Abstract
Free full text

Single dose of an adenovirus vectored mouse interferon-α protects mice from lethal EV71 challenge
Associated Data
Abstract
Enterovirus 71 (EV71) causes hand-foot-and-mouth diseases as well as neurological complications in young children. Interferon (IFN) can inhibit the replication of many viruses with low cytotoxic effects. Previously, an adenovirus vectored mouse interferon-α (DEF201), subtype 5, was generated by Wu et al, 2007. In this study, the antiviral effects of DEF201 against EV71 were evaluated in a murine model. 6–day-old BALB/c mice were administered a single dose of DEF201 before or after infection with lethal dose of EV71. The survival rate, clinical symptoms, tissue viral loads and histology pathogenesis were evaluated. IFN gene expression following a single dose of DEF201 maintained high concentrations of 100–9000 pg/mL for more than 7 days in mice serum. Pre-infection administration of a single dose of 106 PFU of DEF201 offered full protection of the mice against EV71 infection compared with the empty Ad5 vector control. In addition, virus load in DEF201-treated mice muscle tissue was significantly decreased as compared with empty vector control. Histopathology analysis revealed that DEF201 significantly prevented the development of severe tissue damage with reduction of viral antigen in the murine muscle tissue. Post-infection treatment at 6 h offered full protection and partial protection at 12 h, indicating that DEF201 could be used as an anti-EV71 therapeutic agent in early stage of EV71 infection. In addition, our study showed that DEF201 enhanced the neutralization ability of serum in EV71-vaccinated mice, implying that DEF201 could promote the production of specific anti-EV71 antibodies. In conclusion, single dose of DEF201 is highly efficacious as a prophylactic agent against EV71 infection in vivo.
1. Introduction
Enterovirus 71 (EV71) causes hand-foot-and-mouth diseases and neurological complications in young children and immuno-compromised adults. EV71 belongs to Enterovirus genus, Picornaviridae family. EV71 is a non-enveloped virus with a single-strand, positive-sense RNA genome. Currently, there are no specific drugs for EV71 infection. Five inactivated whole virus vaccines are currently being developed and showed good safety and immunogenicity in clinical trials (Li et al., 2014). One of the five vaccines has been approved by the China FDA recently (Sinovac Obtains New Drug, 2016). In addition, a formalin-inactivated EV71 vaccine, in a Phase I clinical trial, showed strong cross neutralizing ability against EV71 B1, B4, B5 and C4A subgenotypes but not C4B, C2 and coxsackievirus A16 (Chou et al., 2013). Another EV71 subgenotype C4 vaccine also showed cross neutralizing ability against B4, B5, C2 and C5 subgenotypes (Zhu et al., 2013). The subgenotype restriction of these vaccines compromises their vaccination efficacy and limits their application in the clinics.
Type I interferons are a group of broad spectrum antiviral cytokines released by host cells during viral infection. Upon binding to the receptor, type I IFNs induce hundreds of downstream genes, known as IFN stimulated genes (ISGs), and confers an antiviral state of host cells (van Boxel-Dezaire et al., 2006). The specific roles of most of these ISGs in host antiviral response are still largely unknown. Among these ISGs, PKR and p53 promote the apoptosis of virus-infected cells (Balachandran et al, 2000, Takaoka et al, 2003). 2′-5′ oligoadenylate synthases (OAS) are activated by IFN during viral infection and produce 2′-5′ oligoadenylates which activate RNase L and further lead to the degradation of viral RNA (Stark et al., 1998).
Type I IFNs are effective in inhibiting viral infection, however, their application in the clinics is restricted due to their short half-life. To maintain elevated serum IFN without requiring repeated injections, an adenovirus vectored interferon-α, named as DEF201, was generated (Wu et al., 2007). Interferon is species specific, so mouse interferon-α gene was constructed into adenovirus type 5 (Ad5) vector under the control of a strong promoter PCMV. Upon intranasal administration, the adenovirus vector transduces the IFN-α gene into nasal epithelial cells where IFN-α is expressed constitutively. In vivo studies showed that DEF201 could significantly increase the half-life of IFN-α and was protective for mice against viruses or bacteria infection including western equine encephalitis virus (Wu et al., 2007), vaccinia virus (Smee et al., 2011), arenavirus (Gowen et al., 2011), rift valley fever virus (Scharton et al., 2015), SARS coronavirus (Kumaki et al., 2011), yellow fever virus (Julander et al., 2011), chikungunya virus (Dagley et al., 2014) and Streptococcus pneumoniae (Damjanovic et al., 2014).
To date, the antiviral effects of type I IFNs against EV71, especially in vivo effects, have not been well studied. Previous studies showed that poly (I:C), an interferon inducer, significantly protected neonate mice from lethal EV71 challenge (Liu et al., 2005). In this study, the anti-EV71 effects of single dose of DEF201 were evaluated in a murine model.
2. Materials and methods
2.1. Cell lines and virus strain
Rhabdomyosarcoma cells (RD) were used for EV71 quantification by plaque assay. Human embryo kidney cells (HEK293) were used for Ad5 vector preparation. Enterovirus 71 (EV71) strain 41 (5865/SIN/000009) (GenBank accession no. AF316321) was used to infect mice in this study.
2.2. DEF201 and adenovirus5 empty vector (Ad5)
DEF201 was used to test its antiviral efficacy for EV71 infection. An empty Ad5 vector was used as a test control for DEF201. The stock titer of DEF201 was 1.45 × 109 PFU/mL. Ad5 was generated using the method as described previously by Wu et al, 2007. For Ad5 vector preparation, Ad5 plasmid was cut by PacI restriction endonuclease and linearized plasmid was transfected into HEK293 cells by lipofectamine reagents to generate Ad5 vector. Ad5 culture was harvested when full CPE had developed. Ad5 vector was subsequently propagated in HEK293 cells. Ad5 vector was purified by cesium chloride ultra-centrifugation using the method as described before (Graham et al., 1991). The Ad5 vector stock used in this study was 1.68 × 109 PFU/mL. The viral stock of DEF201 and Ad5 vector was concentrated by 100 K Amicon centrifugal filter units and prepared in PBS before administrating into mice intranasally.
2.3. mIFN quantification by ELISA
For in vitro study, 105 of HEK293 cells were treated with DEF201 at different M.O.I. (0.1, 0.5, 1, 2, and 5) and cultured with DMEM+10%FBS media in 24-well plate. At 24 h post-treatment, cell culture supernatants were collected and mIFN concentrations were quantified by ELISA (R&D system). HEK293 cells infected with Ad5 vector were used as negative control.
For in vivo study, 5 × 107 PFU per mouse DEF201 was administered intranasally to 5–day-old suckling BALB/c mice with same dose of Ad5 vector as control. The blood of DEF201-treated mice was collected at different time points (1, 3, 5, 7 days post-treatment), serum was separated and the concentrations of mouse interferon-α in serum were detected by ELISA (R&D system).
2.4. Cytotoxicity of DEF201 in vivo
DEF201 was administered intranasally to 5–day-old suckling BALB/c mice with dose of 5 × 107 PFU per mouse. The body weight of DEF201-treated mice were weighed daily for 15 days with body weight loss as a marker for cytotoxicity effects (Chen et al., 2010). Naive 5–day-old suckling BALB/c mice were used as negative control for cytotoxicity study.
2.5. Establishment of a murine model for EV71 infection
All animal work was performed in accordance to the IACUC-approved protocol (088/10) and in Animal Biosafety Level 2 (ABSL-2) facility. Suckling BALB/c mice were purchased from Invivos. Mice were infected by enterovirus 71 (EV71) at 6 day old with 3–4 g body weight. EV71 infected and uninfected mice were fed in separated cages to prevent EV71 oral-fecal transmission among different groups of mice. 6 –day-old suckling BALB/c mice were infected with EV71 S41 (2 × 107 PFU per mouse). Virus-infected mice were monitored daily for 14 days post-infection and scored by a mice clinical scoring system (Supplementary Table 1). The mice clinical scoring system was used to score the severity of clinical symptoms in EV71-infected mice. The scoring system involved four items: activity, diarrhea, movement and body weight change. For the individual mouse, each item was scored daily and the sum scores of four items were used as total score. Total score 6 was defined as the endpoint.
2.6. Antiviral effects evaluation of DEF201
DEF201 was intranasally administered into nostrils of mice with serial doses (from 105 to 5 × 107 PFU per mouse). For prophylactic effects, DEF201 was administered to 4–5–day-old suckling BALB/c 48 h or 24 h prior to challenge. Mice were then infected with EV71 S41 (2 × 107 PFU per mouse) via intraperitoneal (i.p.) injection at 6 days old. For therapeutic effects, 6–day-old suckling BALB/c mice were infected with EV71 S41 (2 × 107 PFU per mouse) at 6 days old and DEF201 was intranasally administered at 5 × 107 PFU per mouse at 6 h or 12 h post-infection. 5 × 107 PFU DEF201 per mouse Ad5 empty vector was used as treatment control. Upon EV71 infection, the survival of mice was monitored with the mice scoring system. Mice muscle tissue was harvested on 7 days post-infection (dpi), virus load in muscle tissue were quantified by plaque assay. For histopathology study, hind limb muscle tissues from mice were harvested on 7 dpi, fixed with 4% paraformaldehyde for 1 week at 4 °C, decalcified at room temperature for 2 h, embedded in paraffin and sectioned in 4 μm thickness. Tissue damage was evaluated by haematoxylin and eosin (H&E) staining and EV71 antigen was detected by immunohistochemistry staining (IHC) using bondmax system. The antibody used for IHC was anti-EV71 antibody (MAB979, 1:200 dilution).
2.7. Quantification of EV71 load in mice tissue by plaque assay
EV71-infected mice were sacrificed on 7 days post-infection (dpi) and mice tissue, including hind limb muscle, liver, spleen, brain and sera were collected, homogenized and titrated by plaque forming assay. Briefly, RD cells monolayer was infected by mice tissue lysis supernatant for 1 h at 37°C to absorb EV71, washed twice with PBS and overlaid with DMEM containing 2%FBS and 0.5% Agarose (Firstbase). The monolayer was incubated for 4 days and staining with 10% crystal violet solution containing 4% paraformaldehyde.
2.8. Titration of EV71 antibodies by neutralization assay
EV71 prepared in DMEM+2%FBS media was inactivated by UV. The inactivation of EV71 was confirmed by plaque assay. 5–day-old mice were pre-treated with 5 × 107 PFU DEF201 (Ad5 Vector as control) 24 h prior to challenge and 6–day-old DEF201-treated mice was injected with 2 × 107 PFU UV-inactivated EV71. Mice serum was collected on 4, 8 and 12 days post-injection, incubated at 56 °C for 30min to inactivate complements. The neutralization ability of mice serum was evaluated by neutralization assay. Briefly, 50 PFU of EV71 in 50 μl DMEM media was mixed with 50 μl serial diluted DEF201-treated mice serum and incubated at 37°C for 1 h. After incubation, 105 RD cells in 24-well plate were infected with incubated mixture at 37°C for 1 h for virus absorption. Virus mixture was removed, RD cells were washed twice with PBS and DMEM media containing 0.5% agarose was added into 24-well plate for plaque formation. RD cells were incubated for 3–4 days and stained with 10% crystal violet solution containing 4% paraformaldehyde. Serum from mice without pre-infection treatment and injected with DMEM media was used as serum control. Plaques were counted and the effects of neutralization were determined.
3. Results
3.1. DEF201 produces abundant mIFN both in vitro and in vivo
The expression of mIFN driven by DEF201 is shown in Fig. 1 . DEF201 transfected HEK293 cells cultured for 24 h (Fig. 1a), showed a robust mIFN expression, 103–105 pg/mL in the supernatant. These mIFN concentrations increased in a DEF201 multiplicity of infection (M.O.I.) -dependent manner. Minimal mIFN expression (10 pg/mL) was detected upon treatment with Ad5 vector control. The expression of mIFN stimulated by DEF201 was further evaluated in vivo (Fig. 1b). Upon intranasal treatment of DEF201 at dose of 5 × 107 PFU per mouse, abundant mIFN (300–9000 pg/mL) was detected in mice serum on 1, 3 and 5 day post-infection treatment. mIFN concentrations in the serum of Ad5 vector-treated mice were within the range of 10–100 pg/mL which was used as baseline in this study. On 7 day post-infection treatment, the mIFN concentrations in the serum of DEF201-treated mice decreased to 70–500 pg/mL. These results showed that DEF201 could drive robust expression of mIFN both in vitro and in vivo and the in vivo expression of mIFN stimulated by DEF201 could last for at least 7 days.

Mouse interferon-α expression driven by DEF201 both in vitro and in vivo. (a) HEK293 cells were treated with DEF201 at different multiplicities of infection (M.O.I.) for 24 h, interferon-α concentrations in HEK293 culture supernatant were detected by ELISA. (b) 5–day-old BALB/c mice were treated intranasally with 5 × 107 PFU DEF201 per mouse and serum IFN-α concentration was quantified at different time points by ELISA. Ad5 empty vector was used as control for both in vitro and in vivo studies. In vitro data was presented as mean ± standard deviation of three biological repeats. In vivo data was presented as mean and individual value of five mice. *p < 0.05, paired t-test, compared to Ad5 Vector control.
3.2. DEF201 has minimal cytotoxic effects on suckling BALB/c mice
To evaluated the in vivo cytotoxicity effects of DEF201, 5–day-old BALB/c mice were intranasally administered DEF201 at the dose of 5 × 107 PFU per mouse, the body weight of DEF201-treated mice was measured daily for 15 days and the body weight change was used as a marker for cytotoxic effects (Chen et al., 2010). Non-treated naive mice were used as negative control for cytotoxic effects. As shown in Fig. 2 , DEF201-treated suckling mice showed no significant body weight difference compared with naive mice. No clinical symptoms, including diarrhea, ruffled fur, abnormal movement and inactivity, were observed for DEF201-treated mice. Together, the result showed that DEF201 had minimal cytotoxic effects on the suckling BALB/c mice.

Body weight changes as a measure of cytotoxic effects of DEF201 on suckling BALB/c mice. 5–day-old BALB/c mice were treated intranasally with 5 × 107 PFU DEF201, with 5–day-old naive mice used as control, and body weight was used as a marker for cytotoxic effects. The body weight of DEF201-treated mice was measured daily for 15 days and compared with untreated control. Data was presented as body weight mean ± standard deviation of six mice.
3.3. Murine model for EV71 infection
6–day-old BALB/c mice were infected with EV71 S41 via intraperitoneal injection (i.p.) with titer of 2 × 107 PFU per mouse. EV71 was prepared in DMEM media containing 2%FBS and mice injected with same volume of DMEM + 2%FBS media were used as mock-infected control. EV71-infected mice were monitored and scored daily using the scoring system mentioned above. As shown in Fig. 3 a, 100% mortality was observed by 9 days post-infection (dpi) for EV71-infected mice. EV71-infected mice developed severe clinical symptoms including inactivity, loss of body weight, ruffled fur and hind limbs paralysis. Fig. 3b showed the high clinical scores of EV71-infected mice as compared with mock control which showed minimal clinical symptoms. To detect viral load in EV71-infected mice tissues, the liver, spleen, brain, sera and hind limb muscle were harvested on 7 dpi and EV71 viral load was quantified by plaque assay. EV71 viral load was detected in hind limb muscle tissue (~104 PFU per gram) while no virus was detected in spleen, liver, brain and sera (Fig. 3c).

EV71 BALB/c mice model. 6–day-old BALB/c mice were infected with 2 × 107 PFU EV71 S41 per mouse, DMEM+2%FBS was used as mock-infected control. (a) The survival of EV71-infected mice was monitored daily for 14 days by a clinical scoring system and (b) the clinical symptoms of EV71-infected mice, including body weight change, activity, diarrhea, and movement, were scored daily for 14 day according to the severity of symptoms and (c) viral load in mice tissues, including hind limb muscle, blood, spleen, liver and brain tissues, was detected at 7 days post-infection by plaque assay. Each group contained 6 mice. Survival curve was generated and significance was calculated by Graphpad software. Clinical scores were presented as the mean ± standard deviation. The viral load from five mice was presented as the mean and individual values. Tissue damage and viral antigen in the hind limb muscle tissue of EV71-infected mice were detected at 7 days post-infection. (d) Muscle tissue damage of EV71-infected mice was detected by H&E staining and (e) muscle tissues from mice injected with DMEM+2%FBS media were used as mock control. (f) EV71 viral antigen in muscle of infected mice was detected by immunohistochemistry staining (IHC) using bondmax system with (g) muscle tissue of DMEM+2%FBS media-injected mice as control. Anti-EV71 antibody, MAB979, was used for IHC with 1:200 dilution.
To further confirm the presence of EV71 in hind limb muscle tissue, haematoxylin and eosin (H&E) staining was performed to detect the tissue damage and immunohistochemistry (IHC) staining was performed to detect viral antigen (Fig. 3d–e). H&E staining revealed that, by 7 dpi, EV71 caused severe tissue damage in hind limb muscle tissue which was indicated by the destruction of muscle fibers. Massive cells infiltration was also observed, suggesting that EV71 infection caused severe inflammation in hind limb muscle tissue. As reported, during EV71 infection, immune cells, mainly neutrophils and macrophages, were recruited to the EV71 infection sites and released abundant pro-inflammatory cytokines which further led to tissue damage (Wang et al., 2003). IHC study further revealed that abundant viral antigen was presented in hind limb muscle tissue of infected mice (Fig. 3f) as compared to the mock-infected mice (Fig. 3g), indicating EV71 established significant infection in the hind limb muscle tissue of mice.
3.4. DEF201 has dose-dependent prophylactic effects against EV71 on suckling BALB/c mice
To evaluate the prophylactic effects of DEF201, 5–day-old BALB/c suckling mice were intranasally administered DEF201 at different doses (105 to 5 × 107 PFU per mouse) at 24 h prior to infection. Ad5 empty vector was given at a similar dose to the treatment control. DEF201-treated BALB/c suckling mice were infected with EV71 at 6 days old and were monitored for 14 days by the clinical scoring system mentioned in Supplementary Table 1. As shown in Fig. 4 a, DEF201 at 106 PFU to 5 × 107 PFU offered full protection for mice against EV71 lethal challenge as compared to Ad5 vector or PBS-treated control which showed no protective effects. The low dose of 105 PFU DEF201 showed a >50% death rate indicating 105 PFU DEF201 was not effective in protecting the mice against EV71 infection. Fig. 4b showed the clinical scores of groups of the DEF201 mice. Consistent with the survival rate, the clinical symptoms and scores decreased in a DEF201 dose-dependent manner while Ad5 vector- or PBS-treated EV71-infected mice developed severe infection symptoms with significant clinical scores. Ad5 vector had no antiviral effects compared with PBS indicating that the antiviral effects of DEF201 were mainly due to the expression of mIFN. Fig. 4c showed the infectious EV71 viral loads in the muscle tissue and compared with the Ad5 empty vector administered control, DEF201 significantly decreased infectious EV71 viral loads in the hind limb muscle tissue in a dose-dependent manner. This was consistent with clinical scores of EV71-infected mice which also decreased in a DEF201 dose-dependent manner.

DEF201 dose-response. Survival curve, clinical scores and viral load detection upon intranasal DEF201 administration in murine model. Suckling BALB/c mice were dosed at 24hr prior to challenge with different levels of DEF201, from 105 to 5 × 107 PFU per mouse, and infected with 2 × 107 PFU per mouse EV71. DEF201 was prepared in PBS, Ad5 empty vector and PBS were used as treatment controls. (a) Survival of the mice were monitored daily for 2 weeks by a mice scoring system (b) clinical symptoms were scored and (c) the virus load in hind limb muscle tissue was detected by plaque assay. Each group contains ≥6 mice. Survival curve was generated and significance was calculated by Graphpad software. Clinical scores were presented as the mean of clinical scores. Viral load was presented as the mean and individual values.
3.5. DEF201 prophylaxis prevents tissue damage caused by EV71 infection and inhibits the replication of EV71 in muscle tissue
To further confirm the prophylactic effects of DEF201, H&E staining was performed in EV71-infected mice which were administered DEF201 24 h before challenge. As compared to Ad5 empty control mice which developed massive loss of muscle fiber, no tissue damage was observed upon pre-infection administration with a 106 PFU or higher dose of DEF201, suggesting that DEF201 completely inhibited the muscle tissue damage caused by EV71 infection (Fig. 5 a–h). Consistent with the inhibition of tissue damage, no immune cells infiltration was detected in the muscle tissue of EV71-infected mice with 106 PFU DEF201, suggesting that DEF201 inhibited the tissue inflammation induced by EV71 infection.
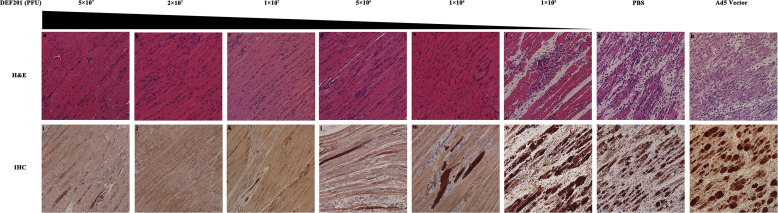
Muscle tissue damage and viral antigen in the muscle tissue of EV71-infected mice upon intranasal DEF201 administration at 24 h pre-challenge. Hind limb muscle tissue of the mice was harvested at 7 days post-infection, fixed by 4% paraformaldehyde for 7 days at 4 °C, embedded in paraffin and sectioned with 4 μm thickness. (a–f) Muscle tissue damage of the mice was detected by H&E staining, with (g) PBS and (h) Ad5 vector as treatment controls. (i–p) EV71 viral antigen in muscle tissue of EV71-infected mice was detected by IHC using bondmax system and anti-EV71 antibody (MAB979, 1:200 dilution).
To detect whether DEF201 could inhibit the replication of EV71 in mice muscle tissue, IHC was performed (Fig. 5i–p). Consistent with the virus load detection, with administration of DEF201 24 h prior to challenge, viral antigen was significantly reduced in the muscle tissue of EV71-infected mice, indicating that DEF201 could potently inhibit the replication of EV71 in the muscle tissue of mice.
3.6. Extended prophylactic effects of DEF201 on EV71 infection
As shown in Fig. 1b, mIFN stimulated by DEF201 could last for 7 days in mice serum. Therefore, the antiviral effects of DEF201 might be able to last for weeks. One group of mice were administered DEF201 at 48 h prior to challenge at a dose of 5 × 107 PFU per mouse and full protection as well as minimal clinical scores were observed (Fig. 6 a–b). In addition, with DEF201 administration at 48 h pre-challenge, the virus load in the muscle tissue of EV71-infected mice was significantly decreased (Fig. 6c). Furthermore, DEF201 administration 48 h pre-challenge protected mice from tissue damage and inhibited EV71 replication in the muscle tissue (Fig. 6d–g). The data clearly indicated that 2-day pre-challenge dosing with DEF201 had prophylactic effects on the mice. However, it was worth noting that the long-term prophylactic effects of DEF201 could not be determined in this murine model for EV71 due to the age limit of the suckling mice.

DEF201 prophylactic effects measured by survival curve, clinical scores and viral load detection upon 48hr pre-challenge administration of DEF201in murine model. 4–day-old suckling BALB/c mice were dosed at 48 h prior to challenge with 5 × 107 PFU per mouse DEF201 and infected with EV71 S41 at 2 × 107 PFU per mouse, Ad5 vector was used as treatment control. (a) The survival of the mice was monitored for 14 days using a clinical scoring system and (b) clinical symptoms were scored and (c) the virus load in muscle tissue was detected by plaque assay. Each group contains 6 mice. Survival curve was generated and significance was calculated by Graphpad software. Clinical scores were presented as mean ± standard deviation of clinical scores of the mice. Viral load of five mice was presented as mean and individual values. Tissue damage and viral antigen in the muscle tissue were detected at 7 days post-infection. (d) Muscle tissue damage of the mice was detected by H&E staining and (e) muscle tissues from mice treated with Ad5 vector were used as control. (f) EV71 viral antigen in muscle tissue of the mice was detected by IHC with using bondmax system and anti-EV71 antibody (MAB979, 1:200 dilution) and (g) Ad5 vector was used as treatment control.
3.7. DEF201 has therapeutic effects during early stage of EV71 infection
To evaluate the therapeutic effects of DEF201, 6–day-old BALB/c suckling mice were infected by EV71 and at 6 h or 12 h post-infection, mice were intranasally dosed with DEF201 (5 × 107 PFU per mouse). The same dose of Ad5 empty vector was used as treatment control. As shown in Fig. 7 a–b, full protection and minimal clinical symptoms were observed at 6 h post-infection treatment compared with the Ad5 vector which showed no protection. For 12 h post-infection treatment with DEF201, a 2-day delay of death and 33.3% survival rate were observed for the murine model. Further study showed that DEF201 post-infection treatment did not significantly decrease the virus load in EV71-infected mice muscle tissue (Fig. 7c). However, consistent with survival study, histopathology analysis further revealed that DEF201 reduced EV71 antigen in the muscle tissue and prevented mice from developing severe tissue damage and inflammation (Fig. 7d–k). The results strongly demonstrated that DEF201 had therapeutic effects against EV71 by inhibiting tissue damage and viral replication in muscle tissue in early stage of EV71 infection.

DEF201 treatment effects measured by survival curve, clinical scores and viral load detection upon DEF201 post-infection treatment in murine model. 6–day-old suckling BALB/c mice were infected with EV71 at 2 × 107 PFU per mouse and treated with DEF201 at 5 × 107 PFU per mouse at 6 h or 12 h post-infection and the same dose of Ad5 vector was used as treatment control. (a) Survival of the mice were monitored daily for 2 weeks by a mice scoring system and (b) clinical symptoms were scored and (c) the virus load in muscle tissue was detected by plaque assay. Each group contained ≥6 mice. Survival curve was generated and significance was calculated by Graphpad software. Clinical scores were presented as mean + standard deviation of clinical scores of the mice. The viral load from five mice was presented as mean and individual value. Tissue damage and viral antigen in the muscle tissue of EV71-infected mice were detected at 7 days post-infection. No tissue damage was detected by H&E staining for (d) 6 h or (f) 12 h post-infection treatment of DEF201, compared with (e, g) Ad5 vector controls. Consistent with tissue damage detection, significantly decreased viral antigen was shown by IHC in mice with (h) 6 h or (j) 12 h post-infection treatment of DEF201, compared with (i, k) Ad5 vector controls. MAB979 was used for IHC with 1:200 dilution.
3.8. DEF201 enhances the production of specific anti-EV71 neutralizing antibodies
IFN is mainly involved in innate immune system by directly inhibiting the replication of viruses. However, previous studies reported that IFN also played a role in acquired immune system. IFN could enhance antibody production by enhancing B cell development and differentiation (Neubauer et al., 1985, Jego et al, 2003). IFN also promoted immunoglobulin class switching from IgM to IgG and enhanced the specificity of antibodies (Finkelman et al., 1991). Long term antibody production and immune memory were also induced by IFN (Le et al., 2001). Thus, DEF201 might be able to enhance the neutralizing ability of anti-EV71 antibodies in mice serum during EV71 infection. To evaluate this, 5–day-old BALB/c suckling mice were intranasally dosed with DEF201 (5 × 107 PFU per mouse) at 24 h prior to challenge. Ad5 empty vector was as control. DEF201-treated mice were vaccinated by UV-inactivated non-replicating EV71 (2 × 107 PFU per mouse) via i.p. injection, mice serum was collected at different time points post-vaccination and the neutralizing ability of mice serum was evaluated by neutralization assay. As shown in Fig. 8 a–c, in comparison with Ad5 vector control, serum of mice given DEF201 showed 20%–30% higher neutralizing ability against EV71 at day 4 (8a), 8 (8b) and 12 (8c) post-injection of UV-inactivated EV71. Statistical studies showed that the percentage of EV71 which was not neutralized by mice serum was significantly lower upon DEF201 treatment, in comparison to Ad5 control (Fig. 8d). The result supported that DEF201 could enhance the production of specific anti-EV71 neutralizing antibodies in vivo.

Neutralization assay of serum from DEF201-treated EV71-vaccinated mice. 5–day-old mice were dosed with 5 × 107 PFU DEF201 (Ad5 Vector as control) at 24 h prior to challenge and injected with 2 × 107 PFU UV-inactivated EV71 at 6 day old. Mice serum was collected on 4, 8 and 12 days post-injection and incubated at 56 °C for 30min to inactivate complement. The neutralization ability of mice serum was evaluated by neutralization assay. Serum from the mice showed enhanced neutralization ability against EV71 compared Ad5 Vector control on (a) day 4, (b) day 8 and (c) day 12 post-injection. (d) Percentage of non-neutralized viruses upon incubation with DEF201- and Ad5-treated mice serum was calculated by Graphpad according to neutralization curves. Data was presented as mean ± standard deviation and significance was calculated by t-test, *p < 0.05.
4. Discussion
In this study, we have shown for the first time that a single dose of DEF201 is highly efficacious as a prophylactic agent, in a dose-dependent manner, against EV71 infection in vivo. In the murine model, 106 PFU DEF201 offered full protection for mice against lethal EV71 infection. For therapeutic application, DEF201 can only offer protection for mice at early stage of EV71 infection (less than 12 h), indicating that DEF201 has limited effects at late stage of mouse EV71 infection when symptoms have already developed. Clinically, the latency of EV71 infection is several days and the limited antiviral effects of DEF201 suggesting that the application of DEF201 in the treatment of EV71 infection is restricted. However, one important point was that, in this murine model, a lethal dose of EV71 virus was used which quickly established a significant infection in mice tissues and led to death of the mice while clinically, EV71 infection is usually a self-limiting disease with mild symptoms featuring hand, foot and mouth symptoms. Thus, there is still a chance that DEF201 might be effective to treat EV71 infected patients developed symptoms as the diseases progression is less severe. To evaluate the clinical therapeutic efficacy of DEF201 against EV71 infection, further studies are necessary.
In addition to antiviral effects, IFN also plays a role in the regulation of immune cells development, such as B cells. Previous studies have shown that IFN is important for B cell activation, antibody production and antibody class switching (Le et al., 2001). In this study, we have shown that DEF201 is able to enhance the production of neutralizing antibodies against EV71. Thus, DEF201 can be used as a broad-spectrum immune stimulating agent to prevent viral infection as well as other infectious diseases. Furthermore, DEF201 might be used as an adjuvant to enhance the production of antibodies and the efficacy of vaccines.
In addition to EV71, other viruses have been reported to be inhibited by DEF201. Previously, it has been reported that pre-infection dosing with 107 PFU DEF201 could offer 100% protection for mice against different strains of Western equine encephalitis virus (WEEV), including a highly-virulent strain (Fleming) (Wu et al., 2007). Post-infection treatment of DEF201 at 6 h post WEEV infection was also protective. Similar inhibition pattern by DEF201 was also observed for vaccinia virus (Smee et al., 2011), arenavirus (Gowen et al., 2011), SARS coronavirus (Kumaki et al., 2011), yellow fever virus (Julander et al., 2011) and chikungunya virus (Dagley et al., 2014). Pre-infection treatment with 106-108 PFU DEF201 offered significant protection in vivo in a dose-dependent manner while post-infection treatment at early time-point post infection was effective. Together, these results demonstrate that pre-infection treatment with DEF201 is able to inhibit the infection of varieties of viruses and can be used as a broad-spectrum antiviral agent.
The use of adenovirus vector as an in vivo constitutive IFN expression platform evaluates serum IFN and induces long term antiviral effects with a single dose. In our study, we showed that IFN produced by 5 × 107 PFU DEF201 was maintained at high levels for at least 7 days in mice serum. This demonstrates that the effective half-life of IFN has been significantly prolonged by DEF201 which is continually releasing the IFN into the bloodstream. To further illustrate whether DEF201 has long term antiviral effects, we tested the antiviral effects of 48 h pre-infection treatment of DEF201 in our EV71 murine model. Due to the age limit of suckling mice model, we were only able to show that DEF201 pre-infection treatment is effective for at least 48 h against EV71 infection. However, previous studies have shown that the antiviral effects of DEF201 could last for at least 13 weeks for WEEV and 56 days for vaccinia virus. This might be due to three reasons: firstly, antiviral state of host cells can last for long term upon IFN stimulation, secondly, the IFN is continually produced by the transduced cells, and thirdly, the immune system stimulated by DEF201 offers long term antiviral protection. In conclusion, these results demonstrate that DEF201 is able to offer long term antiviral protection in vivo.
However, the robust and lasting expression of IFN induced by DEF201 might cause IFN-related side effects, such as headache, diarrhea and nausea. Although we did not observe any side effect in suckling mice, the difference between animal models and clinical application should be considered. Thus, further clinical evaluation of DEF201-related side effects is necessary, especially for children since they are more vulnerable for HFMD. In addition, the use of adenovirus 5 vector might stimulate immune response against the vector which might lead to inflammation at the inoculation sites of DEF201 especially for those people who have pre-existing immune response to adenovirus 5, though the inoculation via intranasal administration has minimized the exposure of adenovirus 5 vector to immune system. Thus, further DEF201-related immune response should be evaluated in clinical trials. One approach to avoid immune response to DEF201 for people with pre-existing immune response to adenovirus 5 is to change another delivery vector for DEF201.
In addition, the IFN subtype in DEF201 is mouse α5. However, for EV71 inhibition, a previous study showed that α4, α6, α14 and α16 were more effective than other type I IFNs although all type I IFNs had inhibition effects against EV71 (Yi et al., 2011). Thus, for the humanized version of DEF201, α4, α6, α14 and α16 should be considered for treatment of EV71 infection.
In conclusion, we have shown that a single dose of DEF201 was highly efficacious as a prophylactic agent against EV71 infection in vivo. Furthermore, DEF201 can significantly enhance the neutralizing ability of anti-EV71 antibody in mice serum. The clinical prophylactic effects of DEF201 is worth being further evaluated.
Acknowledgments
This study was supported by MINDEF DIRP Grant [R182-000-210-232].
Footnotes
Appendix ASupplementary data related to this article can be found at http://dx.doi.org/10.1016/j.antiviral.2016.09.003.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Balachandran S. Alpha/beta interferons potentiate virus-induced apoptosis through activation of the FADD/Caspase-8 death signaling pathway. J. Virol. 2000;74:1513–1523. [Europe PMC free article] [Abstract] [Google Scholar]
- Chen Y.L. Inhibition of dengue virus by an ester prodrug of an adenosine analog. Antimicrob. Agents Chemother. 2010;54:3255–3261. [Europe PMC free article] [Abstract] [Google Scholar]
- Chou A.H. Formalin-inactivated EV71 vaccine candidate induced cross-neutralizing antibody against subgenotypes B1, B4, B5 and C4A in adult volunteers. PloS One. 2013;8:e79783. [Europe PMC free article] [Abstract] [Google Scholar]
- Dagley A. Protection against Chikungunya virus induced arthralgia following prophylactic treatment with adenovirus vectored interferon (mDEF201) Antivir. Res. 2014;108:1–9. [Europe PMC free article] [Abstract] [Google Scholar]
- Damjanovic D. Type 1 interferon gene transfer enhances host defense against pulmonary Streptococcus pneumoniae infection via activating innate leukocytes. Mol. Ther. — Methods & Clin. Dev. 2014;1 [Europe PMC free article] [Abstract] [Google Scholar]
- Finkelman F.D. Regulation by interferon alpha of immunoglobulin isotype selection and lymphokine production in mice. J. Exp. Med. 1991;174:1179–1188. [Europe PMC free article] [Abstract] [Google Scholar]
- Gowen B.B. Use of recombinant adenovirus vectored consensus IFN-a to avert severe arenavirus infection. PloS One. 2011;6:e26072. [Europe PMC free article] [Abstract] [Google Scholar]
- Graham F.L., Prevec L. Manipulation of adenovirus vectors. In: Murray E.J., editor. vol. 7. Humana Press; Clinton, NJ: 1991. pp. 109–128. (Methods in Molecular Biology). [Abstract] [Google Scholar]
- Jego G. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19:225–234. [Abstract] [Google Scholar]
- Julander J.G., Ennis J., Turner J., Morrey J.D. Treatment of yellow fever virus with an adenovirus-vectored interferon, DEF201, in a hamster model. Antimicrob. Agents Chemother. 2011;55:2067–2073. [Europe PMC free article] [Abstract] [Google Scholar]
- Kumaki Y. Single-dose intranasal administration with mDEF201 (adenovirus vectored mouse interferon-alpha) confers protection from mortality in a lethal SARS-CoV BALB/c mouse mode. Antivir. Res. 2011;89:75–82. [Europe PMC free article] [Abstract] [Google Scholar]
- Le B.A. Type I interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity. 2001;14:461–470. [Abstract] [Google Scholar]
- Li L., Yin H., An Z., Feng Z. Considerations for developing an immunization strategy with enterovirus 71 vaccine. Vaccine. 2014;33:1107–1112. [Abstract] [Google Scholar]
- Liu M.-L. Type I interferons protect mice against enterovirus71 infection. J. General Virol. 2005;86:3263–3269. [Abstract] [Google Scholar]
- Neubauer R.H., Goldstein L., Rabin H., Stebbing N. Stimulation of in vitro immunoglobulin production by interferon-alpha. J. Immunol. 1985;134:299–304. [Abstract] [Google Scholar]
- Scharton D. Rift valley fever virus infection in golden syrian hamsters. PloS One. 2015;10:e0116722. [Europe PMC free article] [Abstract] [Google Scholar]
- Sinovac Obtains New Drug Certificate and Production License for EV71 Vaccine. 2016. http://www.sinovac.com/?optionid=754&auto_id=803 Sinovac Website Press Releases. (accessed 04.01.16) [Google Scholar]
- Smee D.F. Therapy and long-term prophylaxis of vaccinia virus respiratory infections in mice with an adenovirus-vectored interferon alpha (mDEF201) PloS One. 2011;6:e26330. [Europe PMC free article] [Abstract] [Google Scholar]
- Stark G.R. How cells respond to interferons. Ann. Rev. Biochem. 1998;67:227–264. [Abstract] [Google Scholar]
- Takaoka A. Integration of interferon-alpha/beta signalling to p53 responses in tumour suppression and antiviral defence. Nature. 2003;424:516–523. [Abstract] [Google Scholar]
- van Boxel-Dezaire A.H., Rani M.R.S., Stark G.R. Complex modulation of cell type-specific signaling in response to type I interferons. Immunity. 2006;25:361–372. [Abstract] [Google Scholar]
- Wang S.M. Pathogenesis of enterovirus 71 brainstem encephalitis in pediatric patients: roles of cytokines and cellular immune activation in patients with pulmonary edema. J. Infect. Dis. 2003;188:564–570. [Abstract] [Google Scholar]
- Wu J.Q.H. Pre- and post-exposure protection against Western equine encephalitis virus after single inoculation with adenovirus vector expressing interferon alpha. Virology. 2007;369:206–213. [Abstract] [Google Scholar]
- Yi L. Potent inhibition of human enterovirus 71 replication by type I interferon subtypes. Antivir. Ther. 2011;16:51–58. [Abstract] [Google Scholar]
- Zhu F.-C. Immunogenicity and safety of an enterovirus 71 vaccine in healthy Chinese children and infants: a randomised, double-blind, placebo-controlled phase 2 clinical trial. Lancet. 2013;381:1037–1045. [Abstract] [Google Scholar]
Citations & impact
Impact metrics
Citations of article over time
Article citations
Research progress on pathogenic and therapeutic mechanisms of Enterovirus A71.
Arch Virol, 168(10):260, 29 Sep 2023
Cited by: 0 articles | PMID: 37773227
Review
Experimental animal models for development of human enterovirus vaccine.
Clin Exp Vaccine Res, 12(4):291-297, 31 Oct 2023
Cited by: 0 articles | PMID: 38025911 | PMCID: PMC10655152
Review Free full text in Europe PMC
AAV-CRISPR-Cas13 eliminates human enterovirus and prevents death of infected mice.
EBioMedicine, 93:104682, 28 Jun 2023
Cited by: 4 articles | PMID: 37390772 | PMCID: PMC10363442
TBK1 and IRF3 are potential therapeutic targets in Enterovirus A71-associated diseases.
PLoS Negl Trop Dis, 17(1):e0011001, 10 Jan 2023
Cited by: 4 articles | PMID: 36626364 | PMCID: PMC9831319
Pharmacological perturbation of CXCL1 signaling alleviates neuropathogenesis in a model of HEVA71 infection.
Nat Commun, 13(1):890, 16 Feb 2022
Cited by: 1 article | PMID: 35173169 | PMCID: PMC8850555
Go to all (13) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Nucleotide Sequences
- (1 citation) ENA - AF316321
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
In Vivo Imaging with Bioluminescent Enterovirus 71 Allows for Real-Time Visualization of Tissue Tropism and Viral Spread.
J Virol, 91(5):e01759-16, 14 Feb 2017
Cited by: 15 articles | PMID: 27974562 | PMCID: PMC5309956
Single-dose intranasal treatment with DEF201 (adenovirus vectored consensus interferon) prevents lethal disease due to Rift Valley fever virus challenge.
Viruses, 6(3):1410-1423, 24 Mar 2014
Cited by: 8 articles | PMID: 24662673 | PMCID: PMC3970158
Use of recombinant adenovirus vectored consensus IFN-α to avert severe arenavirus infection.
PLoS One, 6(10):e26072, 24 Oct 2011
Cited by: 10 articles | PMID: 22039436 | PMCID: PMC3200317
Diseases caused by enterovirus 71 infection.
Pediatr Infect Dis J, 28(10):904-910, 01 Oct 2009
Cited by: 79 articles | PMID: 20118685
Review




