Abstract
Background
Some patients with COVID-19 pneumonia also present with kidney injury, and autopsy findings of patients who died from the illness sometimes show renal damage. However, little is known about the clinical characteristics of kidney-related complications, including hematuria, proteinuria, and AKI.Methods
In this retrospective, single-center study in China, we analyzed data from electronic medical records of 333 hospitalized patients with COVID-19 pneumonia, including information about clinical, laboratory, radiologic, and other characteristics, as well as information about renal outcomes.Results
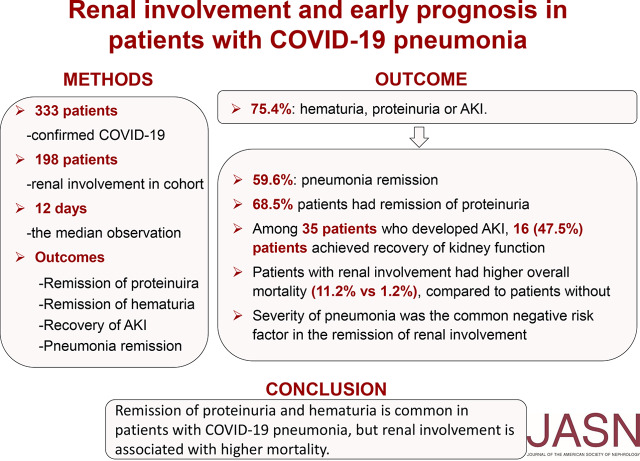
We found that 251 of the 333 patients (75.4%) had abnormal urine dipstick tests or AKI. Of 198 patients with renal involvement for the median duration of 12 days, 118 (59.6%) experienced remission of pneumonia during this period, and 111 of 162 (68.5%) patients experienced remission of proteinuria. Among 35 patients who developed AKI (with AKI identified by criteria expanded somewhat beyond the 2012 Kidney Disease: Improving Global Outcomes definition), 16 (45.7%) experienced complete recovery of kidney function. We suspect that most AKI cases were intrinsic AKI. Patients with renal involvement had higher overall mortality compared with those without renal involvement (28 of 251 [11.2%] versus one of 82 [1.2%], respectively). Stepwise multivariate binary logistic regression analyses showed that severity of pneumonia was the risk factor most commonly associated with lower odds of proteinuric or hematuric remission and recovery from AKI.Conclusions
Renal abnormalities occurred in the majority of patients with COVID-19 pneumonia. Although proteinuria, hematuria, and AKI often resolved in such patients within 3 weeks after the onset of symptoms, renal complications in COVID-19 were associated with higher mortality.Free full text

Renal Involvement and Early Prognosis in Patients with COVID-19 Pneumonia
Significance Statement
Some cases of COVID-19 pneumonia have presented with kidney injury, and autopsy findings for patients with COVID-19 have revealed renal involvement. In this retrospective, single-center study of Chinese patients with COVID-19 pneumonia, 251 of 333 patients (75.4%) presented with renal complications, including proteinuria, hematuria, and AKI. After the median duration of 12 days of follow-up, nearly half of patients with AKI recovered from AKI within 3 weeks of onset of infection. However, patients with renal involvement had higher overall mortality (11.2%) compared with patients without renal involvement (1.2%). Adverse short-term outcomes of renal involvement were associated with severity of COVID-19 pneumonia. These findings indicate that although early renal abnormalities often resolve in such patients, intensive support and careful monitoring of severe or critical illness is appropriate for COVID-19 pneumonia with renal complications.
Abstract
Background
Some patients with COVID-19 pneumonia also present with kidney injury, and autopsy findings of patients who died from the illness sometimes show renal damage. However, little is known about the clinical characteristics of kidney-related complications, including hematuria, proteinuria, and AKI.
Methods
In this retrospective, single-center study in China, we analyzed data from electronic medical records of 333 hospitalized patients with COVID-19 pneumonia, including information about clinical, laboratory, radiologic, and other characteristics, as well as information about renal outcomes.
Results
We found that 251 of the 333 patients (75.4%) had abnormal urine dipstick tests or AKI. Of 198 patients with renal involvement for the median duration of 12 days, 118 (59.6%) experienced remission of pneumonia during this period, and 111 of 162 (68.5%) patients experienced remission of proteinuria. Among 35 patients who developed AKI (with AKI identified by criteria expanded somewhat beyond the 2012 Kidney Disease: Improving Global Outcomes definition), 16 (45.7%) experienced complete recovery of kidney function. We suspect that most AKI cases were intrinsic AKI. Patients with renal involvement had higher overall mortality compared with those without renal involvement (28 of 251 [11.2%] versus one of 82 [1.2%], respectively). Stepwise multivariate binary logistic regression analyses showed that severity of pneumonia was the risk factor most commonly associated with lower odds of proteinuric or hematuric remission and recovery from AKI.
Conclusions
Renal abnormalities occurred in the majority of patients with COVID-19 pneumonia. Although proteinuria, hematuria, and AKI often resolved in such patients within 3 weeks after the onset of symptoms, renal complications in COVID-19 were associated with higher mortality.
The outbreak of coronavirus disease has rapidly evolved into a global pandemic.1–7 This novel coronavirus is named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).8 The World Health Organization (WHO) officially named the disease caused by SARS-CoV-2 as coronavirus disease 2019 (COVID-19). The initial clinical sign for the detection of COVID-19 was pneumonia.3 However, other organ damages were also reported.6,9 Some cases of COVID-19 pneumonia presented with kidney injury,4 and pathologic findings from autopsies also revealed renal damage from the corpses of patients with COVID-1910; thus, SARS-CoV-2 may include kidney tropism. In this study, we reported renal involvement and their early prognosis of patients with COVID-19 pneumonia admitted to Tongji Hospital in Wuhan, China, in terms of proteinuria, hematuria, and AKI.
Methods
Study Design and Participants
For this retrospective, single-center study, we included adult patients from January 28 to February 9, 2020, at Tongji Hospital of Sino-French New City District, which was one of the designated hospitals solely for the treatment of patients with COVID-19 in Wuhan, China. The final follow-up for this study ended on February 23, 2020. All patients who were diagnosed as having COVID-19 pneumonia according to the Diagnosis and Treatment Protocols of Pneumonia caused by Novel Coronavirus (SARS-CoV-2) by the National Health Commission of China (Trial Version 7)10 were screened. Specifically, the diagnosis criteria were as follows: (1) fever or respiratory symptoms, (2) leukopenia or lymphopenia, and (3) computed tomography (CT) scan showing radiographic abnormalities in lung. Those with two or more diagnosis criteria (lung involvement is necessary) and a positive result on high-throughput sequencing or RT-PCR assay were diagnosed as having COVID-19 pneumonia.10 Exclusion criteria were as follows: (1) patients with CKD or suspected CKD, with at least one abnormal urine test including proteinuria and hematuria in the 3 months before admission; (2) patients with maintenance dialysis, renal transplantation, or peak serum creatinine of <53 μmol/L; (3) patients with time from onset of disease to admission date of >14 days, given that most patients achieved radiologic remission after 14 days from onset.11 The study was approved by Tongji Hospital Ethics Committee of Huazhong University of Science and Technology (approval number TJ-C20200132). Informed consent was waived in the light of the urgency of data collection.
Data Collection and Measurements
We obtained demographic, epidemiologic, clinical, laboratory, and radiologic characteristics, and treatment and renal outcome data from electronic medical records. The data were reviewed by two physicians (G.P. and R.Z.). The onset date was defined as the day when the symptom was noticed. As of February 9, 2020, a total of 467 patients with COVID-19 were admitted and screened. A total of 333 patients with urine dipstick test on the first morning after admission, or with AKI on admission and during the hospital stay, were included in this study. Among the 333 patients, 82 patients without proteinuria or hematuria on admission and without AKI were excluded. Further, 53 patients without serial monitoring of urine dipstick tests were excluded. Finally, 198 patients with serial monitoring of urine dipstick tests, or with AKI, were included in the cohort (Supplemental Figure 1). It should be noted that 19 of the 35 patients with AKI and 235 of the 298 patients without AKI have been described previously by Cheng et al.12
Patients with possible COVID-19 pneumonia were admitted and quarantined, and throat swab samples were collected and detected in the hospital using a quantitative real-time RT-PCR to confirm SARS-CoV-2, as follows: A Viral Nucleic Acid Kit (Tianlong Science & Technology Co., Ltd., Xi’An, China) was used to extract nucleic acids from throat swab samples and a SARS-CoV-2 detection kit (DAAN GENE Co., Guangzhou, China) was used to detect the ORF1ab gene (nCovORF1ab) and the N gene (nCoV-NP) according to the manufacturer’s instructions, using real-time RT-PCR. If the circulation threshold (Ct) values for both genes were >40 or had no typical amplification curves, and the internal control performed well, it was considered that the SARS-CoV-2 RNA was not present. If the Ct values for both genes were ≤40, the gene detection results were considered as positive. If only one gene had a Ct value of ≤40, and the other one had no typical amplification curve, a repeat experiment was performed, with consistent results considered as positive for SARS-CoV-2 RNA.
All patients were given a chest CT scan before or after admission to hospital, and a repeat chest CT scan was obtained at 5–10 day intervals. Laboratory data consisted of complete blood counts, liver and renal function, coagulation function, high-sensitivity C-reactive protein (CRP), erythrocyte sedimentation rate, and serum cytokines. Serial monitoring of these laboratory tests was performed for each patient according to the patient’s clinical progress.
Definitions
COVID-19 pneumonia was classified into four types, namely, mild, moderate, severe, and critically ill, according to Diagnosis and Treatment Protocols of Pneumonia caused by Novel Coronavirus (SARS-CoV-2) by the National Health Commission of China (Trial Version 7).10 Moderate COVID-19 pneumonia was defined as fever, respiratory syndrome, and radiologic lung findings.10 Severe COVID-19 pneumonia was defined as meeting any of the following conditions: (1) respiratory rate ≥30 breaths/min, (2) oxygen saturation ≤93% in a resting state, (3) Arterial oxygen partial pressure(PaO2) / Fractional inspired oxygen(FiO2) ratio ≤300 mm Hg, and (4) a 50% increase in chest radiologic abnormalities in 24–48 hours.10,12 Critically ill COVID-19 pneumonia was defined as either (1) respiratory failure need for mechanical ventilation, (2) shock, or (3) organ failure need for ICU admission.10,12
For the diagnosis of AKI, we referred to the 2012 Kidney Disease: Improving Global Outcomes (KDIGO) definition and the expanded criteria used by Yang et al.13,14 First, we screened patients with suspected AKI using 2012 KDIGO definition as the major screening criteria. However, patients without previous baseline serum creatinine and those who did not meet the 2012 KDIGO criteria at admission but their increased serum creatinine was 1.5 times above the baseline with intervals longer than 7 days, would be excluded according to the 2012 KDIGO definition, which might potentially underestimate overall occurrence of AKI. For those who had no repeated serum creatinine within 7 days or with recovering AKI, we expanded the screening criteria to an increase or decrease in serum creatinine by ≥0.3 mg/dl during hospital stay. Second, these suspected AKI were reviewed on a case-by-case basis to confirm the diagnosis. The identification criteria included the 2012 KDIGO definition of AKI (KDIGO criteria). For those who had no repeated serum creatinine within 7 days or with recovering AKI, we expanded the identification criteria of AKI to an increase or decrease in serum creatinine by 50% during hospital stay (using serum creatinine concentration at admission as a baseline; we looked for increases or decreases in creatinine relative to this value), with or without oliguria. We defined renal recovery as serum creatinine decreasing to below threshold or to the baseline. The staging of AKI was on the basis of 2012 KDIGO criteria.14
Patients were considered to have prerenal AKI when the rise in serum creatinine concentration had been caused by low renal perfusion, and creatinine recovered rapidly to baseline after volume administration within 3 days.15 Rhabdomyolysis was diagnosed on the basis of medical history, and elevated serum creatinine phosphokinase (CK) levels more than five times the upper limit of normal and/or serum myoglobin >150 ng/ml.16,17 The elevations in CK were not caused by myocardial infarction, cerebral vascular disease, or neuromuscular disease. As no obstructive AKI was diagnosed, we classified the other AKI in this study as suspected intrinsic AKI, which might include acute tubular necrosis (ATN) and nephrotoxic AKI.
Patients with CKD were excluded. CKD was defined as eGFR<60 ml/min per 1.73 m2 or urine microalbumin-creatinine ratio ≥30 μg/mg at least 3 months before admission.18 If there was only one outpatient GFR <60 ml/min per 1.73 m2, we evaluated CKD-related complications such as anemia and mineral and bone disorders. The patients who had CKD-related complications were considered as having suspected CKD and were also excluded.
Proteinuria and hematuria were defined as more than trace albumin or blood on urine dipstick tests, which were collected and detected on the first morning after admission and during the observation period. Remission of proteinuria and hematuria was defined as protein and blood were negative on urine dipsticks. Recovery of AKI was defined as complete recovery of kidney function.
Statistical Analyses
Data conforming to normal distribution were presented as mean±SD, or median and quartiles for non-normal distribution. Rate comparisons were performed by chi-squared test. t Test, Wilcoxon rank-sum test, Wilcoxon signed-rank test, or Kruskal–Wallis test were used to compare means across groups according to the number of group and distribution of variable. To estimate the degree of correlation between proteinuria or hematuria and clinical variables, Spearman rank correlation coefficient was used. Stepwise multivariate binary logistic regression was used to select and estimate the association between proteinuria, hematuria, or AKI remission and variables that were clinically relevant on grounds of professional knowledge and were statistically significant in preliminary univariate binary logistic regression. Specifically, variables with P<0.05 in the univariate analysis were entered into multivariate analysis to select the predictors (inclusion criterion was P<0.05 and exclusion criterion was P<0.10). Results are presented as odds ratios (ORs) with 95% confidence intervals (95% CIs) and P values. All statistical analyses were performed using SPSS version 20.0 software (IBM Corp.).
Results
Baseline Characteristics
A total of 333 patients were included in the study. The mean of age was 56.3 years (Table 1), and 54.7% (182 of 333) patients were men. The median duration from onset to admission was 9 (7–11) days (Table 1). The prevalence of hypertension and diabetes was 32.2% (107 of 333) and 22.9% (76 of 333), respectively. Compared with moderate cases, patients with severe or critically ill COVID-19 pneumonia were older, more likely to experience dyspnea, and more likely to have hypertension, diabetes, and angiotensin-converting enzyme inhibitor/angiotensin receptor blocker (ACEI/ARB) treatment history (Table 1).
Table 1.
Clinical characteristics of patients with COVID-19
| Variables | All Patients | Moderate | Severe | Critically Ill | P Value |
|---|---|---|---|---|---|
| N | 333 | 144 (43.2%) | 133 (39.9%) | 56 (16.8%) | |
| Days from onset | 9 (7–11) | 8 (6–11) | 10 (7–11) | 8 (6–11) | 0.12a |
| Age, yr | 56.3±13.4 | 50.9±12.5 | 58.1±12.3 | 63.1±11.0 | <0.001a |
| Male patient, % | 182/333 (54.7%) | 67/144 (46.5%) | 79/133 (59.4%) | 36/56 (64.3%) | 0.03b |
| Fever, % | 301/333 (90.4%) | 126/144 (87.5%) | 123/133 (92.5%) | 52/56 (92.9%) | 0.16b |
| Cough, % | 229/333 (68.8%) | 98/144 (68.1%) | 94/133 (70.7%) | 37/56 (66.1%) | 0.94b |
| Dyspnea, % | 190/333 (57.1%) | 71/144 (49.3%) | 85/133 (63.9%) | 34/56 (60.7%) | 0.05b |
| Diarrhea, % | 108/333 (32.4%) | 42/144 (29.2%) | 41/133 (30.8%) | 25/55 (49.1%) | 0.06b |
| Hypertension, % | 107/332 (32.2%) | 37/144 (25.7%) | 43/133 (32.3%) | 27/55 (49.1%) | 0.003b |
| ACEI/ARB history, % | 37/321 (11.5%) | 12/143 (8.4%) | 14/126 (11.1%) | 11/52 (21.2%) | 0.02b |
| ACEI/ARB | 11/26 | 0/12 | 6/8 | 5/6 | |
| Diabetes, % | 76/332 (22.9%) | 20/144 (13.9%) | 32/133 (24.1%) | 24/55 (43.6%) | <0.001b |
| SPO2 (%) | 93 (91.0–97.0) | 97.0 (96.0–98.0) | 92.0 (90.0–93.0) | 89.5 (80.0–93.0) | <0.001a |
| Systolic BP, mm Hg | 126 (115–138) | 124 (114–132) | 128 (116–141) | 135 (118–145) | 0.001a |
| Diastolic BP, mm Hg | 78 (72–86) | 77 (72–86) | 78 (71–87) | 79 (73–86) | 0.76a |
| Blood sugar, mmol/L | 6.6 (5.7–8.1) | 6.0 (5.4–7.1) | 6.9 (5.9–8.6) | 7.8 (6.3–12.4) | <0.001a |
| CRP, mg/L | 44.1 (16.3–90.8) | 22.1 (7.5–45.3) | 58.1 (32.5–101.4) | 69.7 (42.9–119.6) | <0.001a |
| Erythrocyte sedimentation rate, mm/h | 31.0 (18.0–58.8) | 27.0 (14.3–50.3) | 39.0 (22.0–64.0) | 31.0 (22.0–58.0) | 0.004a |
| ALT, U/L | 24.0 (15.0–38.3) | 20.0 (13.0–33.0) | 26.0 (18.5–39.5) | 29.0 (18.0–43.0) | 0.001a |
| AST, U/L | 31.0 (22.0–48.0) | 24.0 (20.0–38.0) | 33.0 (24.0–50.5) | 40.5 (25.5–62.3) | <0.001a |
| HsTnI, pg/ml | 5.4 (3.3–14.0) | 4.6 (2.5–7.0) | 4.8 (3.1–11.7) | 11.4 (6.3–21.6) | <0.001a |
| NT-proBNP, pg/ml | 139.0 (52.0–392.5) | 76.0 (23.0–152.0) | 157.5 (55.5–333.8) | 372.5 (155.3–758.3) | 0.002a |
| Serum albumin, g/L | 34.5±4.7 | 36.6±4.9 | 33.6±4.2 | 32.9±4.3 | <0.001a |
| BUN, mmol/L | 4.3 (3.2–5.7) | 3.9 (3.1–5.0) | 4.4 (3.2–5.5) | 5.9 (4.6–8.6) | <0.001a |
| SCR, μmol/L | 70.0 (57.0–84.0) | 66.5 (56.0–81.0) | 69.0 (57.0–84.0) | 77.0 (60.0–89.0) | <0.001a |
| Prothrombin time, sec | 14.1 (13.5–14.8) | 14.0 (13.2–14.5) | 14.3 (13.5–14.9) | 14.4 (13.8–14.9) | 0.004a |
| D-dimer, mg/L | 0.73 (0.40–1.33) | 0.47 (0.32–1.10) | 0.80 (0.57–1.33) | 1.25 (0.64–5.90) | <0.001a |
| Neutrophils, 109/L | 3.79 (2.57–5.45) | 2.99 (2.14–4.13) | 4.06 (2.84–5.56) | 5.79 (3.79–8.59) | <0.001a |
| Lymphocytes, 109/L | 0.86 (0.63–1.20) | 1.01 (0.72–1.44) | 0.78 (0.60–1.05) | 0.55 (0.43–0.80) | <0.001a |
| Eosinophils, 109/L | 0.00 (0.00–0.01) | 0.00 (0.00–0.09) | 0.00 (0.00–0.06) | 0.00 (0.00–0.01) | <0.001a |
| Monocytes, 109/L | 0.39 (0.28–0.51) | 0.41 (0.30–0.53) | 0.36 (0.26–0.52) | 0.38 (0.22–0.49) | 0.16a |
| Serum TNFα, pg/ml | 8.6 (7.0–10.7) | 8.1 (6.8–9.8) | 9.3 (7.6–11.6) | 9.3 (6.8–11.1) | 0.09a |
| Serum IL-10, pg/ml | 5.7 (5.0–10.2) | 5.0 (5.0–8.1) | 6.4 (5.0–10.4) | 8.0 (5.0–15.1) | 0.01a |
| Serum IL-6, pg/ml | 19.9 (8.0–45.4) | 13.2 (3.8–23.1) | 27.1 (11.8–60.0) | 32.8 (17.8–62.6) | <0.001a |
| Serum IL-2R, U/ml | 669 (459–963) | 546 (455–743) | 766 (595–1050) | 1026 (378–1260) | <0.001a |
| Proteinuria, % | 219/333 (65.8%) | 63/144 (43.8%) | 108/133 (81.2%) | 48/56 (85.7%) | <0.001b |
| Hematuria, % | 139/333 (41.7%) | 48/144 (33.3%) | 52/133 (39.1%) | 39/56 (69.6%) | <0.001b |
| AKI, % | 35/333 (10.5%) | 5/144 (3.5%) | 6/133 (4.5%) | 24/56 (42.9%) | <0.001b |
| Renal involvement, % | 251/333 (75.4%) | 89/144 (61.8%) | 111/133 (83.5%) | 51/56 (91.1%) | <0.001b |
| Death, % | 29/333 (8.7%) | 0 | 0 | 29/56 (52.8%) | <0.001b |
Data are presented as a number and percentage or mean±SD or median (25th–75th percentiles); eosinophils are presented as median (fifth–95th percentiles). SPO2, pulse oxygen saturation; ALT, alanine transaminase; AST, aspartate transaminase; HsTnI, high-sensitivity troponin; NT-proBNP, pro-brain natriuretic protein, N-terminal; SCR, serum creatinine.
Proteinuria, Hematuria, and AKI
On admission, of the 333 patients, 75.4% (251 of 333) patients had renal involvement, 65.8% (219 of 333) patients presented with proteinuria, and 41.7% (139 of 333) patients had hematuria (Table 1). The incidence of AKI in the overall cohort was 4.7% (22 of 467) by KIDGO criteria and 7.5% (35 of 467) by expanded criteria. Greater incidence of proteinuria (81.2% and 85.7%, respectively, versus 43.8%) and hematuria (39.1% and 69.6%, respectively, versus 33.3%) were demonstrated in patients with severe or critically ill COVID-19 pneumonia (Table 1). Among the 333 patients, the patients with AKI had higher incidence rate of proteinuria (88.6% versus 63.1%) and hematuria (60% versus 41.7%) compared with the non-AKI group (Supplemental Table 1). A total of 42.9% (24 of 56) critically ill cases developed AKI during the hospital stay (Table 1).
The number of patients with AKI was 22 by KDIGO criteria and 35 by expanded criteria (Table 2). According to KDIGO criteria, AKI occurred after admission in 19 (86.4%) of 22 patients with AKI. Rhabdomyolysis-induced AKI accounted for 18.2% (four of 22), but no prerenal AKI occurred. Suspected intrinsic AKI that accounted for 81.8% (18 of 22) was the most frequent form. Stage 2 comprised seven (31.8%) of 22 patients with AKI, and 50% (11 of 22) reached stage 3. The patients with AKI identified by the expanded criteria had more AKI occurrence on admission and more AKI in stage 1 than patients with AKI who met KDIGO criteria (Table 2).
Table 2.
Characteristics of patients with AKI according to two different criteria
| Variables | KDIGO AKI Criteria | Expanded Criteria |
|---|---|---|
| N | 22 | 35 |
| AKI occurrence on admission | 3/22 (13.6%) | 13/35 (37.1%) |
| AKI occurrence during hospital stay | 19/22 (86.4%) | 22/35 (62.9%) |
| Classification | ||
 Prerenal AKI Prerenal AKI | 0/22 (0%) | 2/35 (5.7%) |
 Rhabdomyolysis-induced AKI Rhabdomyolysis-induced AKI | 4/22 (18.2%) | 4/35 (11.4%) |
 Suspected intrinsic AKI Suspected intrinsic AKI | 18/22 (81.8%) | 29/35 (82.9%) |
| AKI stage | ||
 1 1 | 4/22 (18.2%) | 16/35 (45.7%) |
 2 2 | 7/22 (31.8%) | 8/35 (22.9%) |
 3 3 | 11/22 (50.0%) | 11/35 (31.4%) |
| AKI recovery (total) | 4/22 (18.2%) | 16/35 (45.7%) |
 Prerenal AKI recovery Prerenal AKI recovery | — | 2/2 (100%) |
 Rhabdomyolysis-induced AKI recovery Rhabdomyolysis-induced AKI recovery | 1/4 (25%) | 1/4 (25%) |
 Suspected intrinsic AKI recovery Suspected intrinsic AKI recovery | 3/18 (16.7%) | 13/29 (44.8%) |
 Stage 1 recovery Stage 1 recovery | 1/4 (25.0%) | 12/16 (75.0%) |
 Stage 2 recovery Stage 2 recovery | 3/7 (42.9%) | 4/8 (50.0%) |
 Stage 3 recovery Stage 3 recovery | 0/11 (0%) | 0/11 (0%) |
| The mean time for AKI recovery | 6 (5–8)a | 6 (5–11)a |
 Total death Total death | 19/22 (86.4%) | 20/35 (57.1%) |
  Death in prerenal AKI Death in prerenal AKI | 0/0 (0%) | 0/2 (0%) |
  Death in rhabdomyolysis-induced AKI Death in rhabdomyolysis-induced AKI | 3/4 (75.0%) | 3/4 (75.0%) |
  Death in suspected intrinsic AKI Death in suspected intrinsic AKI | 16/18 (88.9%) | 17/29 (58.6%) |
  Death in AKI stage 1 Death in AKI stage 1 | 3/4 (75.0%) | 4/16 (25.0%) |
  Death in AKI stage 2 Death in AKI stage 2 | 6/7 (85.7%) | 6/8 (75.0%) |
  Death in AKI stage 3 Death in AKI stage 3 | 10/11 (90.9%) | 10/11 (90.9%) |
Data are presented as number and percentage.
Several clinical parameters were identified as being associated with proteinuria, hematuria, and AKI in patients with COVID-19 pneumonia at the time of admission, which were shown in Supplemental Tables 2– 4. Among 198 patients, one patient with AKI had a urine albumin-creatinine ratio of 238.7 μg/mg, and his urine protein electrophoresis showed a high proportion of the renal tubular protein (40.2%).
Renal Prognosis and Risk Factors
The clinical characteristics and treatment of the 198 patients followed up on are shown in Supplemental Table 4. The mean of age was 57.1 years, 57.1% (113 of 198) patients were men, and the basic serum creatinine level was 74 μmol/L. A total of 98 (49.5%) of 198 patients presented with severe pneumonia and 37 (18.7%) of 198 had critical illness. Most patients received antibacterial therapy (84.8%, including moxifloxacin, levofloxacin, and cefoperazone-sulbactam), and many received antiviral therapy (umifenovir, 70.2%; lopinavir/ritonavir, 23.2%; ribavirin, 1.0%; remdesivir, 5.1%) and glucocorticoid therapy (71.7%, including at least one dose of dexamethasone in 5 mg or methylprednisolone in 20 mg, respectively).
The 198 patients were followed up on for a median duration of 12 days (Supplemental Table 5), during which 59.6% (118 of 198) patients with COVID-19 experienced pneumonia remission. Urine dipstick in 111 (68.5%) of 162 patients with proteinuria and in 44 (43.1%) of 102 patients with hematuria were reported as negative after follow-up (Table 3). According to KDIGO criteria, four (18.2%) of 22 patients with AKI achieved complete recovery of kidney function during the observation, including one of four patients with rhabdomyolysis-induced AKI, and three of 18 patients with suspected intrinsic AKI (Table 2). The mean time for AKI recovery was 6 days. The patients with AKI identified by the expanded criteria had higher rate of AKI recovery (45.7%, 16 of 35) and lower in-hospital mortality (57.1% versus 86.4%) than patients with AKI who met KDIGO criteria (Table 2). According to expanded criteria, patients with nonrecovered AKI presented with higher incidence of critical illness and severe pneumonia, and severe AKI (78.0% versus 25.0% in stage 2–3), compared with that in patients with recovered AKI (Table 4).
Table 3.
Change in kidney function and biomarkers among participants in the follow-up study (n=198)
| Variables | Patients before Follow-Up | Patients after Follow-Up | P Value |
|---|---|---|---|
| CRP, mg/L | 56.2 (26.9–105.5) | 3.9 (1.3–17.8) | <0.001a |
| Serum albumin, g/L | 34.4±5.0 | 33.4±5.3 | 0.10b |
| BUN, mmol/L | 4.7 (3.5–5.9) | 4.6 (3.8–6.6) | 0.07a |
| SCR, μmol/L | 74.0 (61.0–89.0) | 72.5 (60.0–85.0) | 0.29a |
| Lymphocytes, 109/L | 0.80 (0.59–1.12) | 1.36 (0.87–1.82) | <0.001a |
| Eosinophils, 109/L | 0.00 (0.00–0.06) | 0.00 (0.07–0.29) | <0.001a |
| Serum IL-10, pg/ml | 6.7 (5.0–13.1) | 5.0 (5.0–5.2) | 0.01a |
| Serum IL-6, pg/ml | 24.3 (12.6–57.6) | 4.8 (2.0–22.4) | 0.008a |
| Serum IL-2R, U/ml | 794 (552–1065) | 505 (277–720) | <0.001a |
| Proteinuria | <0.001a | ||
 None None | 21/198 (10.6%) | 132/198 (66.7%) | |
 ±/+ ±/+ | 146/198 (73.8%) | 40/198(20.2%) | |
 ++/+++ ++/+++ | 31/198 (15.6%) | 4/198 (2.0%) | |
| Hematuria | <0.001a | ||
 None None | 89/198 (44.9%) | 133/198 (67.2%) | |
 ±/+ ±/+ | 84/198 (42.4%) | 33/198 (16.7%) | |
 ++/+++ ++/+++ | 25/198 (12.6%) | 10/198 (5.1%) | |
| AKI | <0.001a | ||
 No No | 163/198 (82.3%) | 179/198 (90.4%) | |
 AKI stage 1 AKI stage 1 | 16/198 (8.1%) | 6/198 (3.0%) | |
 AKI stage 2 AKI stage 2 | 8/198 (4.0%) | 4/198 (2.0%) | |
 AKI stage 3 AKI stage 3 | 11/198 (5.6%) | 9/198 (4.5%) | |
| Resolution on lung involvement | 118/198 (59.6%) | ||
| Death | 29/198 (14.6%) | ||
| Death in critically ill group | 29/56 (51.8%) |
Data are presented as mean±SD or median (25th–75th percentiles) or as number and percentage. SCR, serum creatinine; ±/+, ± approximately 1+; ++/+++, 2+ approximately 3+.
Table 4.
Characteristics of patients in AKI nonrecovered and recovered group during follow-up periods
| Variables | AKI Nonrecovery | AKI Recovery | P Value |
|---|---|---|---|
| N | 19 | 16 | |
| Days from onset | 9 (7–14) | 7 (5–12) | 0.22a |
| Age | 64.0±8.1 | 64.5±14.7 | 0.89b |
| Sex (male) | 14/19 (73.7%) | 8/16 (50.0%) | 0.15c |
| Hypertension, % | 9/19 (47.4%) | 7/16 (43.8%) | 0.83c |
| Diabetes, % | 8/19 (42.1%) | 6/16 (37.5%) | 0.78bc |
| ACEI/ARB treatment history, % | 4/19 (21.1%) | 5/16 (31.3%) | 0.49c |
| Glucocorticoids treatment, % | 18/19 (94.7%) | 13/16 (81.3%) | 0.21c |
| Antibiotic treatment, % | 19/19 (100.0%) | 14/16 (87.5%) | 0.11c |
| Umifenovir treatment, % | 12/19 (63.2%) | 8/16 (50.0%) | 0.43c |
| Intravenous immunoglobulin therapy, % | 9/19 (47.4%) | 9/16 (56.3%) | 0.60c |
| Continuous KRT, % | 5/19 (26.3%) | 1/16 (6.3%) | 0.19d |
| Reexamined serum albumin, g/L | 25.9±5.9 | 31.3±5.9 | 0.01b |
| Reexamined lymphocytes, 109/L | 0.47 (0.32–0.85) | 0.93 (0.35–1.45) | 0.07a |
| Reexamined eosinophils, 109/L | 0.03 (0.00–0.13) | 0.04 (0.00–0.08) | 0.67a |
| SCR of baseline, μmol/L | 74.0 (61.0–89.0) | 72.5 (60.0–85.0) | 0.81a |
| COVID-19 grade | <0.001a | ||
 Moderate, % Moderate, % | 1/19 (5.3%) | 4/16 (25%) | |
 Severe, % Severe, % | 0/19 (0.0%) | 6/16 (37.5%) | |
 Critically ill, % Critically ill, % | 18/19 (94.7%) | 6/16 (37.5%) | |
| Stage of AKI | 0.001a | ||
 Stage 1 Stage 1 | 4/19 (21.1%) | 12/16 (75.0%) | |
 Stage 2 Stage 2 | 4/19 (21.1%) | 4/16 (25.0) | |
 Stage 3 Stage 3 | 11/19 (57.9%) | 0 | |
| Classification of AKI | 0.27d | ||
 Prerenal AKI Prerenal AKI | 0/19 (0) | 2/16 (12.5%) | |
 Rhabdomyolysis-induced AKI Rhabdomyolysis-induced AKI | 3/19 (15.8%) | 1/16 (6.3%) | |
 Intrinsic AKI Intrinsic AKI | 16/19 (84.2%) | 13/16 (81.3%) | |
| Remission of proteinuria | 1/7 (14.3%) | 7/10 (70.0%) | 0.05d |
| Remission of hematuria | 1/7 (14.3%) | 2/6 (33.3%) | 0.56d |
Data are presented as number and percentage, mean±SD, or median (25th–75th percentiles). SCR, serum creatinine.
Stepwise multivariate binary logistic regressions were used to select significant predictors and estimate its ORs. Specifically, it showed that, compared with patients who achieved a decrease of CRP of >10 mg/L, patients whose decrease of CRP was ≤10 mg/L had a 9.20-fold odds (95% CI, 1.62 to 52.41) of proteinuric remission (Supplemental Table 6). Moderate versus critically ill, and severe versus critically ill groups had a 4.08-fold odds (95% CI, 0.58 to 28.55) and 41.18-fold odds (95% CI, 5.36 to 316.44) of proteinuric remission, respectively. Old age (≥60 years) and ACEI/ARB treatment before admission were negative risk factors in proteinuric remission (old age: OR, 0.12 [95% CI, 0.02 to 0.70; ACEI/ARB: OR, 0.08 [95% CI, 0.01 to 0.68]) (Supplemental Table 6). Old age (≥60 years), AKI, and ACEI/ARB treatment before admission were also negative risk factors in hematuric remission (old age: OR, 0.28 [95% CI, 0.08 to 0.90]; AKI: OR, 0.12 [95% CI, 0.02 to 0.81]; ACEI/ARB: OR, 0.11 [95% CI, 0.02 to 0.73]) (Supplemental Table 7). Moderate versus critically ill, and severe versus critically ill had a 1.05-fold odds (95% CI, 0.21 to 5.39) and 9.16-fold odds (95% CI, 1.53 to 54.97) of hematuric remission, respectively (Supplemental Table 7). Critical illness was the independently negative risk factor in the recovery of AKI in the multivariate analysis (OR, 0.03; 95% CI, 0.004 to 0.32) (Supplemental Table 8).
In the other 135 patients, 82 presented with no renal involvement at admission. The remaining 53 patients were lost to follow-up because they refused to or did not recheck urine analysis during follow-up. We compared the characteristics of the 53 patients lost to follow-up and 163 patients without AKI with follow-up. It showed no significant difference existing between the two groups (Supplemental Table 9). There was also no significant difference between the un-reported patients in this cohort and the overlapped patients in a previous cohort12 (Supplemental Table 10).
Mortality
In this cohort study, 29 patients died during the observation period, out of 333 patients. All deaths occurred in patients with critical illness (Table 3). The patients with renal involvement, including hematuria, proteinuria, and AKI, had higher overall mortality (11.2%, 28 of 251) compared with that (1.2%, one of 82) of patients without renal involvement (Supplemental Table 11). No one with prerenal AKI died, whereas 75% (three of four) of patients with rhabdomyolysis-induced AKI died and 58.6% (17 of 29) of patients with suspected intrinsic AKI according to expanded AKI definition died (Table 2). The mortality was 25% (four of 16) in stage 1, 75% (six of eight) in stage 2, and 90.9% (ten of 11) in stage 3, respectively (Table 2). Among patients with proteinuric remission, no one died, whereas among patients without remission, nine (17.6%) of 51 died. There were no deaths among patients with hematuric remission; however, nine (20.3%) of 44 patients without remission died. Among patients with recovery of AKI, three (18.8%) of 16 died, whereas among patients with nonrecovery of AKI or new onset of AKI, 17 (89.5%) of 19 died (Supplemental Figure 2), suggesting that short-term outcomes of renal complications are associated with mortality in COVID-19 pneumonia.
Discussion
Like Middle Eastern respiratory syndrome coronavirus (MERS-CoV), all coronaviruses have high renal involvement. The prevalence of proteinuria in this cohort study is similar to that reported in patients with MERS-CoV,19 although a quantitative assessment of urinary protein excretion was not performed and patients with CKD were excluded. In the early phase (3 weeks after symptom onset) of MERS-CoV infection, proteinuria excretion is related to fever and systemic inflammatory status,19 which is consistent with our results that half of proteinuria cases were mitigated with the recovery of pneumonia and systemic inflammation in 3 weeks after symptom onset. Therefore, we assume that proteinuria in early phase is probably transient febrile/illness-related proteinuria.
The high frequency of renal abnormalities in this study, including 75.4% with renal involvement, 65.8% with proteinuria, and 41.7% with hematuria, were similar to findings in other patients with critical illness, in which 67.4% had proteinuria and 77.5% had hematuria,20 suggesting proteinuria and hematuria in COVID-19 is not different from patients with other critical illnesses. However, the incidence of AKI is lower.
About 7.5% (35 of 467) of the patients in the overall cohort had AKI according to the expanded criteria; and 45.7% of these patients had stage 1 AKI and 45.7% (16 of 35) achieved complete remission in 3 weeks after onset of disease. The mean time for AKI recovery was 6 days. When compared with patients with AKI who met KDIGO criteria, although the patients with AKI identified by the expanded criteria presented with more AKI on admission, they had a higher rate of AKI recovery and lower in-hospital mortality. As expanded criteria helped to identify the recovery of AKI, these data suggested that half of the early AKI in patients with COVID-19 was mild and easily recovered.
Using either KDIGO criteria or expanded criteria, suspected intrinsic AKI accounted for the most frequent form of AKI (>80%). Pathology from autopsies of patients with COVID-19 with renal function impairment demonstrated the direct evidence of intrinsic renal involvement, and revealed that the kidneys had varying degrees of ATN, luminal brush border sloughing, hyaline casts, microthrombi, and mild fibrosis in the interstitium, whereas severe glomerular injury and lymphocyte infiltration were absent,10,21 suggesting the major form of intrarenal AKI in COVID-19 is ATN, which is consistent with the high proportion of renal tubular protein in urine findings. The high rate of resolution of proteinuria, hematuria, and AKI in the early stage (3 weeks after onset of symptom) is also consistent with the postmortem data that ATN appears to be the most common cause. However, the mortality in patients with renal involvement, especially in those with no improvement of kidney function during the follow-up period (89.5%, 17 of 19), was extremely high in the early stage, suggesting that despite good short-term renal prognosis, renal complications in COVID-19 still remain associated with poor mortality.
Risk factors and causes of AKI in COVID-19 are diverse and multifactorial. On the basis of our multivariate binary regression analysis, we suggest that severity of pneumonia is the most important factor in development of AKI in patients with COVID-19. The fundamental pathophysiology of pneumonia in critically ill patients is severe acute respiratory distress syndrome, which has been identified as an independent risk factor associated with occurrence of AKI.22 These data suggest it is necessary for intensively supporting and carefully monitoring patients with severe and critically ill pneumonia to ameliorate renal complications.
This study also has several limitations. First, the number of patients included in this study is limited, and there were some missing data. Second, because of the strain on medical resources in the epicenter of the COVID-19 outbreak, we could not get full laboratory support to obtain more sufficient evidence for evaluating the urine. Third, the duration of observation is not long enough to implement survival analysis to predict the probabilities for remission of renal damage in the long term and risks for mortality. Fourth, we did not detect SARS-CoV-2 in urine samples. Therefore, we could not assess correlations between urine virus and renal complications. Fifth, we did not have renal pathology data to assess the direct effect of the virus on renal outcomes. We did not detect biomarkers, such as urinary NGAL and KIM-1, to accurately distinguish prerenal AKI from ATN. Sixth, as the admission creatinine was used to define AKI in expanded criteria, some AKI at admission that did not resolve may have not been included, which might potentially overestimate overall recovery as well as underestimate severity in some patients (those with AKI at admission that continued to worsen).
In conclusion, despite high morbidity of renal involvement, the short-term renal prognosis of patients is good, as half of them achieved remission in 3 weeks after onset of symptoms. However, adverse short-term outcomes of renal involvement are also associated with mortality in COVID-19. Severity of pneumonia was identified as an independent negative prognostic indicator for renal complications. Therefore, the strategy on treatment and prevention of severe or critically ill pneumonia is appropriate for COVID-19–related renal complications.
DisclosureS
All authors declare that they have no competing interests, no support from any organization for the submitted work, and no financial relationships with any organizations that might have an interest in the submitted work.
Funding
This work was financially supported by international (regional) cooperation and exchange projects (National Natural Science Foundation of China-Deutsche Forschungsgemeinschaft, grant 81761138041), Major Research Plan of the National Natural Science Foundation of China grant 91742204, National Natural Science Foundation of China grants 81670633, 81600556, 81770681, and 81974086, and National Key Research and Development Program grants 2016YFC0906103 and 2018YFC1314000.
Acknowledgments
The authors greatly appreciate all of the hospital staff for their efforts in recruiting and treating patients and thank all patients involved in this study.
Dr. Xu, Dr. Yao, and Dr. Zeng designed the study. Dr. Pei, Dr. Liu, Dr. Zhang, Dr. Yu, Dr. Ma, Dr. Huang, and Dr. Liu collected the data. Dr. Pei, Dr. Zhang, and Dr. Zeng prepared figures and tables. Dr. Pei and Dr. Zhang contributed analytical tools. Dr. Pei, Dr. Zhang, and Dr. Zeng wrote the paper. Dr. Zeng and Dr. Yao conceived the project and supervised and coordinated all of the work.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/10.1681/ASN.2020030276/-/DCSupplemental.
Supplemental Table 1. Hematuria and proteinuria in the non-AKI group versus the AKI group.
Supplemental Table 2. The correlation between proteinuria and variables.
Supplemental Table 3. The correlation between hematuria and variables.
Supplemental Table 4. The correlation between AKI occurrence and variables.
Supplemental Table 5. Baseline and treatment characteristics among participants in the follow-up study (N=198).
Supplemental Table 6. Risk factors associated with proteinuria remission during follow-up periods.
Supplemental Table 7. Risk factors associated with hematuria remission during follow-up periods.
Supplemental Table 8. Risk factors associated with AKI recovery during follow-up periods.
Supplemental Table 9. Characteristics of patients lost to follow-up and patients with follow-up.
Supplemental Table 10. Comparison of unreported patients in this cohort and the overlapped patients in a previous cohort.
Supplemental Table 11. Mortality of the patients with renal involvement during follow-up periods.
Supplemental Figure 1. Schematic figure.
Supplemental Figure 2. Relationship between outcomes of renal involvement and mortality during follow-up periods.
References
Articles from Journal of the American Society of Nephrology : JASN are provided here courtesy of American Society of Nephrology
Full text links
Read article at publisher's site: https://doi.org/10.1681/asn.2020030276
Read article for free, from open access legal sources, via Unpaywall:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7269350
Citations & impact
Impact metrics
Article citations
Predicting Clinical Outcomes in COVID-19 and Pneumonia Patients: A Machine Learning Approach.
Viruses, 16(10):1624, 17 Oct 2024
Cited by: 0 articles | PMID: 39459956 | PMCID: PMC11512216
Acute kidney injury in coronavirus disease: a comparative study of the two waves in Brazil.
Einstein (Sao Paulo), 22:eAO0687, 30 Sep 2024
Cited by: 0 articles | PMID: 39356942 | PMCID: PMC11461013
A scoping review on adult patients with de novo glomerular diseases following COVID-19 infection or vaccine.
Int Urol Nephrol, 03 Sep 2024
Cited by: 0 articles | PMID: 39225763
Review
COVID-19 Recovery Time and Its Predictors among Hospitalized Patients in Designated Hospitals in the Madhesh Province of Nepal: A Multicentric Study.
Healthcare (Basel), 12(17):1691, 24 Aug 2024
Cited by: 0 articles | PMID: 39273716 | PMCID: PMC11395077
Construction and validation of a risk model of proteinuria in patients with omicron COVID-19: retrospective cohort study.
Ren Fail, 46(2):2365979, 06 Aug 2024
Cited by: 0 articles | PMID: 39108141 | PMCID: PMC11308959
Go to all (423) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Prevalence of Kidney Injury and Associations with Critical Illness and Death in Patients with COVID-19.
Clin J Am Soc Nephrol, 15(11):1549-1556, 17 Sep 2020
Cited by: 31 articles | PMID: 32943396 | PMCID: PMC7646240
Renal changes and acute kidney injury in covid-19: a systematic review.
Rev Assoc Med Bras (1992), 66Suppl 2(suppl 2):112-117, 21 Sep 2020
Cited by: 19 articles | PMID: 32965368
Review
Kidney disease is associated with in-hospital death of patients with COVID-19.
Kidney Int, 97(5):829-838, 20 Mar 2020
Cited by: 1539 articles | PMID: 32247631 | PMCID: PMC7110296
Acute kidney injury in critically ill patients with COVID-19.
Intensive Care Med, 46(7):1339-1348, 12 Jun 2020
Cited by: 278 articles | PMID: 32533197 | PMCID: PMC7290076
Review Free full text in Europe PMC
Funding
Funders who supported this work.
Major Research Plan of the National Natural Science Foundation of China (1)
Grant ID: 91742204
National Key Research and Development Program (2)
Grant ID: 2016YFC0906103
Grant ID: 2018YFC1314000
National Natural Science Foundation of China (4)
Grant ID: 81770681
Grant ID: 81670633
Grant ID: 81600556
Grant ID: 81974086
National Natural Science Foundation of China-Deutsche Forschungsgemeinschaft (1)
Grant ID: 81761138041
 1
1