Abstract
Background
Activated eosinophils have been deemed to affect carcinogenesis and tumor progression via various mechanisms in tumor microenvironment. However, the prognostic role of tumor-associated tissue eosinophilia (TATE) in human cancers remains controversial. Therefore, we conducted this meta-analysis to better comprehend the association between TATE and clinical outcomes of patients.Methods
We searched PubMed, Embase and EBSCO to determine the researches assessing the association between TATE and overall survival (OS) and/or disease-free survival (DFS) in patients with cancer, then combined relevant data into hazard ratios (HRs) or odds ratio (OR) for OS, DFS and clinicopathological features including lymph node metastasis etc. with STATA 12.0.Results
Twenty six researches with 6384 patients were included in this meta-analysis. We found that the presence of TATE was significantly associated with improved OS, but not with DFS in all types of cancers. In stratified analyses based on cancer types, pooled results manifested that the infiltration of eosinophils was remarkably associated with better OS in esophageal carcinoma and colorectal cancer. In addition, TATE significantly inversely correlated with lymph node metastasis, tumor stage and lymphatic invasion of cancer.Conclusion
TATE promotes survival in cancer patients, suggesting that it is a valuable prognostic biomarker and clinical application of biological response modifiers or agonists promoting TATE may be the novel therapeutic strategy for patients.Free full text

Tumor-associated tissue eosinophilia predicts favorable clinical outcome in solid tumors: a meta-analysis
Abstract
Background
Activated eosinophils have been deemed to affect carcinogenesis and tumor progression via various mechanisms in tumor microenvironment. However, the prognostic role of tumor-associated tissue eosinophilia (TATE) in human cancers remains controversial. Therefore, we conducted this meta-analysis to better comprehend the association between TATE and clinical outcomes of patients.
Methods
We searched PubMed, Embase and EBSCO to determine the researches assessing the association between TATE and overall survival (OS) and/or disease-free survival (DFS) in patients with cancer, then combined relevant data into hazard ratios (HRs) or odds ratio (OR) for OS, DFS and clinicopathological features including lymph node metastasis etc. with STATA 12.0.
Results
Twenty six researches with 6384 patients were included in this meta-analysis. We found that the presence of TATE was significantly associated with improved OS, but not with DFS in all types of cancers. In stratified analyses based on cancer types, pooled results manifested that the infiltration of eosinophils was remarkably associated with better OS in esophageal carcinoma and colorectal cancer. In addition, TATE significantly inversely correlated with lymph node metastasis, tumor stage and lymphatic invasion of cancer.
Conclusion
TATE promotes survival in cancer patients, suggesting that it is a valuable prognostic biomarker and clinical application of biological response modifiers or agonists promoting TATE may be the novel therapeutic strategy for patients.
Background
Tumor microenvironment (TME) linked closely with the initiation, promotion, and progression of cancer [1]. Innate and adaptive immunocytes such as mast cells, macrophages and memory T lymphocytes etc. are the vital components of TME [2]. Multitudinous studies have demonstrated that these immune cells were significantly associated with survival in solid tumors [3, 4]. However, it is essential to distinguish among different types of immune cells as they may play differential roles in the TME. Eosinophils, as the important component of innate immune cells, have proven to play significant roles in a multitude of solid tumors.
Eosinophils are granulocytic leukocytes that are associated with multitudinous pathologic conditions including allergic reactions, parasitic and bacterial infections etc. [5] These cells secrete massive proteins and cytokines upon activation and are involved in a variety of other functions including inducing tissue remodeling and promoting antigen presentation [6]. In the last decade, activated eosinophils have been deemed to affect carcinogenesis and tumor progression via various mechanisms including modulating innate and adaptive immune responses in TME [7]. Eosinophils infiltrating into tumor is also called tumor-associated tissue eosinophilia (TATE) [8]. Recent researches have investigated the TATE in tumor progression and survival, but their results were inconsistent even contradictory [9]. Hence, it needs further evaluation. In addition, the potential of TATE as prognostic biomarker and therapeutic strategy is also required to be investigated.
Herein, we carried out this meta-analysis to expound the relation between TATE and clinical outcomes including overall survival (OS) and disease-free survival (DFS) in patients with cancer.
Methods
Search strategy
This meta-analysis was guided by the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) Statement issued in 2009 (Checklist S1). PubMed, Embase and EBSCO were searched for researches from 1980 to May 15th 2019. The keywords applied for search were: (eosinophil [Title/Abstract] OR eosinophilia [Title/Abstract]) AND (neoplasms [Title/Abstract] OR tumor [Title/Abstract] OR cancer [Title/Abstract] OR carcinoma [Title/Abstract]).
Inclusion and exclusion criteria
Researches included in this meta-analysis should meet the following inclusion criteria: (1) been published as original articles; (2) investigated human subjects; (3) examined eosinophils in primary tumor tissues; (4) reported hazard ratios (HRs) with 95% confidence interval (CI), or Kaplan – Meier curves of eosinophil infiltration with clinical outcomes.
The exclusion criteria were as follows: researches (1) were not published as research articles or full texts including commentaries, case reports, letters to the editors and meeting abstracts; (2) didn’t offer ample data to obtain HRs; (3) investigated eosinophils in metastases or not in tumor tissues.
Endpoints
In this study, OS and DFS were regarded as the primary and second endpoint respectively.
Data extraction
GM.H. and SM.W. reviewed and recorded data including number of patients, method to quantify eosinophils, cutoff value to determine TATE and time of follow-up etc. independently. OS, DFS and clinicopathological features such as tumor, node, metastasis (TNM) stage and lymphatic invasion were extracted from the text, tables, or Kaplan – Meier curves.
Quality assessment
Two authors independently assessed the quality of included cohort researches with Newcastle–Ottawa Scale (NOS), [10] and achieved consensus for each item under the help of third or more authors. Research scored 6 or above was regarded as high quality.
Statistical analysis
We combined extracted data using STATA 12.0 analysis software, and estimated statistical heterogeneity with the chi-squared based Q-test or I2 (25% was considered low-level heterogeneity, 25–50% moderate-level heterogeneity, and 50% high-level heterogeneity) [11]. Data were pooled based on the random-effect model in the presence of heterogeneity, [12] otherwise, the fixed-effect model was applied [13]. In addition, stratified analyses were conducted based on tumor types; sensitivity analysis, Begg’s funnel plot and Egger’s test [14] were employed to explore the impact of each research on the overall result and potential publication bias respectively. All P values were two-sided and below 0.05 was treated as statistical significance.
Results
Search results and description of studies
Flow chart diagram of research selection was displayed in Fig. S1. Twenty six researches with 6384 patients were ultimately included in this meta-analysis [15–40]. And all the researches were scored 6 or above after careful evaluation with the Newcastle–Ottawa Scale (NOS); Characteristics of those researches being in the light of the inclusion criteria and suitable for data incorporation were exhibited in Table Table11 and Table S1.
Table 1
Main characteristics of the included studies
| Study | Year | Tumor type | No. of Patients | Male/Female | median age (range) (year) | Staining | TATE: Present / absent | Tumor stage | median follow-up date (months) | Survival | Quality Score (NOS) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Peurala, E. etal [15] | 2018 | Oral cancer | 99 | 55/44 | 65.3 | H&E | 51/47 | I - III | 40.7 | OS | 8 |
| Oliveira, D. T. etal [16] | 2012 | Oral cancer | 71 | 55/16 | 59 (35, 77) | H&E | 35/36 | I - II | NR | DFS | 7 |
| Tostes Oliveira, D. etal [19] | 2009 | Oral cancer | 43 | 27/16 | 55.79 (28, 83) | H&E | 21/22 | I - IV | (3, 229) | OS | 7 |
| Dorta, R. G. etal [17] | 2002 | Oral cancer | 125 | 105/20 | 58 (30, 95) | H&E | 57/68 | II - III | 88.2 (0, 287.4) | OS, DFS | 7 |
| Dante, P. etal [40] | 2019 | Tongue Carcinoma | 259 | 223/36 | 53.0 ± ± 12.2 12.2 | H&E | NR | I - IV | NR | OS, DFS | 8 |
| Alrawi, S. J. etal [18] | 2005 | Head and neck carcinoma | 87 | NR | (41, 76) | H&E | 13/7 | II - IV | 36 (6, 216) | OS, DFS | 7 |
| Ercan, I. etal [20] | 2005 | Laryngeal carcinoma | 78 | 78/0 | 55.9 (35, 80) | H&E | 25/53 | NR | 41.91 | OS | 7 |
| Sassler, A. M. etal [21] | 1995 | Laryngeal carcinoma | 248 | NR | NR | H&E | 56/192 | III - IV | 48 | OS, DFS | 6 |
| Thompson, A. C. etal [22] | 1994 | Laryngeal carcinoma | 104 | 85/19 | 64.6 (39, 91) | H&E | 31/73 | NR | ≥ 60 | OS | 6 |
| Fujii, M. etal [23] | 2002 | Nasopharyngeal carcinoma | 53 | 40/13 | 49.4 (15, 81) | H&E | 26/27 | I - IV | 90.5 (35.3, 199.9) | DFS | 7 |
| Leighton, S. E. etal [24] | 1996 | Nasopharyngeal carcinoma | 96 | 68/28 | NR | H&E | 65/31 | NR | 57 | OS, DFS | 6 |
| Harbaum, L. etal [25] | 2015 | Colorectal cancer | 381 | 166/215 | 68.5 | H&E | 101/280 | I - IV | 45 (1, 182) | OS | 8 |
| Fernandez-Acenero, M. J. etal [26] | 2000 | Colorectal cancer | 126 | 70/56 | 67.35 (32, 87) | H&E | 29/97 | Duke’s A-C | ≥ 60 | OS, DFS | 8 |
| Nielsen, H.J. etal [27] | 1999 | Colorectal cancer | 584 | 240/344 | 61 (49, 75) | H&E | 150/115 | Duke’s A-D | 61 (49, 75) | OS | 7 |
| Prizment, A. E etal [28] | 2016 | Colorectal cancer | 441 | 0/441 | (55, 69) | H&E; EPX | 197 /244 | NR | 60 | OS | 8 |
| Zhang, Y. etal [29] | 2014 | Esophageal carcinoma | 36 | 25/11 | 59 (45, 77) | H&E | 18/18 | I - IV | 22 (2, 143) | OS | 7 |
| Ishibashi, S. etal [30] | 2006 | Esophageal carcinoma | 97 | 82/15 | 62.7 ± ± 8.9 8.9 | H&E | 30/31 | NR | 61.7 (5.3, 165.4) | OS | 7 |
| Hollander, P. etal [31] | 2018 | Hodgkin’s lymphoma | 459 | 242/217 | < 45: 68%; ≥45: 32% 45: 68%; ≥45: 32% | H&E | NR | I - IV | 154.8 | OS | 8 |
| Kereszres, K. etal [32] | 2007 | Hodgkin’s lymphoma | 104 | 54/50 | 33 (12, 72) | H&E | 64/40 | I - IV | 110 (24, 214) | OS, DFS | 7 |
| von Wasielewski, R. etal [33] | 2000 | Hodgkin’s lymphoma | 1511 | 745/766 | (15, 75) | H&E | 510/823 | I - IV | 120 | OS | 8 |
| Enblad, G.etal [34] | 1993 | Hodgkin’s lymphoma | 140 | NR | 45 (11, 94) | H&E | 26/114 | I - IV | 48 (20, 85) | DFS | 6 |
| van Driel, W.J. etal [35] | 1996 | Cervical cancer | 83 | 0/83 | 42.1 | H&E | NR | I - IIA | 44.6 (5, 108) | OS, DFS | 7 |
| Bethwaite, P. B. etal [36] | 1993 | Cervical cancer | 67 | 0/67 | 43.7 (25, 76) | H&E | 28/39 | IB | 62.4 (1, 93) | OS | 7 |
| Flamm, J. etal [37] | 1992 | Bladder cancer | 428 | 289/139 | 70.2 (29, 91) | H&E | 99/329 | NR | 84 | OS | 7 |
| Iwasaki, K. etal [38] | 1986 | Gastric cancer | 647 | 364/283 | (22, 84) | H&E | 157/490 | I - IV | (8, 92) | OS | 7 |
| Ono, Y. etal [39] | 2002 | Penile cancer | 17 | 17/0 | 68 (36, 84) | H&E | 9/8 | I - IV | NR | OS | 6 |
H&E haematoxilyn and eosin, EPX eosinophil peroxide, NR not reported
Meta-analyses
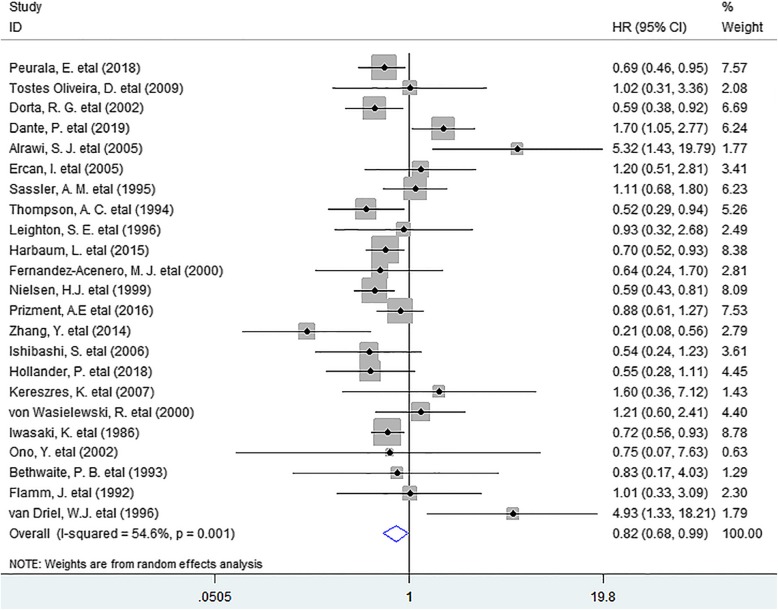
Overall survival (OS)
In this meta-analysis, we discovered that the presence of TATE was notably associated with improved OS (HR =
= 0.82, 95% CI 0.68 to 0.99, P
0.82, 95% CI 0.68 to 0.99, P =
= 0.041) in patients with solid tumor. (Fig. (Fig.11).
0.041) in patients with solid tumor. (Fig. (Fig.11).
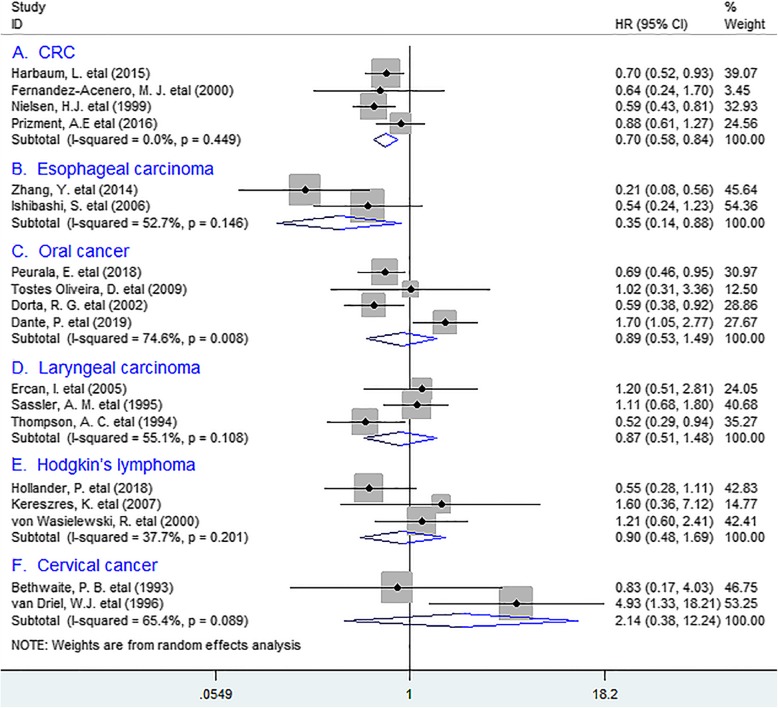
In stratified analyses according to tumor types, the combined results manifested that TATE was markedly associated with better OS in colorectal cancer (CRC) (HR =
= 0.70, 95% CI 0.58 to 0.84, P
0.70, 95% CI 0.58 to 0.84, P =
= 0.000), with no heterogeneity detected (I2 =
0.000), with no heterogeneity detected (I2 = 0%, P
0%, P =
= 0.449). Similar data was obtained between TATE and OS in esophageal carcinoma (EC) (HR
0.449). Similar data was obtained between TATE and OS in esophageal carcinoma (EC) (HR =
= 0.35, 95% CI 0.14 to 0.88, P
0.35, 95% CI 0.14 to 0.88, P =
= 0.026); Whereas no distinct relation existed between eosinophil infiltration and OS in oral cancer (OC) (HR
0.026); Whereas no distinct relation existed between eosinophil infiltration and OS in oral cancer (OC) (HR =
= 0.89, 95% CI 0.53 to 1.49, P
0.89, 95% CI 0.53 to 1.49, P =
= 0.657), laryngeal carcinoma (HR
0.657), laryngeal carcinoma (HR =
= 0.87, 95% CI 0.51 to 1.48, P
0.87, 95% CI 0.51 to 1.48, P =
= 0.599), Hodgkin’s lymphoma (HR
0.599), Hodgkin’s lymphoma (HR =
= 0.90, 95% CI 0.48 to 1.69, P
0.90, 95% CI 0.48 to 1.69, P =
= 0.741) or cervical cancer (HR
0.741) or cervical cancer (HR =
= 2.14, 95% CI 0.38 to 12.24, P
2.14, 95% CI 0.38 to 12.24, P =
= 0.391). (Fig. (Fig.22).
0.391). (Fig. (Fig.22).
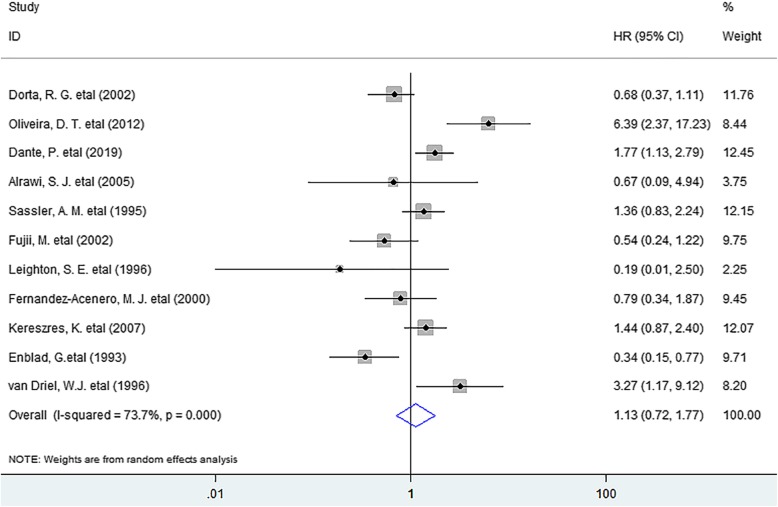
Disease-free survival (DFS)
As for DFS, the meta-analysis indicated that no noticeable association existed between eosinophil infiltration and DFS (HR =
= 1.13, 95% CI 0.72 to 1.77, P
1.13, 95% CI 0.72 to 1.77, P =
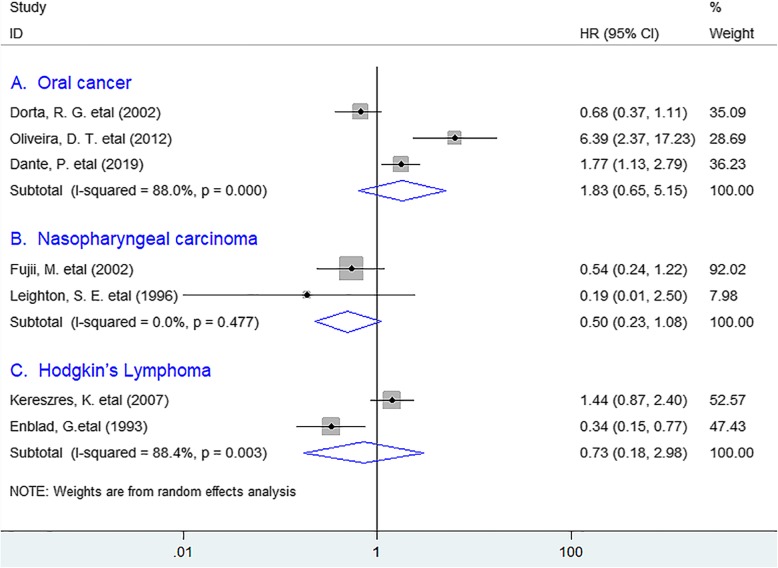
= 0.598) in solid tumors. (Fig. (Fig.3)3) In the stratified analyses, the incorporated results revealed that TATE was not significantly associated with improved DFS in oral cancer (HR
0.598) in solid tumors. (Fig. (Fig.3)3) In the stratified analyses, the incorporated results revealed that TATE was not significantly associated with improved DFS in oral cancer (HR =
= 1.83, 95% CI 0.65 to 5.15, P
1.83, 95% CI 0.65 to 5.15, P =
= 0.253), nasopharyngeal carcinoma (HR
0.253), nasopharyngeal carcinoma (HR =
= 0,50, 95% CI 0.23 to 1.08, P
0,50, 95% CI 0.23 to 1.08, P =
= 0.079) or Hodgkin’s lymphoma (HR
0.079) or Hodgkin’s lymphoma (HR =
= 0.73, 95% CI 0.18 to 2.98, P
0.73, 95% CI 0.18 to 2.98, P =
= 0.657). (Fig. (Fig.44).
0.657). (Fig. (Fig.44).
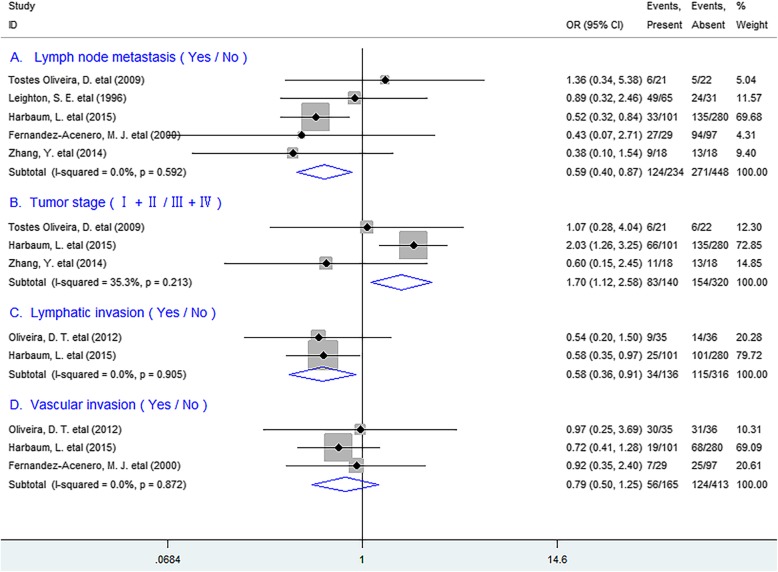
Clinicopathological features
We next tested the relation between TATE and clinicopathological features, and found that TATE was remarkably inversely correlated with lymph node metastasis (OR =
= 0.59, 95% CI 0.40 to 0.87, P
0.59, 95% CI 0.40 to 0.87, P =
= 0.007), TNM stage (OR
0.007), TNM stage (OR =
= 1.70, 95% CI 1.12 to 2.58, P
1.70, 95% CI 1.12 to 2.58, P =
= 0.013) and lymphatic invasion (OR
0.013) and lymphatic invasion (OR =
= 0.58, 95% CI 0.36 to 0.91, P
0.58, 95% CI 0.36 to 0.91, P =
= 0.018), but not with vascular invasion (OR
0.018), but not with vascular invasion (OR =
= 0.79, 95% CI 0.50 to 1.25, P
0.79, 95% CI 0.50 to 1.25, P =
= 0.308) of patients. (Fig. 5).
0.308) of patients. (Fig. 5).
Sensitivity analysis
Sensitivity analysis demonstrated that each included research had no impact on the overall result for OS or DFS. (Fig. S2).
Publication bias
No publication bias existed between TATE and OS (P =
= 0.152) or DFS (P
0.152) or DFS (P =
= 0.876) in patients by Funnel plot (Fig. S3) and Egger’s test.
0.876) in patients by Funnel plot (Fig. S3) and Egger’s test.
Discussion
Eosinophilia is commonly associated with allergies, helminth infections and several inflammatory states. Recently, it has also been noted in human solid tumors. The present meta-analysis revealed that TATE had a positive effect in improving survival in human solid tumors, especially in CRC and EC. Moreover, It significantly inversely correlated with lymph node metastasis etc. of tumor. Hence, these data offered important evidence in uncovering the positive prognostic role of TATE in human solid tumors.
The close relation between TATE and better clinical outcome identified in this study possibly attribute to the following reasons: eosinophils in the TME can express same receptors and mediators such as granzyme A etc. as cytotoxic T lymphocytes (CTLs) and be directly involved in anti-tumor response, [41] and they can also secret several chemokines including CCL5, CXCL9 to promote anti-tumor immunity through attracting CD8+ T cells to the tumor site [42]. In addition, eosinophils are capable of regulating immunity, for instance, they can release major basic protein (MBP), a highly cationic protein to stimulate maturation of dendritic cells by increasing cell surface activation markers including MHC-II, CD80 and CD86, [43] which has the potential to overcome immune tolerance and induce anti-tumor immunity with the powerful antigen-presentation ability [44]. Furthermore, they can induce cell death of various cell lines such as colo-205 cell line with some selectivity in their tumoricidal properties, which are dependent on the CD11a/CD18-mediated stable contacts with target cells [45]. Hence, it is rational to conclude that TATE is capable of regulating tissue homeostasis of the TME and inhibiting tumor growth and metastasis thereby improving survival. However, in other tumor types, TATE as a prognostic marker for survival has been a controversial issue. This may be because of differences in methods of counting TATE as well as heterogeneity of material.
Previous studies have demonstrated that cytokines such as IL-2, IL-4 could recruit eosinophils and lead to eosinophilia and enhanced eosinophil activation, thereby exert potent anti-tumor immune responses [41, 46]. Thus, based on our present result that TATE improving survival in human solid tumors identified in this study and the function of IL-2 and IL-4 stated above, we harbor the idea that clinical application of biological response modifiers (BRM) such as carrier-assisted recombined human IL-2 /or IL-4 may have the potential to treat human solid tumors.
Quite a few limitations should be noted from this study. First, morphometric analyses for TATE adopted in included researches were not exactly consistent. In addition, researches with negative results might not be published, which might result in potential publication bias.
Conclusions
TATE promotes survival in solid tumors especially in CRC and EC, suggesting that it is a valuable prognostic biomarker and clinical application of biological response modifiers or agonists promoting TATE may be a novel therapeutic strategy for patients.
Abbreviations
| TATE | Tumor-associated tissue eosinophilia |
| OS | Overall survival |
| DFS | Disease-free survival |
| HR | Hazard ratio |
| OR | Odds ratio |
| Cl | Confidence interval |
| TNM | Tumor, node, metastasis |
| OC | Oral cancer |
| CRC | Colorectal cancer |
| EC | Esophageal carcinoma |
| NR | Not reported |
| TME | Tumor microenvironment |
| BRM | Biological response modifier |
Authors’ contributions
GM.H. conceived of the study, participated in its design, extracted data, performed the statistical analysis and drafted the manuscript. SM.W. participated in data extraction; KF.Z., F. X. and LM.H. participated in statistical analysis and manuscript revision. W.C. and P.C. participated in its design and manuscript revision. All authors read and approved the final manuscript.
Funding
This work was funded by the National Natural Science Foundation of China (Grant No. 81702803, GMH) and was also partly supported by Shaoxing Science and TechnologyPlanProject (2018C30055, LMH; 2018C30075, KFZ; 2017B70036, FX). We used the funding to perform data collection, analysis and interpretation.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article.
Ethics approval and consent to participate
The ethical approval was unnecessary because this study based on summary and analysis of the results of previous studies.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guoming Hu and Shimin Wang contributed equally to this work.
Contributor Information
Guoming Hu, Email: moc.621@jlpmgh.
Wei Chen, Email: moc.361@8105xsjzwc.
Pu Cheng, Email: nc.ude.ujz@upgnehcrd.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12885-020-06966-3.
References
Articles from BMC Cancer are provided here courtesy of BMC
Full text links
Read article at publisher's site: https://doi.org/10.1186/s12885-020-06966-3
Read article for free, from open access legal sources, via Unpaywall:
https://bmccancer.biomedcentral.com/track/pdf/10.1186/s12885-020-06966-3
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1186/s12885-020-06966-3
Article citations
Squamous Cell Carcinoma with Prominent Eosinophils.
Head Neck Pathol, 18(1):115, 28 Oct 2024
Cited by: 0 articles | PMID: 39466476
Review
TYK2 Protein Expression and Its Potential as a Tissue-Based Biomarker for the Diagnosis of Colorectal Cancer.
Cancers (Basel), 16(21):3665, 30 Oct 2024
Cited by: 0 articles | PMID: 39518103 | PMCID: PMC11545102
Polo-like kinase 1 as a biomarker predicts the prognosis and immunotherapy of breast invasive carcinoma patients.
Oncol Res, 32(2):339-351, 28 Dec 2023
Cited by: 0 articles | PMID: 38186570 | PMCID: PMC10765123
An increased relative eosinophil count as a predictive dynamic biomarker in non-small cell lung cancer patients treated with immune checkpoint inhibitors.
Thorac Cancer, 15(3):248-257, 12 Dec 2023
Cited by: 3 articles | PMID: 38087769 | PMCID: PMC10803223
Ultrastructural Evidence of Eosinophil Clustering and ETosis in Association with Damage to Single Tumour Cells in a Case of Poorly Cohesive NOS Gastric Carcinoma.
Eur J Case Rep Intern Med, 10(10):004016, 31 Aug 2023
Cited by: 0 articles | PMID: 37789981 | PMCID: PMC10545153
Go to all (20) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Tumor-Infiltrating Podoplanin+ Fibroblasts Predict Worse Outcome in Solid Tumors.
Cell Physiol Biochem, 51(3):1041-1050, 26 Nov 2018
Cited by: 21 articles | PMID: 30476924
Tumor-associated tissue eosinophilia promotes angiogenesis and metastasis in head and neck squamous cell carcinoma.
Neoplasia, 35:100855, 19 Nov 2022
Cited by: 5 articles | PMID: 36410227 | PMCID: PMC9677212
Tumor associated tissue eosinophilia in oral squamous cell carcinoma: A systematic review and meta-analysis.
J Oral Biol Craniofac Res, 11(1):33-39, 23 Nov 2020
Cited by: 2 articles | PMID: 33344159 | PMCID: PMC7736986
Review Free full text in Europe PMC
Galectin-9 Expression Predicts Favorable Clinical Outcome in Solid Tumors: A Systematic Review and Meta-Analysis.
Front Physiol, 9:452, 26 Apr 2018
Cited by: 36 articles | PMID: 29765332 | PMCID: PMC5939667
Funding
Funders who supported this work.
National Natural Science Foundation of China (1)
Grant ID: 81702803

 #1
#1