Abstract
Free full text

Going to extremes: determinants of extraordinary response and survival in patients with cancer
Abstract
Research into factors affecting treatment response or survival in patients with cancer frequently involves cohorts that span the most common range of clinical outcomes, as such patients are most readily available for study. However, attention has turned to highly unusual patients who have exceptionally favourable or atypically poor responses to treatment and/or overall survival, with the expectation that patients at the extremes may provide insights that could ultimately improve the outcome of individuals with more typical disease trajectories. While clinicians can often recount surprising patients whose clinical journey was very unusual, given known clinical characteristics and prognostic indicators, there is a lack of consensus among researchers on how best to define exceptional patients and little has been proposed for the optimal design of studies to identify factors that dictate unusual outcome. In this Opinion article, we review different approaches to identifying exceptional patients with cancer, and possible study designs to investigate extraordinary clinical outcomes. We discuss pitfalls with finding these rare patients, including challenges associated with accrual of patients across different treatment centres and time periods. We describe recent molecular and immunological factors that have been identified as contributing to unusual patient outcome, and make recommendations for future studies on these intriguing patients.
Introduction
Treasure your exceptions! William Bateson (1861–1926)1.
Determining a patient’s prognosis is central to their initial clinical management and subsequent treatment decision-making. Tumour type, pathological features such as tumour grade, extent of cancer spread, adequacy of surgical clearance and patient age are measures routinely assessed to determine possible patient outcome. Increasingly, molecular data such as the expression of individual biomarkers, gene signatures, and mutations in driver genes provide information about tumour aggressiveness and potential responses to conventional and targeted therapies2. However, even when all these factors are taken into consideration, and patients with similar clinical and molecular characteristics are compared, substantial unexplained variation in patient survival time often remains.
At either end of the range of clinical outcomes for a given cancer type are patients with atypically poor or unusually favourable responses to treatment and survival (FIG. 1). Understanding the biological determinants of survival in extreme outliers may provide a route to improving responses in more typical patients, particularly if these studies identify new biomarkers to guide drug selection or novel pathways that are targetable3. Furthermore, seeking determinants of prolonged survival is particularly important in cancers with generally poor outcomes.
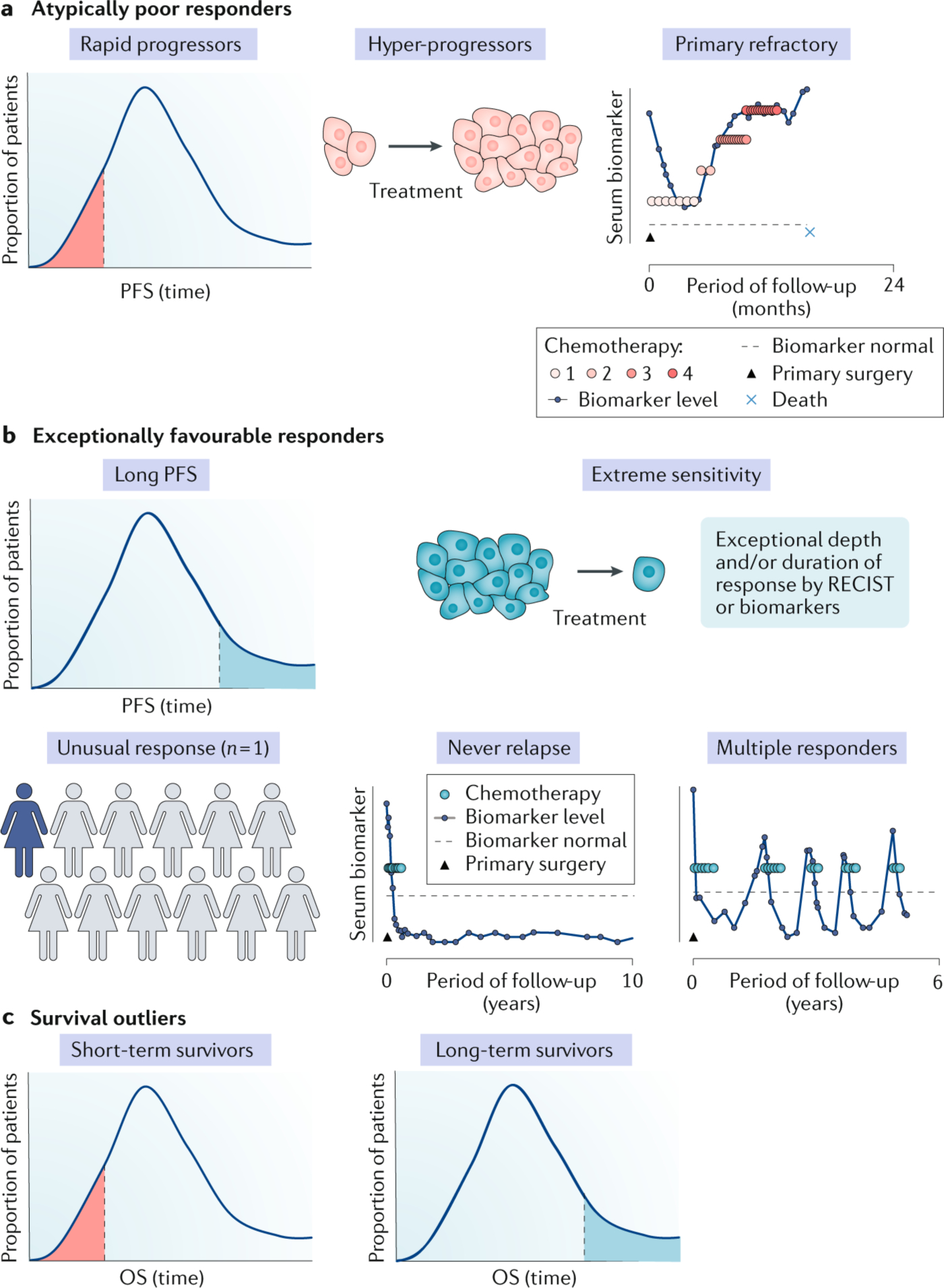
Patients with cancer with an exceptional outcome can be classified based on either an atypically good or bad treatment response or on their unusual length of overall survival (OS). a | Rapid progression is observed in a proportion of patients who are expected to respond favorably to conventional or novel therapy. Hyper-progression has been observed in some patients treated with immune checkpoint inhibitors, with apparent accelerated tumour growth on treatment. An example of a patient with primary refractory high-grade serous ovarian cancer, where progression occurs on or within 4 weeks of the end of treatment, is depicted. b | Exceptionally favorable responses can reflect the duration, depth, or proportion of patients responding to therapy, and is most commonly a feature of new treatment approaches. An unusual response (n = 1) can occur when there is a durable response in the context of very few other patients responding to a novel treatment. Alternatively, some patients never relapse: an example of a patient with ovarian cancer in which surgery failed to clear all disease, and who therefore would be expected to relapse in 12–18 months, but who remained disease free for many years after a single line of chemotherapy is depicted. Multiple responders are a clinically distinct subgroup of exceptional responders, showing repetitive profound responses to several lines of chemotherapy. Some but not all exceptional responders may become long-term survivors. c | Most information on short-term and long-term cancer survival relates to conventional therapy in which data from a large number of patients, collected over many years, are available. PFS, progression-free survival; RECIST, Response Evaluation Criteria in Solid Tumours.
The broad insights into tumour suppressor function obtained through studying families with rare high penetrance inherited mutations provides a potent past example of how unusual patients with cancer can inform cancer biology more generally4. Accordingly, the National Cancer Institute (NCI) launched the Exceptional Responders Initiative (ERI) in 2014 with the goal of discovering the molecular underpinnings of exceptional responses to treatment in patients with cancer5. Other funding agencies, including the US Department of Defense Congressionally Directed Ovarian Cancer Research Program (OCRP), have also proactively supported research on exceptional cancer survivors. BOX 1 shows additional examples of exceptional responder studies in cancer patients internationally. In this Opinion article, we discuss various criteria used for defining exceptional patients, consider the challenges and cautionary lessons, and review insights gained to date from some of these extraordinary patients with cancer.
Identifying exceptional patients
It is often said that beauty is easy to recognize but hard to define, and this principle may also apply to exceptional responders. Although most cancer clinicians can readily recount patients who have surprised them with an unexpected (favourable or unfavourable) response to treatment and/or their overall survival, there is a lack of consensus regarding the definition of an exceptional patient with cancer6.
Broadly, exceptional patients can be categorized as either having an unusually favourable response to treatment, an atypically long survival, or at the other end of the spectrum, unexpected rapid progression on treatment and short survival7. Objective measures of tumour response to treatment are provided by Response Evaluation Criteria in Solid Tumours (RECIST), the internationally recognised standard methodology to assess changes in solid tumour burden8, and iRECIST, the modified criteria for measuring response to immune-oncology therapies9. In some cancer types, response definitions incorporate the extent or rate of fall of a biomarker10 and patient symptom improvement or alleviation has also been used to indicate unusual responses11,12. The eligibility criteria of the NCI ERI require patients with complete response (CR) or partial response (PR) of at least 6 months defined by RECIST, in a treatment where fewer than 10% of patients respond, or a CR or PR at least three times longer than the published median. Case reports, often involving a single patient (N=1) with an unusually profound or durable response to a novel agent in a setting of very low rates of response to conventional therapy are among the most commonly reported examples of exceptional patients (FIG. 1b). For example, publication of an anecdotal account of a dramatic response to anti-hormonal therapy in a patient with metastatic prostate cancer13 led to a treatment that has since become standard of care. Similarly, the NCI ERI study was prompted by the exceptional response of a patient with tuberous sclerosis complex 1 (TSC1) mutant bladder cancer to the mTOR inhibitor everolimus14, an observation subsequently validated in a more extended analysis of patients from a phase II trial15. While the trial can be regarded as molecularly-targeted, the subsequent identification of TSC1 mutations as a marker of exceptional response in bladder cancer shows that a more accurate biomarker can refine a precision medicine approach.
In a recent study of glioblastoma long-term survivors, a cut-off of ≥36 months was chosen as it was >3 standard deviations above the mean survival for the non-long-term survivors group and because it was a cut-off that had been employed by earlier studies16. The OCRP-funded studies of ovarian cancer long-term survivors focuses on those who have survived 10 years or more, which is 2 standard deviations above the median overall survival (~36 months) for women with advanced stage, high-grade serous ovarian, fallopian or primary peritoneal cancer (HGSC)17.
Clearly what constitutes an exceptional duration of survival is highly dependent on cancer type, with generally poor outcome cancers such as lung or pancreatic cancer requiring a shorter survival period than for example, breast cancer or lymphoma (FIG. 2). The NCI ERI criterion of a CR or PR in a setting where fewer than 10% of patients show these objective responses to treatment is most suited to patients being treated in clinical trials of novel agents, as few conventional therapies with such a low response rate are standard of care. Conversely, exceptional response based on long overall survival most commonly relates to established therapies, where there is extensive data on the median duration of survival accrued over many years from a large series of patients.
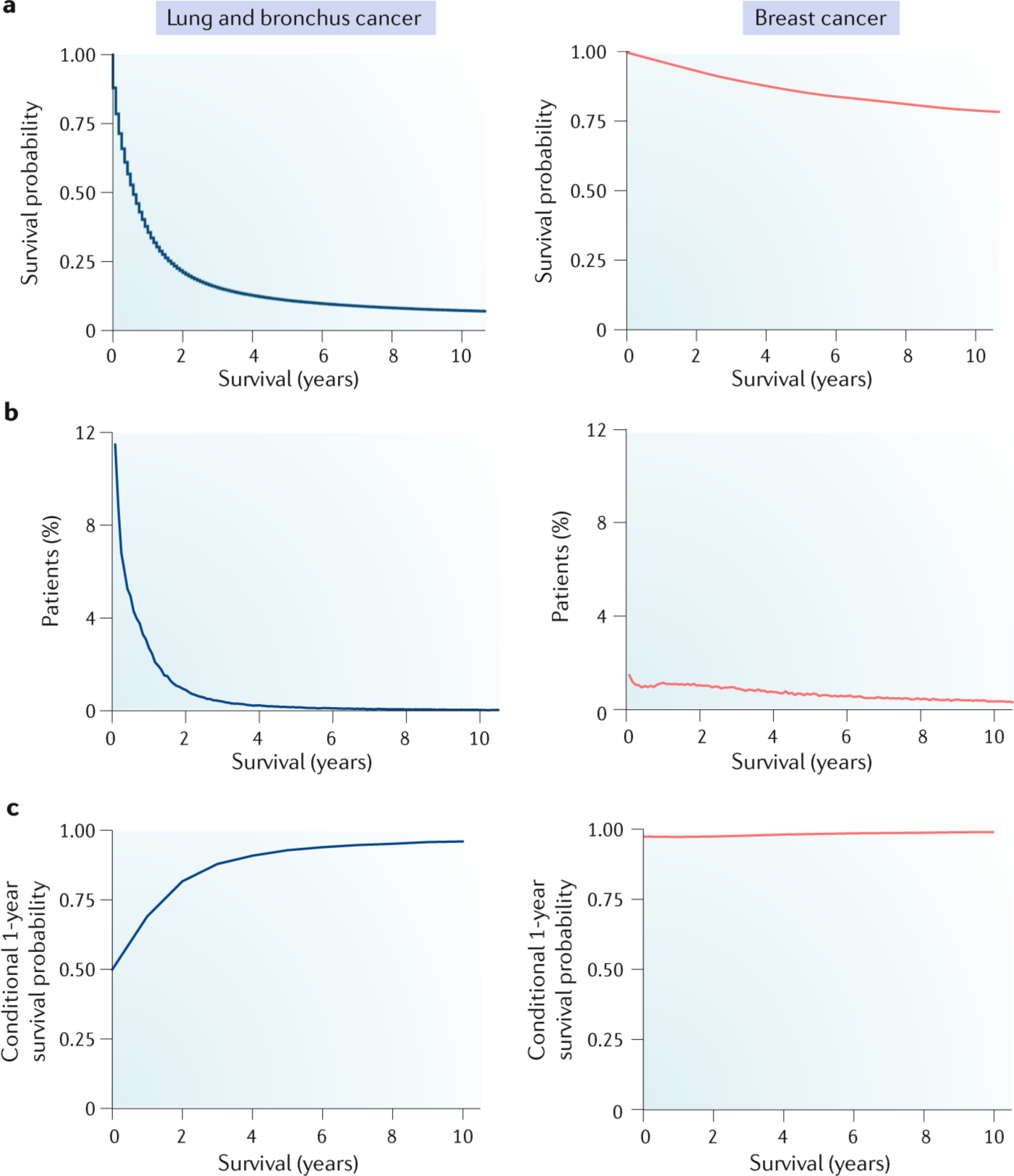
a | Kaplan-Meier survival curves for patients with lung (blue) and breast (red) cancer, showing distinctly different patterns of patient survival over a 10-year period. Survival time of those patients who were alive at last follow-up was censored at their last follow-up. b | Distribution of disease-specific deaths, showing the proportion of patients who die within specific time intervals. In patients with lung cancer, the peak death rate occurs sharply within the first few months after diagnosis, whereas breast cancer-specific deaths occur over an extended time period. Survival distributions vary between cancer types and show that overall survival is not normally distributed. Patients who were alive at last contact were excluded from this analysis. c | Conditional survival analyses indicating the probability of surviving an additional year if a patient has already survived a certain amount of time since diagnosis. In breast cancer, the risk of death remains remarkably constant, whereas in lung cancer, the chance of surviving increases substantially over time. The shape of the curves associated with disease-specific deaths and conditional survival for lung cancer suggest that the greatest difference in determinants of outcome may be found between patients that survive less than 2 years with those who are alive after 4 or more years. Data for this figure were extracted from Surveillance, Epidemiology and End Results Program (SEER; www.seer.cancer.gov) Research Data (1973–2015), National Cancer Institute, DCCPS, Surveillance Research Program released April 2018, on the basis of the November 2017 submission. All patients diagnosed with lung (n = 266,779) or female breast cancer (n = 521,857) between 1995 and 2005 and available follow-up data were included to allow a >10-year follow-up period.
Patients who experience exceptional response and survive for an unusually long period may have had quite varied clinical courses. For example, some patients may remain disease free after initial treatment, whereas others experience cycles of remission followed by relapse, retreatment, and response17 (FIG. 1b), perhaps reflecting a contribution by the immune system in partially holding the disease in check18. Multiple complete responses to platinum-based chemotherapy have been seen in some unusual patients with HGSC17, indicating an impaired ability of the tumour to develop resistance to carboplatin, so commonly seen in patients with recurrent HGSC. Conversely, patients have been observed whose initial treatment was highly successful with an unusually extended period of remission but when the disease returned it was resistant to treatment and they succumbed rapidly, suggesting significant tumour evolution17. These distinct patterns show that there are likely to be multiple genetic, biological, clinical, and possibly lifestyle factors contributing to exceptional response (FIG. 3). Careful stratification of patient subgroups based on their clinical patterns may therefore facilitate the discovery of novel molecular biomarkers and clinical associations.
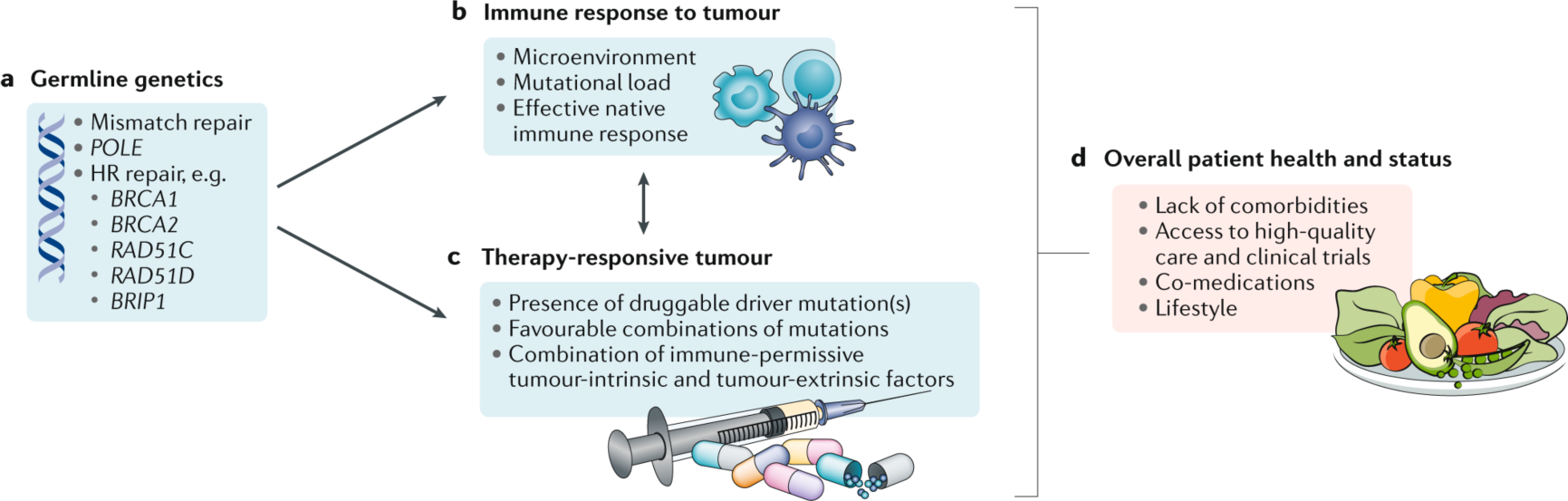
a | Germline genetics can influence response to therapy via imparting a high mutational load, for example through mutations in DNA mismatch repair or polymerase ε (POLE) genes, or by leading to the development of tumours that are vulnerable to platinum-based chemotherapy and poly(ADP-ribose) polymerase (PARP) inhibitors, such as with mutations in BRCA1, BRCA2, or other genes involved in homologous recombination (HR) DNA repair. b | Endogenous antitumour immune responses orchestrated by tumour infiltrating lymphocytes (TILs) are associated with longer survival. Both the tumour mutational load and characteristics of the microenvironment influence response to immunotherapy. c | Presence of a driver mutation or combinations of mutations can determine durable responses to targeted therapies. d | Optimal patient health, access to quality care, and certain co-medications may impart long-term survival in a patient who may otherwise have had a more typical disease trajectory.
Analysing exceptional patients
Although the use of fixed thresholds to define survival or response groups provides reassuring certainty, the biological and clinical determinants of exceptional outcome may not be so precise. For example, is a trial where 11% of patients respond less interesting and the patients less exceptional than one where only 10% of patients have a CR or PR? – a criterion for the NIH ERI program. Similarly, factors such as patient age and comorbidities could be the differences between two individuals who fall either side of a sharp survival boundary of for example, 10 years, even though their cancers may share the same molecular properties.
In a case-control study, the inclusion of patients who fall just short of a predetermined cut-off as part of a comparator group may affect the ability of the study to distinguish differences between exceptional and typical survival groups. This problem may be partially resolved by providing a temporal buffer between exceptional and comparator groups to compensate for confounding factors influencing survival. For example, in a multicentre OCRP study of HGSC long-term survivors (>10 years)19 the long-term survivors are compared to those with short (2–4.99 years) or moderate (5–7.99 years) survival. Whether this is sufficient to focus attention on the most interesting biological or epidemiological survival factors remains to be determined.
The major advantage of a case-control study where an exceptional group is compared to one or more control groups lies in over-sampling the exceptional group for a given total sample size. This is particularly useful when the total sample size is dictated by a limited research budget. Case-control series are most suited to a situation where a few determinants are present that have a large effect on survival or response. This is similar to previous case-control studies that were used to discover highly penetrant germline mutations, which have a major impact on cancer risk4. One limitation is that the effect size of any identified survival determinants are not measured with this type of analysis: the actual impact, such as the number of months of prolonged survival associated with the risk factor, can only be estimated.
It is also possible that numerous factors influence exceptional treatment response or survival, and that these operate across the whole time frame, from cancer formation to diagnosis and onwards. Here an exceptional responder is simply a patient with an unusual combination of many different prognostic factors. This situation may be analogous to polygenic risk, where individual loci with small effects can summate to impart a substantially increased risk of developing cancer. Under this model, overall survival should be regarded as a continuous variable that is best analysed within the entire cohort of patients available for study, similar to genome wide association studies (GWAS) performed in very large patient populations20. While an all comers approach avoids the need to define survival groups, there often remain practical issues of cost and logistics associated with accessing samples from an unselected cohort that inevitably will have few exceptional patients, unless very large cohorts are considered. As there is no way for an investigator to know a priori whether a few factors with large effects or many small factors determine exceptional response in the cancer under study, designing a study that includes only a few patients at the extremes can be a limitation. To counter this problem, a more even representation across survival time points may be preferred. Such an approach would allow an adequate number of patients with unusually long survival times to be included in the analysis, even though they are just a fraction of the overall cohort, and would aid in the estimation of the impact of the risk factors.
Just how large should such a study be? The statistical power of a study to detect an effect of a biomarker on survival is related to the effect size. Effect size can be thought of as the strength of the relationship between a biomarker result and the individual hazard. Although one cannot know at the outset how large the effect size may be for an individual biomarker, investigators can predetermine how large it would need to be to achieve adequate power for a given sample size (and research budget).
Compare like with like
Increased use of antibody-based and other molecular markers has improved the accuracy of pathological diagnoses; however, investigators should consider the possibility that misdiagnosed patients may reside among their exceptional responders. Misclassification is particularly relevant to studies of long-term survivors where their diagnosis may have been made many years ago when a lack of molecular biomarkers could have contributed to pathological reporting errors. Hence, re-review of patients selected for a study, preferably using multiplex immunohistochemistry on stored diagnostic pathological material, is highly desirable to eliminate past errors in histopathological assessment and the potential for misdiagnosis21.
Due to the rarity of exceptional patients, study participants are often drawn from multiple cohorts, accrued across different treatment centres and/or diagnosed over an extensive period of time in order to obtain sufficient numbers for investigation. Variation in treatment practises between centres can influence survival times, as shown for regional variation in survival of patients with ovarian cancer in the United Kingdom22. Similarly advances in patient care over time can mean that what was an unusually long survival in the past could now be considered typical, as seen in certain childhood leukemias23. A range of other clinical parameters can influence patient survival, such as variation in surgical effort between centers24, and where possible these should be taken into account. In addition, clinical data may be incompletely collected or inconsistently defined between the cohorts contributing to a study making the practicalities of collating patients from multiple cohorts difficult. However, the relatively accessible and objective parameters of year and age at diagnosis may be available for most studies. Therefore, to counter the potential for regional and temporal differences in clinical care in a case-control study, analysis should be stratified by study and the findings then combined.
While determinants that are already known to influence survival can be accounted for in multivariable analyses, there are advantages to ensuring from the outset that patients are as similar as possible, except for differences in response or survival. For example, there may be little value in performing expensive whole genome sequence analysis on a group of patients with mixed histologies or molecular subtypes that are already known to be associated with highly differential outcome. Similarly, including patients with more indolent forms of a cancer type within a group of long-term survivors is likely to distract from those with truly unusual outcomes. Potential gains made by progressively homogenizing the study cohort must be balanced against reduced statistical power associated with restricted sample size. The rarity of exceptional patients (by definition) may limit the statistical power of association studies and consequently increase the risk of overfitting, particularly of high dimensional data such as genomic information. Therefore, studies of exceptional responders are likely to benefit from nationally- and internationally-coordinated efforts in specific cancer types. Approaches to validate findings in independent cohort(s) should be considered at the outset. Logistic elements to be considered in multisite collaborative studies of exceptional responders are outlined in BOX 2.
Patients with particularly poor response or survival can comprise a valuable group to contrast with those at the other end of the clinical spectrum. However, care is needed to ensure that such patients have not had a poor outcome because they were unable to complete treatment, have comorbidities that negatively influenced their survival, or with respect to the cancer type, are molecularly unrelated to the exceptionally favourable group to which they are to be compared. Inclusion of patients using a time point of death due to disease rather than all causes may avoid confusion in studies of exceptional response.
Exceptional outcome determinants
Molecular factors influencing exceptional response and survival
Sequencing the tumour genomes of exceptional responders has enabled considerable progress to be made in our understanding of the genetic causes of tumour sensitivity to novel cancer therapies. For example, mutations in serine–threonine protein kinase 11 (STK11), TSC1, TSC2 or neurofibromatosis type 2 (NF2), or activating mutations in the mTOR pathway are associated with an exceptional response to mTOR inhibitor everolimus in metastatic breast, bladder and thyroid cancer14,25–28. Exceptional responses have been seen in women with metastatic low-grade serous ovarian carcinoma, including response to BRAF inhibitors in BRAF-mutant tumours29. Profound responses to MEK inhibitors such as trametinib or selumetinib have been seen in tumours with rare activating somatic mutations, including a patient with a melanoma that had a GOLGA-4-RAF1 fusion30 and in another with low-grade serous ovarian carcinoma that had an in-frame deletion in the negative regulatory helix of MAP2K1, a gene encoding for MEK131.
Mutations in genes involved in DNA repair and synthesis can contribute to exceptional response to therapy and long-term survival. For example, inactivating mutations in the proof reading enzyme polymerase ε (POLE) leads to a hypermutation phenotype and is associated with unusually long survival in some patients with glioblastoma and high-grade uterine cancer32,33. Similarly, germline mutations in genes encoding mis-match repair (MMR) proteins have been associated with long term survival in a small series of patients with clear cell ovarian cancer34. Mutations in genes encoding proteins involved in homologous recombination (HR) DNA repair are also frequently found to be associated with favourable responses to therapy and overall survival. HR-deficiency, associated with germline or somatic mutations in BRCA1, BRCA2 and other pathway-related genes, is particularly common in HGSC where it is seen in approximately half of all tumours35,36, and it is one of the most important predictors for treatment response to platinum-based chemotherapy. HR-deficiency caused by mutations of the gene encoding partner and localizer of BRCA2 (PALB2), a binding partner of BRCA2, is also found to contribute to exceptional chemosensitivity in other advanced cancers such as metastatic gastric carcinoma12. In a small series of patients with breast cancer, exceptional responses to capecitabine were associated with HR-deficiency and/or mutations in chromatin remodelling genes37.
Patients with ovarian cancer who carry germline mutations in BRCA1 or BRCA2 show a more favourable initial response and overall survival 5 years after diagnosis than noncarriers38. At 10 years, however, a survival advantage is only seen in BRCA2 mutation carriers39,40, demonstrating that mutations in the HR pathway are non-equivalent. Moreover, HR-deficiency is important but insufficient to impart enhanced response to treatment or prolonged survival, as a substantial proportion of patients with HGSC with inherited BRCA1 or BRCA2 mutations have a poor response and overall survival41. Hypomorphic germline alleles, such as the BRCA1-Δ11q alternative splice isoform42, or tumours that have retained the wild type BRCA1 or BRCA2 allele41 or an unmethylated BRCA1 allele43, appear to impart sufficient HR-repair activity to confer resistance to chemotherapy and may account for less durable responses. Hence, the prediction of response to chemotherapy requires a more specific assessment than simply knowing that a patient carries a BRCA germline mutation, or that in another patient, some methylation of BRCA1 has been detected in a tumour. It is conceivable that specific mutations in individual HR pathway genes may be associated with a more favourable outcome, such as large deletions that are unlikely to undergo reverting mutations. However, in our study of patients with mutation-positive ovarian cancer we were not able to associate the type or position of mutation in BRCA1 or BRCA2 with a pattern of particularly favourable survival44.
BRCA1 and BRCA2 mutant tumours are also sensitive to poly(ADP-ribose) polymerase (PARP) inhibitors. Inhibition of PARP enzymes leads to the accumulation of DNA damage by impairing single-strand DNA repair, which causes synthetic lethality in cells with BRCA1 or BRCA2 mutations due to the accumulation of DNA damage and subsequent cell death45,46. Recently, a patient with metastatic urothelial carcinoma that had a somatic homozygous deletion of BRCA2 experienced an exceptional response to the PARP inhibitor olaparib, having previously failed to respond to a programmed cell death protein 1 ligand 1 (PDL1) inhibitor despite this tumour type having a high mutational burden47. PARP inhibitors are particularly important in the treatment of HGSC due to the prevalence of BRCA1 and BRCA2 mutations in this disease. In a study of long-term responders (>2 years) on olaparib maintenance therapy, patients with durable responses were more likely to have BRCA2 mutations, PTEN alterations and high HR-deficiency scores compared to those with short (<3 months) responses48.
However, in these examples not all patients whose tumour has a given mutation have profound responses, suggesting that additional factors influence the depth of the observed response. It is plausible that a chance combination of alterations is required for exceptional response, explaining why such patients are rare and why a single biomarker has not yet been found that tracks precisely with unusually favourable outcome. We investigated this possibility by performing whole-genome sequencing and immunohistochemical analysis on long-term survivors of HGSC and found that cooccurrence of loss of the tumour suppressor RB1 with BRCA1 or BRCA2 mutation is associated with long term survival17. Loss of RB1 and HR-deficiency was particularly enriched in patients who were suboptimally debulked and yet had exceptional responses, suggesting that the combined mutations may confer enhanced chemotherapeutic or subsequent immune response.
Immunological factors influencing exceptional response and survival
The impact of tumour infiltrating lymphocytes (TILs) on long-term survival has been demonstrated for several cancer types such as breast, ovarian, lung and colorectal cancer and melanoma49. While most studies to date have focused on CD3+ and/or CD8+ TILs, favourable prognostic effects are also associated with markers of T cell phenotype and function (e.g., CD103 (also known as integrin α-E), T cell-restricted intracellular antigen-1 (TIA-1), and programmed cell death protein 1 (PD1))50–52 and other lymphocyte subsets, in particular B-cells and plasma cells53, suggesting that a multi-modal immune response is required for effective tumour control. In the same way that a combination of driver mutations may influence exceptional response, the concerted impact of multiple tumour cell-intrinsic and extrinsic microenvironmental factors can govern overall response54. TILs may be both direct mediators of favourable prognosis (e.g., via cytolytic effects against tumours) as well as serving as biomarkers of other prognostic drivers, such as DNA repair defects or oncogenic signalling profiles. For example, in endometrial cancer TILs are more prevalent in the highly mutated molecular subtypes (i.e., the microsatellite instable and POLE subtypes); yet, in multivariable analyses, molecular subtype carried stronger prognostic significance than TIL density55, suggesting a predominant role for non-immunological factors in determining outcome in this cancer type.
Given the recent success of cytotoxic T lymphocyte antigen 4 (CTLA4)- and PD1-directed immune checkpoint blockade against a wide range of cancer types56, intense effort is underway to identify determinants of profound response. Somatic mutation load has emerged as a strong correlate of response both between57 and within58 cancer types. Immune checkpoint blockade has been approved for the treatment of any MMR-defective adult or paediatric cancer owing to the higher somatic mutation loads caused by defective DNA repair and the high rates of response to immune checkpoint inhibitors in MMR-defective cancers59–62. Although mutational load is among the strongest predictors of response to immune checkpoint blockade, studies of rare cancer types have revealed the importance of other factors. PDL1 expression by tumour cells is regulated by multiple mechanisms at the environmental, genomic, transcriptional, and protein levels63,64 providing many opportunities for exceptional response. Genomic amplification65 or rearrangement66 of PDL1 can lead to enforced expression and has been associated with exceptional response to anti-PD1 therapy67,68. Classical Hodgkin lymphoma has a relatively low mutational burden yet frequently shows elevated PDL1 expression due to amplification of the PDL1 gene locus on chromosome arm 9p24 (REF.69), with a substantial and long-lasting response rate to PD1 inhibitors in the relapse setting70.
Impaired chromatin remodelling may represent another mechanism by which tumours become sensitive to immune checkpoint blockade. In the setting of clear-cell renal cell carcinoma, response to anti-PD1 monotherapy is associated with loss-of-function mutations in the PBRM1 (also known as BAF180) gene, which encodes a subunit of the SWI/SNF chromatin remodelling complex71. Findings from a rare gynaecological cancer further implicate the SWI/SNF complex in influencing the response to immune checkpoint blockade; small cell carcinoma of the ovary, hypercalcemic type (SCCOHT) has a low mutation load and shows a very high prevalence of deactivating mutations in the SMARCA4 (also known as BRG1) gene72, which encodes another subunit of the SWI/SNF complex. SCCOHT tumours generally exhibit high expression of PDL1 and, based on a small series of patients treated to date, appear to be exceptionally responsive to anti-PD1 immune checkpoint blockade73. Clear cell ovarian cancer, which also appears to be very responsive to immune checkpoint blockade74, show a high prevalence of mutations in AT-rich interactive domain 1A (ARID1A)75, a gene that encodes yet another subunit of the SWI/SNF complex. These findings demonstrate that even in tumours with low mutational burden and therefore an expected poor response to immunotherapy, enforced expression of PDL1 can predict exceptional response to immune checkpoint inhibitors.
The recent description of hyper-progression in patients undergoing immune checkpoint blockade76 provides an interesting contrast to those who benefit from this therapy (FIG. 1a). A comprehensive analysis of 155 consecutive patients with different types of stage IV cancer revealed that alterations of the MDM2, MDM4 or epidermal growth factor receptor (EGFR) genes were significantly associated with rapid progression on immunotherapy77. The mechanism by which immune checkpoint blockade may promote tumour growth is unclear although a recent study found increased macrophage infiltration in a series of lung cancer patients with evidence of accelerated tumour growth follow anti-PD1/PD-L1 therapy78. Similar to studies of patients with exceptional response or survival, investigation of hyper-progression is hampered by a lack of a standard definition of such patients. Additionally, where hyper-progression is suspected in a patient with late stage disease it may be difficult to distinguish therapy-related progression from an apparent acceleration of tumours in a rapidly failing individual.
Ex vivo expansion and adoptive transfer of TILs has yielded profound clinical responses in a substantial proportion of patients with metastatic melanoma; however, the approach has been far less effective against epithelial cancers. Nonetheless, there have been exceptional responders that may provide useful insights into the determinants of success. For example, a patient with metastatic cholangiocarcinoma experienced significant tumour regression at two different points in her disease trajectory after treatment with a TIL product that was highly enriched for CD4+ T-helper 1 (TH1) cells specific for a mutation in ERBB2 interacting protein (ERBB2IP)79. In another example, a patient with chemo-refractory, metastatic, hormone receptor positive breast cancer experienced durable complete regression of all tumour masses after infusion of TILs reactive against mutant versions of four proteins together with systemic interleukin-2 (IL-2) and anti-PD1 immune checkpoint blockade80. The response of this heavily pre-treated patient suggests that refinement of relatively established methodology, such as the adoptive transfer of TILs, through the integration of genome sequencing and directed antitumour vaccination may lead to more durable responses.
Chimeric antigen receptor (CAR) T-cells have shown profound efficacy in certain haematological malignancies, including acute lymphoblastic leukemia (ALL) where targeting of the cell surface protein CD19 has been associated with complete remission in a large proportion of patients81,82. Despite this success, responses have been much more variable in other cancer types, including solid cancers83. Why exceptional responses to CAR T-cell therapy should be seen in ALL compared other cancers is still being determined, however, the uniformity of expression of CD19 in ALL and favourable access of engineered cells to the tumour cell population appear to be important84,85. Recently, a patient with chronic lymphocytic leukaemia was reported who appeared to benefit from a chance integration of the CAR transgene into the tet methylcytosine dioxygenase 2 (TET2) gene locus of a T cell clone that, at the peak of the response, comprised 94% of the CAR-T-cell population86. Additional reduction in TET2 activity arose from a hypomorphic mutation in the second TET2 allele of the patient. TET2 dysfunction was associated with epigenetic changes that enhanced T-cell expansion and sustained cytokine production. This patient illustrates how a chance effect can focus attention on a novel pathway influencing CAR-T cell survival and function.
Epidemiological determinants of long-term survival
Life-style factors including diet87,88, physical activity89–91, obesity92,93, hormone replacement therapy94–96, smoking97,98 and alcohol consumption99 are known to be associated with cancer risk and survival and, by extension, may therefore contribute to exceptional outcomes. These factors are often of special interest to patients with cancer as they provide a perceived opportunity where patients may directly influence their chance of survival through changes in their behaviour or diet.
Despite clear associations between modifiable life-style factors and survival in typical patients, measured both before and after a diagnosis of cancer, there is a paucity of data on their role in exceptional survival. It is currently unclear whether lifestyle factors influence survival through direct effects on tumour characteristics and response to therapy, by altering the ability of the immune system to control tumour growth, and/or by affecting the overall health of the patient and their ability to endure the effects of often physiologically challenging cancer therapy100–102.
Integrated studies that consider the intersection of genetic, genomic, and immune characteristics of a patient’s tumour with epidemiological factors may be of value in untangling complex relationships between a patient’s lifestyle, their genome and physiology, molecular alterations in their tumour, and their body’s ability to mount an immune response against their cancer103. In 2018, the Harvard Medical School launched the Network of Enigmatic Exceptional Responders (NEER) registry of patients with all cancer types who had exceptional responses to treatment104. This study aims to not only comprehensively profile blood (germline DNA), tumour and stool (gut microbiome) samples, but to also incorporate extensive epidemiological data, including environmental factors (inferred by postcode history), real-time physical activity and sleeping patterns (participants will wear personal activity trackers), as well as obtain complete medical records and posts on social media in order to extend data collection on the personality, communication and social interactions of exceptional responders. Assembling large, complete, multidimensional datasets of this kind presents unique challenges that will require interdisciplinary expertise. Analyses may focus on the joint contribution of lifestyle factors and the investigation of whether the individual factors act in isolation or interact positively to influence survival.
Conclusion and recommendations
Much remains to be learned about the factors that influence exceptional response and overall survival in patients with cancer. The journeys of patients with cancer are varied and multiple factors are likely to contribute to outcome, some of which may be independent of the characteristics of the tumour, such as the ability of a patient to complete treatment. We recommend large, carefully designed studies, given the likely multifactorial and inter-related influence of survival factors (BOX 3). With attention to detail, these remarkable patients with cancer are likely to provide insights that will be applicable to the wider patient population.
Acknowledgments
F.A.M.S. is supported by a Swiss National Foundation Early Postdoc Mobility Fellowship (P2BEP3-172246), a Swiss Cancer League grant BIL KFS-3942-08-2016 and a Prof. Max Cloëtta foundation grant. B.H.N., A.dF., C.L.P., M.C.P., D.W.G. and D.D.L.B. are supported by U.S. Army Medical Research and Materiel Command grant W81XWH-16-2-0010. D.D.L.B. is supported by the National Health and Medical Research Council of Australia (NHMRC) grants APP1092856 and APP1117044, and the US National Cancer Institute U54 program (U54CA209978). We gratefully acknowledge additional support from Mrs. Margaret Rose AM and the Rose family, The WeirAnderson Foundation, Border Ovarian Cancer Awareness Group, donors to the Garvan Institute of Medical Research’s Ovarian Cancer Research Program, the Peter MacCallum Cancer Centre Foundation, Wendy Taylor and Arthur Coombs and family. The Australian Ovarian Cancer Study (AOCS) was supported by the U.S. Army Medical Research and Materiel Command under DAMD17-01-1-0729.
Glossary
| Complete response (CR) | The disappearance of all target lesions in response to treatment. |
| Genome-wide association studies (GWAS) | Studies that compare genetic markers across the genome in individuals with and without disease traits. |
| Hyper-progression | Accelerated disease progression associated with immune checkpoint inhibitor therapy. |
| Hypomorphic germline alleles | Also called hypomorphic mutations; inherited genetic variants that cause partial (not complete) loss of gene function through reduced expression or function. |
| Maintenance therapy | Treatment provided following initial therapy to prevent relapse. |
| Multivariable analyses | Statistical models taking into account the impact of multiple explanatory variables that may influence a single outcome. |
| Partial response (PR) | A decrease of at least 30% in the sum of the diameters of target lesions in response to treatment. |
| Polygenic risk | A genetic susceptibility score based on the combination of multiple, often lowpenetrance, disease susceptibility alleles. |
| Synthetic lethality | A phenomenon in cells or organisms whereby two gene or pathway defects occurring in isolation have little or no effect on survival but for which the combination of both leads to death. |
Footnotes
Competing interests
The authors declare no competing interests.
Related links
References
Full text links
Read article at publisher's site: https://doi.org/10.1038/s41568-019-0145-5
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc7255796?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1038/s41568-019-0145-5
Article citations
Concurrent RB1 Loss and BRCA Deficiency Predicts Enhanced Immunologic Response and Long-term Survival in Tubo-ovarian High-grade Serous Carcinoma.
Clin Cancer Res, 30(16):3481-3498, 01 Aug 2024
Cited by: 0 articles | PMID: 38837893 | PMCID: PMC11325151
Comprehensive Clinical Characterization of Decade-Long Survivors of Metastatic Breast Cancer.
Cancers (Basel), 15(19):4720, 25 Sep 2023
Cited by: 2 articles | PMID: 37835414 | PMCID: PMC10571750
Relationship between homologous recombination deficiency and clinical features of breast cancer based on genomic scar score.
Breast, 69:392-400, 21 Apr 2023
Cited by: 2 articles | PMID: 37116400 | PMCID: PMC10165146
Leveraging high-resolution omics data for predicting responses and adverse events to immune checkpoint inhibitors.
Comput Struct Biotechnol J, 21:3912-3919, 24 Jul 2023
Cited by: 0 articles | PMID: 37602228 | PMCID: PMC10432706
Review Free full text in Europe PMC
Extraction, Structural Characterization, Biological Functions, and Application of Rice Bran Polysaccharides: A Review.
Foods, 12(3):639, 02 Feb 2023
Cited by: 4 articles | PMID: 36766168 | PMCID: PMC9914776
Review Free full text in Europe PMC
Go to all (24) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The future of Cochrane Neonatal.
Early Hum Dev, 150:105191, 12 Sep 2020
Cited by: 5 articles | PMID: 33036834
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.
Cochrane Database Syst Rev, 2(2022), 01 Feb 2022
Cited by: 12 articles | PMID: 36321557 | PMCID: PMC8805585
Review Free full text in Europe PMC
Identification of the immune cell infiltration landscape in pancreatic cancer to assist immunotherapy.
Future Oncol, 17(31):4131-4143, 04 Aug 2021
Cited by: 3 articles | PMID: 34346253
The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer.
Crit Rev Oncol Hematol, 88(1):218-230, 17 Apr 2013
Cited by: 795 articles | PMID: 23602134
Review
Funding
Funders who supported this work.
NCI NIH HHS (2)
Grant ID: U54 CA209978
Grant ID: P30 CA008748





