Abstract
Free full text

The need to manage the risk of thromboembolism in COVID-19 patients
Abstract
COVID-19 first appeared in Wuhan, Hubei Province, China, in December 2019. Thought to be of zoonotic origin, it has been named SARS-CoV-2 (COVID-19) and has spread rapidly. As of April 20, 2020, there have been >2.4 million cases recorded worldwide. The inflammatory process, cytokine storm, and lung injury that are associated with COVID-19 can put patients at an increased risk of thrombosis. The total incidence of thrombotic events in COVID-19 patients is currently uncertain. Those with more severe disease and with other risk factors, including increasing age, male sex, obesity, cancer, comorbidities, and intensive care unit admission, are at higher risk of these events. However, there is little international guidance on managing these risks in COVID-19 patients. In this paper, we explore the current evidence and theories surrounding thrombosis in these unique patients and reflect on experience from our center.
Understanding thrombosis in the context of COVID-19
COVID-19 appears to be associated with both venous and arterial thrombosis. This was observed by Klok et al,1 who found that despite thromboprophylaxis of patients, venous thrombosis developed, with pulmonary embolism (PE) being the most common. Instances of arterial thrombosis including ischemic strokes were also noted.
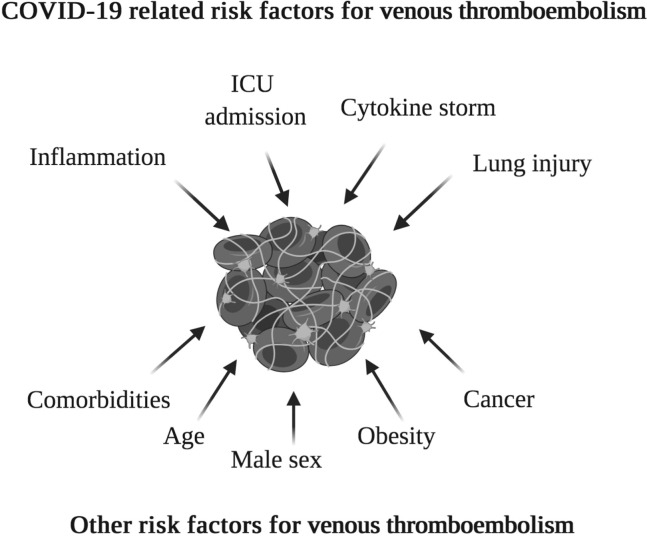
A recent study identified that those who were critically ill with SARS-CoV-2 had elevated levels of D-dimer, fibrinogen, and fibrinogen degradation products compared with healthy controls.2 This may be due to endothelial injury resulting in thrombin generation and fibrinolysis shut down, contributing to a hypercoagulable state. A similar relationship between acute respiratory distress syndrome (which can occur in patients with COVID-19) and deep venous thrombosis (DVT) has previously been shown with influenza A (H1N1).3 The inflammatory disease process, prolonged hospital stay, and pre-existing comorbidities can contribute to venous thromboembolism (VTE).4 A summary of these factors can be seen in the Fig .
SARS-CoV-2 has been shown to gain entry to cells by binding to angiotensin-converting enzyme 2 receptors, and the spike protein plays a vital role in this.5 This protein is cleaved by plasmin, which is found to be higher in individuals with cardiovascular disease, thus potentially making them more susceptible to poorer outcomes.6 , 7 The interaction of SARS-CoV-2 with angiotensin-converting enzyme 2 receptor may cause endothelial damage as a fivefold rise of von Willebrand factor levels has been reported in COVID-19 patients.8 It is well known that endothelial dysfunction is a component of Virchow's triad, driving the development of thrombosis.9
Clinical presentations of thrombosis
In a study including 30 intensive care unit (ICU) patients, 16 were found to have clinical DVT.10 The thrombus was found commonly in the femoropopliteal region (55%), followed by brachial-axillary veins. For upper limb involvement, the authors proposed that continuous positive airway pressure ventilators can often be tied in a way that compresses the superficial or deep vessels of the upper limbs, leading to increased risk of DVT. Zhou et al11 reported a case in which concomitant lower limb venous and arterial thrombosis developed in a COVID-19 patient on the third day of admission. This illustrates the aggressive thrombotic burden in COVID-19 patients.
The Padua Prediction Score takes into consideration multiple factors, as seen in Table I , and can be used to assess patients for VTE.12 Low risk of VTE is defined as a score of <4; a score of ≥4 makes thromboprophylaxis necessary. In a study including 138 patients, Xu et al13 found 23 (16.67%) COVID-19 patients to be at high risk for VTE according to the Padua Prediction Score. A study by Cui et al14 with 81 patients identified 20 (25%) patients to have VTE, of whom eight died. VTE is a risk in COVID-19 patients and may go unnoticed in critical care settings. Early use of scoring systems and risk stratification is paramount in this unique population.
Table I
Padua risk assessment tool used to classify risk of venous thromboembolism (VTE)
| Factors | Score |
|---|---|
| Active cancer | 3 |
| Previous VTE | 3 |
| Reduced mobility | 3 |
| Known thrombophilic condition | 3 |
| Recent trauma or surgery (≤1 month) | 2 |
| Age (≥70 years) | 1 |
| Heart or respiratory failure | 1 |
| Acute MI or ischemic stroke | 1 |
| Acute infection or rheumatologic disorder | 1 |
| Obesity (BMI ≥30 kg/m2) | 1 |
| Currently receiving hormonal therapy | 1 |
| Total score | |
| <4 = Low risk | ≥ 4 = High risk |
BMI, Body mass index; MI, myocardial infarction.
Studies have reported cases of PE in patients with COVID-19. Concomitant PE with COVID-19 should be considered a possibility by clinicians. It requires appropriate management as it may have a profound impact on prognosis. Casey et al15 presented a case of PE in a low-risk COVID-19 patient with no travel history or comorbidities, suggesting that the disease process itself was responsible for PE. Xie et al16 presented two cases from Wuhan of patients in whom PE developed during the hospital stay and who showed respiratory deterioration and raised D-dimer levels. From these case studies, it is evident that PE in the context of COVID-19 is complex and can present with no other risk factors. Furthermore, this is complicated because of the overlap with other respiratory symptoms and adds another layer of diagnostic challenge.
A study evaluating outcomes of 183 patients showed that 71.4% of nonsurvivors met the criteria for disseminated intravascular coagulation (DIC).17 In DIC, there can be a simultaneous derangement of hemostasis and hypercoagulability, resulting in abnormal coagulation profiles. These patients showed elevated D-dimer levels, prolonged prothrombin time (PT) and activated partial thromboplastin time (aPTT), and thrombocytopenia. Another study using thromboelastography to evaluate whole blood from 24 ICU patients showed similar elevated D-dimer levels. However, they showed normal or increased fibrinogen, platelet count, PT, and aPTT, which is consistent with hypercoagulability more than with DIC.18 Although both groups of patients were admitted to the ICU, these differences may be explained by the stage of the disease. DIC may therefore potentially be a late stage of COVID-19. Sepsis is known to cause DIC, and patients with COVID-19 may develop sepsis resulting in DIC. The sepsis-induced coagulopathy (SIC) criteria were created by the International Society on Thrombosis and Haemostasis to categorize sepsis and coagulopathy. SIC values >4 are associated with greater severity of illness, highlighting the importance of prompt recognition and appropriate escalation.19 Iba et al proposed a “two-step system” for the early detection of DIC, with initial screening with the SIC score and, for those who meet these criteria, calculating the DIC score.20 The role of these scoring systems in the context of COVID-19 coagulopathy remains to be demonstrated. The American Society of Hematology reported coagulopathy in COVID-19 to be different from classic DIC, with milder thrombocytopenia and lack of microangiopathy seen. However, the American Society of Hematology recommended the monitoring of platelet count, PT, aPTT, D-dimer, and fibrinogen, emphasizing the role of D-dimer as a marker of severity of infection.21
Myocardial injury can also occur through various mechanisms in COVID-19 patients, especially in those with pre-existing cardiovascular disease. Systemic inflammation as well as shear stress due to increased coronary blood flow can result in plaque rupture, thus causing myocardial infarction.22 A study showed that acute cardiac injury is more common with raised troponin I as well as with significant ST elevation, which could mimic acute coronary occlusion. In patients with COVID-19, there is a higher prevalence of myocardial injury, particularly in patients admitted to the ICU, and it is a poor prognostic factor in the critically ill.23
COVID-19 patients with pre-existing cerebrovascular disease may be at a higher risk of developing strokes. Although there have not been many studies linking stroke and COVID-19, a study with 214 patients identified 5.7% of their patients with severe disease (n = 88) to have developed a stroke, of which four were ischemic.24
Managing thrombosis in COVID-19 patients
A recent study examined two groups of patients, those with COVID-19 and those without COVID-19. The COVID-19 group with elevated D-dimer levels (>6 times the upper limit of normal) showed lower mortality rates with heparin administration (low-molecular-weight heparin [40-60 mg of enoxaparin per day] or unfractionated heparin [10,000-15,000 units/d]) than those without heparin.25 Interestingly, there was no difference in mortality in the COVID-19-negative patients with the use of heparin when stratified by D-dimer level. Heparin has previously been shown to play an anti-inflammatory role and to offer endothelial protection.26 Its antiviral effect is also being studied in experimental models and is an avenue that requires further study.
Generous use of thromboprophylaxis may help improve outcomes, especially in those at high risk of VTE.4 Klok et al1 studied 184 ICU patients; 23 (13%) patients died, and 22 (12%) were discharged. The remaining 139 (76%) were still in the ICU; 31% had thrombotic complications despite receiving low-molecular-weight heparin (subcutaneous doses of 2850 IU or 5700 IU per day, dependent on body weight or date of treatment). From this study, it is evident that thrombotic complications are high despite thromboprophylaxis. Obi et al3 observed during the H1N1 pandemic that those with acute respiratory distress syndrome were at increased risk of VTE. They found that those without anticoagulation were 33 times more likely to develop any VTE than those who were treated with empirical systemic intravenous heparin anticoagulation (aiming for a target partial thromboplastin time of 50-70 seconds). Therefore, a similar approach may be necessary for COVID-19 patients to prevent these complications from occurring.
Challenges
Managing thrombosis in the context of COVID-19 has its own set of novel challenges, including patients with other comorbidities, such as atrial fibrillation. There is the issue of drug interactions. The University of Liverpool in the United Kingdom has developed a list of drug interactions with experimental COVID-19 therapies and suggests that some should not be coadministered with antivirals such as lopinavir and ritonavir. Apixaban, used in atrial fibrillation, may interact with these antivirals.27 A summary of pertinent drug interactions can be seen in Table II . Issues with drugs used for other conditions may continue to emerge as this database populates.
Table II
Drug interactions in COVID-19
| Class | Subtype | Drug | Atazanavira | Lop/Rv | Remdesivirb | Favipiravirb | CLQ | HCLQ | Ribavirinb | Tocilizumabc | IFN-β | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antiplatelets | Thromboxane inhibitor | Aspirin | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | |
| Dipyridamole | ↑↑ | ↓↓ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | |||
| ADP receptor/P2Y12 inhibitors | Thienopyridines | Clopidogrel | ↓↓↓ | ↓↓↓ | ↔ | ↔ | ↔ | ↔ | ↔ | ↓ | ↔ | |
| Prasugrel | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↓ | ↔ | |||
| Nucleotide analogues | Ticagrelor | ↑↑↑ | ↑↑↑ | ↔ | ↔ | ↔ | ↔ | ↔ | ↓ | ↔ | ||
| Anticoagulants | Vitamin K antagonist | Acenocoumarol | ↔ | ↓↓ | ↔ | ↔ | ↔ | ↔ | ↔ | ↓ | ↔ | |
| Phenprocoumon | ↑↑ | ↑↓ | ↔ | ↔ | ↔ | ↔ | ↔ | ↓ | ↔ | |||
| Warfarin | ↑↑ | ↓↓ | ↔ | ↔ | ↔ | ↔ | ↓↓ | ↓ | ↔ | |||
| Factor Xa inhibitors | Heparin/glycosaminoglycans | Dalteparin | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | |
| Enoxaparin | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | |||
| Fondaparinux | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | |||
| Heparin | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | |||
| Direct Xa inhibitor | Apixaban | ↑↑↑ | ↑↑↑ | ↔ | ↔ | ↑ | ↑ | ↔ | ↓ | ↔ | ||
| Betrixaban | ↑↑ | ↑↑ | ↔ | ↔ | ↑↑ | ↑↑ | ↔ | ↔ | ↔ | |||
| Edoxaban | ↑↑ | ↑↑ | ↔ | ↔ | ↑↑ | ↑↑ | ↔ | ↔ | ↔ | |||
| Rivaroxaban | ↑↑↑ | ↑↑↑ | ↔ | ↔ | ↑ | ↑ | ↔ | ↓ | ↔ | |||
| Direct thrombin (IIa) inhibitor | Argatroban | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ||
| Dabigatran | ↑↑↑ | ↓↓ | ↔ | ↔ | ↑↑ | ↑↑ | ↔ | ↔ | ↔ | |||
ADP, Adenosine diphosphate; CLQ, chloroquine (antimalarial); HCLQ, hydroxychloroquine (antimalarial and used in rheumatoid arthritis and lupus); IFN-β, interferon β; Lop/Rv, loprinovir/ritonavir (combination treatment for HIV/AIDS).
Upward arrow (↑) indicates an increased risk of interaction. Downward arrow (↓) indicates a reduced risk of interaction. Single arrow (↓/↑)denotes potential interaction, likely to be of weak intensity—dose adjustment is unlikely. Double arrows (↑↑/↓↓)indicate possible interaction that may require dose adjustment or close monitoring. Triple arrows (↑↑↑/↓↓↓) indicate that these two drugs should not be coadministered. Horizontal arrow (↔) indicates no significant interaction reported.
In a study including 407 patients who were considered at high risk of VTE, it was found that 44 (11%) patients were at a high risk of bleeding.28 Therefore, regular monitoring, adjustment of thromboprophylaxis, and correction of coagulopathy are also crucial in preventing complications.
Our experience
In our unit, as of April 26th, we had 35 COVID-19 patients, and D-dimer level and renal function were used to guide the standard of anticoagulation in the ICU. A D-dimer level of >1000 ng/mL is considered to be a level at which anticoagulation with 1.5 mg/kg body weight of enoxaparin once a day is initiated; if the patient's creatinine clearance is <30 mL/min, 1 mg/kg body weight is used until the D-dimer level is <1000 ng/mL. However, this treatment continues if there is clinical evidence of PE, DVT, or other thrombotic events regardless of D-dimer level. A similar approach was used by colleagues in Massachusetts General Hospital.29 The D-dimer level was evaluated on a daily basis to assess the progress of such patients in terms of VTE risk. At least twice daily, a full clinical assessment of those patients was performed to determine clinical progress and to look for clinical signs of VTE. We reported no instances of PE but one case of DVT.
Future perspectives
Studies have shown the increased risk of mortality associated with VTE.10 A meta-analysis that examined nine studies with >1000 COVID-19 patients looked at the mean differences in platelet count between patients with and patients without severe disease.30 Overall, there was a significantly lower platelet count in patients with more severe COVID-19. The platelet count may be an affordable and accessible biomarker for assessment of disease severity and risk of coagulopathies. Similarly, elevated plasmin has been seen in the setting of acute respiratory distress syndrome and may be useful as a biomarker in COVID-19 patients.31 The use of point-of-care ultrasound screening for DVT may also be necessary, and others have also been suggested this in the context of COVID-19.32
Many hospitals have switched their standard thromboprophylaxis dose to higher doses and even treatment dose thromboprophylaxis based on local expertise. In patients in whom this is contraindicated, it is essential that mechanical thromboprophylaxis (eg, pneumatic compression) devices are used appropriately. Post-discharge thromboprophylaxis also needs to be considered on an individual basis, taking into account the risk of bleeding and packaged with patient education and appropriate follow-up. It is paramount that the outcomes of these experiences be disseminated to help guide clinical judgment.
Conclusions
COVID-19 patients are at increased risk of thrombosis for many reasons, including inflammation, immobility, and other factors that contribute to a hypercoagulable state. It is vital to ensure that early risk stratification is carried out and adequate thromboprophylaxis or anticoagulation is administered to patients. At the same time, we await large-scale studies exploring thrombosis in the context of COVID-19.
Author contributions
Conception and design: IK, SS, ML, AH
Analysis and interpretation: IK, SS, ML, AH
Data collection: IK, SS, ML, AH
Writing the article: IK, SS, ML, AH
Critical revision of the article: IK, SS, ML, AH
Final approval of the article: IK, SS, ML, AH
Statistical analysis: Not applicable
Obtained funding: Not applicable
Overall responsibility: AH
IK and SS participated equally and share co-first authorship.
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.jvs.2020.05.015
Read article for free, from open access legal sources, via Unpaywall:
http://www.jvascsurg.org/article/S0741521420311575/pdf
Citations & impact
Impact metrics
Article citations
Splenic abscess secondary to COVID-19 acute infection: A case report and literature review.
Medicine (Baltimore), 103(31):e39194, 01 Aug 2024
Cited by: 0 articles | PMID: 39093790 | PMCID: PMC11296440
Review Free full text in Europe PMC
Validation of the CoVID-TE model as a tool to predict thrombosis, bleeding, and mortality in the oncology patient with Sars-Cov-2 infection: a study by the SEOM cancer and thrombosis group.
Clin Transl Oncol, 26(1):171-177, 10 Jun 2023
Cited by: 0 articles | PMID: 37301805 | PMCID: PMC10257483
Strategies for the Emergency Treatment of Pregnant Women with Neurological Symptoms during the COVID-19 Pandemic.
Aging Dis, 14(2):290-298, 01 Apr 2023
Cited by: 0 articles | PMID: 37008058 | PMCID: PMC10017149
Safety of COVID-19 Vaccines among Patients with Type 2 Diabetes Mellitus: Real-World Data Analysis.
Diabetes Metab J, 47(3):356-365, 06 Mar 2023
Cited by: 5 articles | PMID: 36872064 | PMCID: PMC10244203
D-dimer trends elaborate the heterogeneity of risk in hospitalized patients with COVID-19: A multi-national case series from different waves.
Front Med (Lausanne), 10:1103842, 17 Mar 2023
Cited by: 0 articles | PMID: 37020675 | PMCID: PMC10068868
Go to all (61) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia.
J Thromb Haemost, 18(6):1421-1424, 06 May 2020
Cited by: 1058 articles | PMID: 32271988 | PMCID: PMC7262324
Extremely High Incidence of Lower Extremity Deep Venous Thrombosis in 48 Patients With Severe COVID-19 in Wuhan.
Circulation, 142(2):181-183, 15 May 2020
Cited by: 132 articles | PMID: 32412320
COVID-19-associated coagulopathy.
Diagnosis (Berl), 7(4):357-363, 01 Nov 2020
Cited by: 37 articles | PMID: 32683333
Review
Coagulopathy in COVID-19: Focus on vascular thrombotic events.
J Mol Cell Cardiol, 146:32-40, 15 Jul 2020
Cited by: 36 articles | PMID: 32681845 | PMCID: PMC7362808
Review Free full text in Europe PMC