximab maintenance when applied after 1L immunochemotherapy induction to patients with previously untreated, high–tumor-burden FL.17 This long-term follow-up strengthens these previously published results,17,18 demonstrating significantly longer PFS, TTNLT, and TTNCT in the rituximab maintenance arm compared with the observation arm. With an updated 9-year median follow-up, projected 10-year PFS was 51.1% in the rituximab maintenance arm and 35.0% in the observation arm, whereas 10-year TTNLT estimates were 53.4% and 41.2%, respectively. Clinically, these results mean that approximately half of patients who receive rituximab maintenance every 8 weeks for 2 years after response to immunochemotherapy induction will remain free from progression or death and free from further antilymphoma treatment after 10 years. Subgroup analyses showed the substantial PFS improvement associated with rituximab maintenance was independent of age, sex, induction immunochemotherapy regimen, response to induction (CR/CRu, or PR), or FLIPI risk score.
The OS estimate at 10 years for the whole patient cohort was approximately 80%, thus confirming how the introduction of rituximab into the therapeutic armamentarium in general, and into the 1L induction setting in particular, has profoundly altered the course of FL, as compared with historical controls.21,22 However, despite significant and sustained PFS prolongation with rituximab maintenance, no OS difference was observed between the two arms. This finding is similar to recently published long-term follow-up studies in which prolonged PFS with the use of rituximab plus CHOP (compared with rituximab plus CVP) or rituximab plus bendamustine (compared with rituximab plus CHOP) fails to translate into prolonged OS23,24 and has important implications for both our understanding of the disease and future research in the field. First, direct extrapolation of PFS as a surrogate marker for OS cannot be made in FL, even with long-term follow-up. Second, PFS and TTNLT prolongation as meaningful clinical and economic end points must be viewed independently of OS. And third, the underlying biologic explanation for PFS improvement not translating into longer OS needs to be addressed.
Recent efforts have been made by the Follicular Lymphoma Analysis of Surrogacy Hypothesis) group to assess if CR at 30 months after initiation of induction therapy can serve as a surrogate end point for PFS in FL, and the initial results look promising.25 However, the evidence for PFS as a surrogate for OS is conflicting. In advanced solid tumors, there is considerable heterogeneity among cancer types and, for a given neoplasm, there are even discrepancies among the same histology subgroups, resulting in a generally low strength of association between PFS and OS.26 In lymphoma, surrogacy has been studied and documented in 1L diffuse large B-cell lymphoma,27 but robust data are lacking in FL. Indeed, statistical modeling indicates that the association between PFS and OS tends to be weaker for malignancies with a long survival after progression, such as FL, which explains how the PFS advantage reported here may have been diluted over subsequent lines of treatment.28 Whether the recently described progression of disease within 24 months of initiating treatment end point is a more reliable surrogate of OS in patients with FL receiving 1L immunochemotherapy with or without maintenance remains to be established.29-32
In our analysis, the proportion of deaths associated with lymphoma progression was almost identical between treatment arms. Response to 2L treatment was also comparable. Shorter survival after first relapse in the maintenance arm helps to explain why OS was similar in both arms despite prolonged PFS. Altogether, these data indicate that rituximab maintenance does not alter the natural course of the disease for patients with aggressive FL and that they will ultimately die as rapidly as if they were observed after induction treatment. Whether the absence of an OS benefit in these patients challenges the appeal of a prolonged 1L remission in most patients with de novo FL with a high tumor burden remains an open question.
No difference in terms of transformation rate was found with this extended follow-up, and these findings confirmed a previous analysis of data from the PRIMA cohort, which showed that rituximab maintenance did not have a significant prognostic impact on histologic transformation.33 Interestingly, detailed analysis of 2L treatments at relapse showed that use of rituximab was significantly less frequent after rituximab maintenance than after observation. Data on the use of anti-CD20 antibodies as maintenance at relapse were lacking, but one could hypothesize that rituximab maintenance may have been more frequently administered in the observation arm, given the established beneficial effect of rituximab maintenance on PFS in the relapsed/resistant setting.34 This could potentially explain, at least in part, the absence of a difference in OS between the two arms.
Consistent with previous analyses,17,18 rituximab maintenance was generally well tolerated, and no unexpected safety signals were observed with the additional 4 years of follow-up. It is worth noting that although the OS rate was not different between the two arms, death due to second neoplasia was almost four times more frequent in the observation arm compared with the maintenance arm. It could be speculated that recurrent use of cytotoxic- and radiation-containing regimens in the context of earlier relapse in the observation arm may have increased the frequency of second neoplasms. Conversely, deaths due to infection, a known consequence of immunotherapy,34 were twice as frequent in the rituximab maintenance arm. However, only two cases of the opportunistic infection, progressive multifocal leukoencephalopathy, were observed, one in each treatment arm. Although rituximab exposure may increase this risk,35 our data suggest there is not a strong effect of maintenance.
In conclusion, this 9-year follow-up of the PRIMA study demonstrates that rituximab maintenance after induction immunochemotherapy provides a significant long-term PFS benefit over observation. Despite the lack of OS advantage, it is noteworthy that more than half of the patients in the rituximab maintenance arm remain free of disease progression and have not required new antilymphoma treatment beyond 10 years.
ACKNOWLEDGMENT
We thank the PRIMA study investigators, coordinators, nurses, and patients. PRIMA was sponsored by the Lymphoma Study Association and supported by F Hoffmann-La Roche and Biogen Idec. We also thank Professor Corinne Haioun for her valuable contribution. Third-party writing assistance was provided by Janis Noonan of Gardiner-Caldwell Communications and was funded by F. Hoffmann-La Roche.
Footnotes
Presented in part at the American Society of Hematology Annual Meeting and Exposition, Atlanta, GA, December 9-12, 2017.
PRIMA was sponsored by the Lymphoma Study Association and supported by F Hoffmann-La Roche and Biogen Idec.
Processed as a Rapid Communication manuscript.
AUTHOR CONTRIBUTIONS
Conception and design: Emmanuel Bachy, John F. Seymour, Pauline Brice, Andrew Lister, Gilles Salles
Financial support: Gilles Salles
Administrative support: Harald Zeuner, Gilles Salles
Provision of study material or patients: John F. Seymour, Armando López-Guillermo, Luc Xerri, John V. Catalano, Pauline Brice, Alejandro Martin, Lars M. Pedersen, Véronique Dorvaux, David Simpson, Jean Gabarre, Steven Le Gouill, Andrew Lister, Gustavo Milone, Hervé Tilly, Gilles Salles
Collection and assembly of data: Emmanuel Bachy, John F. Seymour, Pierre Feugier, Fritz Offner, Armando López-Guillermo, David Belada, Luc Xerri, John V. Catalano, François Lemonnier, Alejandro Martin, Olivier Casasnovas, Lars M. Pedersen, Véronique Dorvaux, David Simpson, Sirpa Leppa, Jean Gabarre, Maria G. da Silva, Sylvie Glaisner, Loic Ysebaert, Anne Vekhoff, Tanin Intragumtornchai, Steven Le Gouill, Andrew Lister, Jane A. Estell, Anne Sonet, Jonathan Farhi, Harald Zeuner, Hervé Tilly, Gilles Salles
Data analysis and interpretation: Emmanuel Bachy, John F. Seymour, Fritz Offner, Armando López-Guillermo, John V. Catalano, Pauline Brice, Olivier Casasnovas, David Simpson, Andrew Lister, Gustavo Milone, Harald Zeuner, Hervé Tilly, Gilles Salles
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Sustained Progression-Free Survival Benefit of Rituximab Maintenance in Patients With Follicular Lymphoma: Long-Term Results of the PRIMA Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Emmanuel Bachy
Honoraria: Gilead Sciences, Roche, Amgen, Janssen-Cilag
Consulting or Advisory Role: Roche
Travel, Accommodations, Expenses: Janssen-Cilag
John F. Seymour
Honoraria: AbbVie, Acerta Pharma, Janssen, Roche, Sunesis Pharmaceuticals, Takeda
Consulting or Advisory Role: AbbVie, Acerta Pharma, Janssen, Roche, Sunesis Pharmaceuticals, Takeda
Speakers' Bureau: AbbVie, Celgene, Roche
Research Funding: AbbVie, Celgene, Janssen, Roche
Expert Testimony: Roche
Travel, Accommodations, Expenses: AbbVie, Roche
Pierre Feugier
Honoraria: Genentech, Janssen, Gilead, Amgen, AbbVie
Research Funding: Genentech, Gilead, Janssen, AbbVie, Amgen
Travel, Accommodations, Expenses: Amgen, Gilead, Janssen, Genentech, AbbVie
Armando López-Guillermo
Consulting or Advisory Role: Roche, Celgene, Gilead/Kite Pharma, Takeda, Janssen, Incyte
Research Funding: Roche (Inst), Gilead Sciences, Celgene (Inst)
Travel, Accommodations, Expenses: Roche
David Belada
Consulting or Advisory Role: Roche, Gilead Sciences, Janssen-Cilag, Takeda
Research Funding: Roche (Inst), Gilead Sciences (Inst), Janssen-Cilag (Inst), Takeda (Inst), MorphoSys (Inst), Pharmacyclics (Inst), Archigen Biotech (Inst)
Travel, Accommodations, Expenses: Gilead Sciences, Takeda, Roche
John V. Catalano
Travel, Accommodations, Expenses: Celgene
Pauline Brice
Research Funding: Takeda, Bristol-Myers Squibb
Travel, Accommodations, Expenses: Roche, Amgen, AbbVie/Genentech
François Lemonnier
Consulting or Advisory Role: Miltenyi Biotec
Travel, Accommodations, Expenses: Janssen
Alejandro Martin
Honoraria: Roche, Janssen-Cilag, Celgene, SERVIER, Gilead Sciences
Consulting or Advisory Role: Roche, Celgene, MorphoSys, Kyowa Hakko Kirin
Research Funding: Celgene, Teva, Janssen-Cilag
Expert Testimony: Gilead Sciences
Travel, Accommodations, Expenses: Roche, Celgene, SERVIER
Olivier Casasnovas
Honoraria: Genentech, Takeda, Gilead Sciences, Bristol-Myers Squibb, Merck, AbbVie, Celgene, Janssen
Consulting or Advisory Role: Genentech, Takeda, Gilead Sciences, Bristol-Myers Squibb, Merck, AbbVie, Celgene, Janssen
Research Funding: Genentech (Inst), Gilead Sciences (Inst), Takeda (Inst)
Travel, Accommodations, Expenses: Genentech, Takeda, Gilead Sciences, Janssen
Véronique Dorvaux
Honoraria: Takeda
Consulting or Advisory Role: Takeda
Travel, Accommodations, Expenses: Janssen Oncology
David Simpson
Honoraria: Celgene, AbbVie, Janssen-Cilag, Roche, Merck Sharp & Dohme
Consulting or Advisory Role: Celgene, Merck, AbbVie, Janssen-Cilag
Research Funding: Amgen, BeiGene (Inst), Sanofi (Inst), Roche (Inst), GlaxoSmithKline (Inst), Acerta Pharma (Inst), Pharmacyclics (Inst), AbbVie (Inst)
Travel, Accommodations, Expenses: Celgene, Gilead Sciences, Janssen
Sirpa Leppa
Honoraria: Takeda
Consulting or Advisory Role: Novartis, Celgene, Takeda, Roche, MSD
Research Funding: Roche (Inst), Janssen-Cilag (Inst), Bayer (Inst), Celgene (Inst)
Maria G. da Silva
Consulting or Advisory Role: Janssen-Cilag, Gilead Sciences, Roche (Inst), Janssen-Cilag (Inst)
Speakers' Bureau: Janssen-Cilag, Takeda
Research Funding: Gilead Sciences
Travel, Accommodations, Expenses: Roche, Celgene, Janssen-Cilag, Gilead Sciences, Takeda
Loic Ysebaert
Consulting or Advisory Role: AbbVie, Janssen-Cilag, Roche, Gilead Sciences, Roche (Inst), Janssen-Cilag (Inst), Gilead Sciences (Inst)
Steven Le Gouill
Honoraria: Genentech
Consulting or Advisory Role: Genentech
Research Funding: Genentech
Travel, Accommodations, Expenses: Genentech
Andrew Lister
Stock and Other Ownership Interests: AstraZeneca, GlaxoSmithKline, AbbVie, Johnson & Johnson, Pfizer, AstraZeneca (I), Dechra Pharmaceuticals (I), Hikma Pharmaceuticals (I)
Consulting or Advisory Role: Genentech (DMC), Gilead Sciences, Millennium, Merck
Travel, Accommodations, Expenses: Genentech, Gilead Sciences, Takeda/Gilead, Merck
Jane A. Estell
Consulting or Advisory Role: Celgene
Research Funding: AbbVie
Gustavo Milone
Honoraria: mAbxience, Gador, Elea
Consulting or Advisory Role: Teva
Travel, Accommodations, Expenses: Elea, Gador, Raffo
Jonathan Farhi
Honoraria: Novartis
Travel, Accommodations, Expenses: Bristol-Myers Squibb, Pfizer, Sandoz, Roche
Harald Zeuner
Employment: Hoffman-LaRoche AG
Hervé Tilly
Honoraria: Bristol-Myers Squibb, SERVIER
Consulting or Advisory Role: Karyopharm Therapeutics, Roche, Janssen
Travel, Accommodations, Expenses: Roche
Gilles Salles
Honoraria: Genentech, Amgen, Janssen, Celgene, SERVIER Gilead Sciences, Novartis, AbbVie, Merck, Takeda, MorphoSys
Consulting or Advisory Role: Genentech, Gilead Sciences, Janssen, Celgene, Novartis, Merck, Pfizer, Acerta Pharma, Kite Pharma, SERVIER, Morphosys, Epizyme
No other potential conflicts of interest were reported.
REFERENCES
Articles from Journal of Clinical Oncology are provided here courtesy of American Society of Clinical Oncology
Citations & impact
Impact metrics
Article citations
Efficacy and Safety of Bendamustine-Rituximab as Frontline Therapy for Indolent Non-Hodgkin Lymphoma: A Real-World, Single-Center, Retrospective Study.
Cureus, 16(8):e66124, 04 Aug 2024
Cited by: 0 articles | PMID: 39229411 | PMCID: PMC11370709
Quality of Life Evaluation in Patients with Follicular Cell Lymphoma: A Real-World Study in Europe and the United States.
Adv Ther, 41(8):3342-3361, 08 Jul 2024
Cited by: 0 articles | PMID: 38976122 | PMCID: PMC11263223
Progression-free survival after front line, second line and third line in patients with follicular lymphoma treated in clinical practice.
Acta Oncol, 63:267-272, 06 May 2024
Cited by: 0 articles | PMID: 38709114 | PMCID: PMC11332539
The contradictory role of febuxostat in ABCG2 expression and potentiating hypericin-mediated photodynamic therapy in colorectal cancers.
Photochem Photobiol Sci, 23(6):1067-1075, 16 Apr 2024
Cited by: 0 articles | PMID: 38625651
What is new in the 5th edition of the World Health Organization classification of mature B and T/NK cell tumors and stromal neoplasms?
J Hematop, 17(2):71-89, 29 Apr 2024
Cited by: 0 articles | PMID: 38683440
Review
Go to all (81) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials
- (2 citations) ClinicalTrials.gov - NCT00140582
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): a phase 3, randomised controlled trial.
Lancet, 377(9759):42-51, 20 Dec 2010
Cited by: 593 articles | PMID: 21176949
Prognostic value of end-of-induction PET response after first-line immunochemotherapy for follicular lymphoma (GALLIUM): secondary analysis of a randomised, phase 3 trial.
Lancet Oncol, 19(11):1530-1542, 08 Oct 2018
Cited by: 50 articles | PMID: 30309758
Rituximab versus a watch-and-wait approach in patients with advanced-stage, asymptomatic, non-bulky follicular lymphoma: an open-label randomised phase 3 trial.
Lancet Oncol, 15(4):424-435, 04 Mar 2014
Cited by: 177 articles | PMID: 24602760
Follicular lymphoma: first-line treatment without chemotherapy for follicular lymphoma.
Curr Treat Options Oncol, 16(7):32, 01 Jul 2015
Cited by: 4 articles | PMID: 26031546
Review
Funding
Funders who supported this work.
Cancer Foundation Finland sr (1)
Grant ID: 180125




