Abstract
Free full text

Coronavirus disease 2019 (COVID-19) pandemic and pregnancy
Abstract
The current coronavirus disease 2019 (COVID-19) pneumonia pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is spreading globally at an accelerated rate, with a basic reproduction number (R0) of 2–2.5, indicating that 2–3 persons will be infected from an index patient. A serious public health emergency, it is particularly deadly in vulnerable populations and communities in which healthcare providers are insufficiently prepared to manage the infection. As of March 16, 2020, there are more than 180,000 confirmed cases of COVID-19 worldwide, with more than 7000 related deaths. The SARS-CoV-2 virus has been isolated from asymptomatic individuals, and affected patients continue to be infectious 2 weeks after cessation of symptoms. The substantial morbidity and socioeconomic impact have necessitated drastic measures across all continents, including nationwide lockdowns and border closures.
Pregnant women and their fetuses represent a high-risk population during infectious disease outbreaks. To date, the outcomes of 55 pregnant women infected with COVID-19 and 46 neonates have been reported in the literature, with no definite evidence of vertical transmission. Physiological and mechanical changes in pregnancy increase susceptibility to infections in general, particularly when the cardiorespiratory system is affected, and encourage rapid progression to respiratory failure in the gravida. Furthermore, the pregnancy bias toward T-helper 2 (Th2) system dominance, which protects the fetus, leaves the mother vulnerable to viral infections, which are more effectively contained by the Th1 system. These unique challenges mandate an integrated approach to pregnancies affected by SARS-CoV-2.
Here we present a review of COVID-19 in pregnancy, bringing together the various factors integral to the understanding of pathophysiology and susceptibility, diagnostic challenges with real-time reverse transcription polymerase chain reaction (RT-PCR) assays, therapeutic controversies, intrauterine transmission, and maternal−fetal complications. We discuss the latest options in antiviral therapy and vaccine development, including the novel use of chloroquine in the management of COVID-19. Fetal surveillance, in view of the predisposition to growth restriction and special considerations during labor and delivery, is addressed. In addition, we focus on keeping frontline obstetric care providers safe while continuing to provide essential services. Our clinical service model is built around the principles of workplace segregation, responsible social distancing, containment of cross-infection to healthcare providers, judicious use of personal protective equipment, and telemedicine. Our aim is to share a framework that can be adopted by tertiary maternity units managing pregnant women in the flux of a pandemic while maintaining the safety of the patient and healthcare provider at its core.
Introduction
A critical component in the management of any communicable disease threat is the care of vulnerable populations. Pregnant women are known to be disproportionately affected by respiratory illnesses, which are associated with increased infectious morbidity and high maternal mortality rates. Although most human coronavirus infections are mild, the severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) epidemics of the past two decades have been especially grave, with approximately one-third of infected pregnant women dying from the illness.1 , 2
The current pneumonia outbreak of coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was declared a pandemic3 by the World Health Organization (WHO) on March 11, 2020, and is predicted to peak around April 2020, without a significant reduction in transmissibility.4 With its indiscriminate and sustained spread across continents, we are likely to see women with COVID-19 canvassed across all trimesters of pregnancy. In this article, we summarize the clinical features of pregnant women with COVID-19, and present a pragmatic and integrated framework that addresses the obstetric complexities of managing this disease in pregnancy.
Clinically Relevant Virology
SARS-CoV-2, a novel enveloped RNA betacoronavirus, infects host respiratory epithelial cells through angiotensin-converting enzyme 2 (ACE2), a membrane-bound aminopeptidase that functions as its putative receptor. Although the expression of ACE2 is predominantly within type II alveolar cells of the lung, the receptor is also present in several extrapulmonary sites across the aerodigestive tract, including the mucosa of the oral cavity.5 Patients with COVID-19 therefore manifest a spectrum of upper and lower respiratory tract symptoms. Sexual diamorphism has been suggested, but not proved; cellular studies reveal that the expression of ACE2 is attenuated in females,6 in keeping with the epidemiological observation that the majority of COVID-19 infections to date have occurred in men.7
Physiological Susceptibility to COVID-19
Cardiorespiratory system
Approximately 80% of infections in COVID-19 are mild or asymptomatic; 15% are severe, requiring supplemental oxygen; and 5% are critical, requiring mechanical ventilation.8 Changes to the cardiorespiratory and immune systems in pregnancy increase a woman’s susceptibility to severe infection and hypoxic compromise, but may also delay diagnosis and source control in those with only innocuous upper respiratory tract symptoms such as sore throat and nasal congestion; the latter are seen in 5% of patients with COVID-19.7 Gestational rhinitis, due to estrogen-mediated hyperemia of the nasopharynx, usually affects one-fifth of healthy women in late pregnancy and results in marked nasal congestion and rhinorrhea; these features may mask the coryzal symptoms of COVID-19, leading to unchecked viral shedding and community transmission.
Shortness of breath occurs in 18% of patients with COVID-19.7 However, physiologic dyspnea due to increased maternal oxygen demands from heightened metabolism, gestational anemia, and fetal oxygen consumption is common in pregnancy9 and must be distinguished from pathologic breathlessness. In addition, pulmonary volumes are altered: functional residual capacity, end-expiratory volumes, and residual volumes decrease steadily from early pregnancy due to diaphragmatic splinting by the gravid uterus, resulting in reduced total lung capacity at term and an inability to clear pulmonary secretions effectively.10 This is pertinent, as COVID-19 pneumonia rapidly progresses from focal to diffuse bilateral consolidation of lung parenchyma,11 which, in the context of the pulmonary changes described above, would more readily predispose to hypoxemic respiratory failure in pregnancy.
Immune system
Cytokines produced by T-helper (Th) lymphocytes regulate immunity and inflammation. Th1-type cytokines12 are microbicidal and proinflammatory and chiefly include interferon-γ (IFN-γ), interleukin (IL)−1α, IL-1β, IL-6, and IL-12. In contrast, Th2-type cytokines12 are anti-inflammatory and comprise IL-4, IL-10, IL-13, and transforming growth factor−β (TGF- β). In pregnancy, the attenuation in cell-mediated immunity by Th1 cells due to the physiological shift to a Th2 dominant environment9 contributes to overall infectious morbidity by increasing maternal susceptibility to intracellular pathogens such as viruses.
Interestingly, the cytokine profiles in SARS-CoV and SARS-CoV-2 infections in nonpregnant patients may be extrapolated to account for the differences in disease severity in affected pregnancies. Patients with SARS showed preferential activation of Th1 immunity, resulting in the marked elevation of proinflammatory cytokines (IFNγ, IL-1β, IL-6, and IL-12) for at least 2 weeks after disease onset, leading to extensive lung damage.13 In contrast, patients with COVID-19 demonstrated activation of both Th1 and Th2 immunity over similar periods in the disease course, culminating in the presence of IFN-γ and IL-1β in addition to IL-4 and IL-10.14 In addition, elevated levels of IL-6 (a predominantly Th1 response) are associated with a significantly increased risk of mortality in COVID-19 patients.15
Murine studies of influenza have demonstrated that pregnancy increases influenza-related pathology via disrupted viral clearance, increased pulmonary IL-6, IL-1α, and Granulocyte-colony stimulating factor (G-CSF) expression and enhanced physiological stress in the lungs, influenced by changes in prostaglandin and progesterone levels.16 However in COVID-19, a range of immune responses has been described, and early adaptive immune responses may be predictive of milder disease severity.17 We postulate that changes in the hormonal milieu in pregnancy, which influence immunological responses to viral pathogens16 together with the physiological transition to a Th2 environment favoring the expression of anti-inflammatory cytokines (IL-4 and IL-10) and other unidentified immune adaptations, may serve as the predominant immune response to SARS-CoV-2, resulting in the lesser severity of COVID-19 compared to that in nonpregnant individuals.18 These immune responses should be further characterized in gravidas and nongravidas with COVID-19 of different disease severities.
Clinical Features
Similar to nonpregnant patients, the predominant features of COVID-19 in pregnant patients are fever, cough, dyspnea, and lymphopenia (Table 1 ).
Table 1
Features of COVID-19 in pregnancy stratified against SARS and MERS
| Characteristics | COVID-19 | SARS | MERS |
|---|---|---|---|
| No. of cases | 55 | 17 | 12 |
| Age (y) | 23−40 | 27−44 | 31−39 |
| Gestational age at infection (wk) | All were in the third trimester except 2 women who were <28 wk gestation | 4-32 | 4-38 |
| Respiratory comorbidities (n) | None | Asthma (1) | Asthma (1), pulmonary fibrosis (1) |
| Symptoms | |||
| Fever (%) | 84b | 100 | 58 |
| Cough (%) | 28b | 76 | 67 |
| Dyspnea (%) | 18b | 35 | 58 |
| Investigationsa | |||
| CXR/CT evidence of pneumonia | 76b | 100b | 100b |
| Leukocytosis (%) | 38b | 40b | 50b |
| Lymphopenia (%) | 22b | 67b | 50b |
| Thrombocytopenia (%) | 13b | 36b | 50b |
| Maternal complications | |||
| Mortality (%) | 0 | 18 | 25 |
| Mechanical ventilation (%) | 2 | 35 | 41 |
| Fetal complications | |||
| Miscarriage/stillbirth (%) | 2 | 25c | 18b |
| IUGR (%) | 9 | 13c | 9b |
| Preterm birth (%) | 43 | 25c | 27b |
| Neonatal complications | |||
| Neonatal death (%) | 2 | 0c | 9b |
Data shown in the table are pooled from references18,35, 36, 37, 38, 39,75, 76, 77 (COVID-19);1,78, 79, 80, 81, 82(SARS);2,27,29,83, 84, 85, 86, 87 (MERS). COVID-19, coronavirus disease 2019; CT, computed tomography; CXR, chest X-ray; IUGR, intrauterine growth restriction; MERS, Middle East respiratory syndrome; SARS, severe acute respiratory syndrome.
Dashraath. COVID-19 pandemic and pregnancy. Am J Obstet Gynecol 2020.
Diagnosis and Imaging
A real-time reverse transcription polymerase chain reaction (RT-PCR) assay is the current gold standard for detecting SARS-CoV-2 from respiratory specimens in patients with suspected COVID-19. At present, it is available in 84 public health laboratories in the United States; these provide in-state testing capacity in all 50 states and the District of Columbia. The test uses specific primers and probes that target the RNA-dependent RNA polymerase (RdRp), envelope, and nucleocapsid genes of SARS-CoV-2, among which the RdRp assay has the highest analytical sensitivity (3.8 RNA copies/reaction at 95% detection probability).19 As RT-PCR is a quantitative method in which the amplification of DNA is detected in real time, the determination of viral load in COVID-19 is theoretically possible. However, this usually requires laboratories to develop in-house test kits and to validate them with internal controls.20
In contrast, most commercially available assays for COVID-19 provide qualitative results, and false-negative results may be due to a low viral load. The practical limitations of RT-PCR testing include the need for a biosafety level-2 (BSL-2) facility, a requirement for kits with specific reagents and primers, the need to maintain a cold chain (as the specimens require storage at 2–8oC), and the use of strict, validated protocols for testing; consequently, countries with resource limitations or acute spikes in the numbers of suspected cases may not be able to meet these demands. However, there are no good alternatives: antigen−antibody detection tests are not validated, and viral culture is impractical, as it takes at least 3 days for SARS-CoV-2 to cause cytopathic effects in selected cell lines (VeroE6 and Huh7 cells).21 In addition, viral culture will require a BSL-3 facility, which are usually found only in tertiary medical or university research centers.
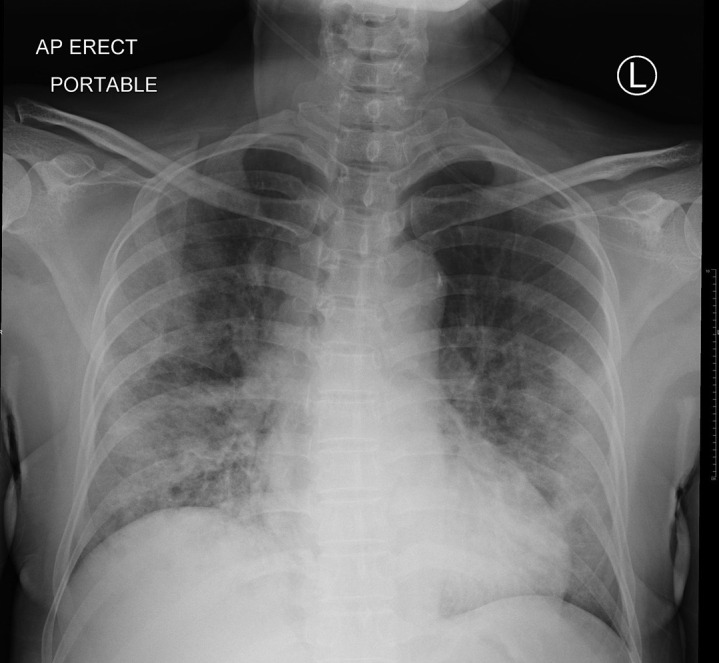
Chest imaging may aid, but not replace, molecular confirmation of COVID-19. The predominant findings are peripheral airspace shadowing on a plain chest radiograph (Figure 1 ) and bilateral, multi-lobar ground-glass opacities or consolidation on a computed tomography (CT) scan of the chest11 , 22; these features are nonspecific and appear to be similar in pregnancy.18 Using RT-PCR as a reference, the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of a CT chest in diagnosing COVID-19 are 97%, 25%, 65%, and 83%, respectively.23 However, when CT scans are performed in pregnancy, concerns regarding the teratogenic effects of ionizing radiation on the fetus are inevitable. It is reassuring that the fetal radiation dose for a routine CT chest is 0.03 mGy, and exposure to radiation doses of <50 mGy is not associated with an increased risk of fetal anomalies or pregnancy loss.24 Although intravenous iodinated contrast medium crosses the placenta, studies have not demonstrated teratogenicity or thyroid dysfunction in the newborn.25
Complications in Pregnancy
The outcomes of coronavirus infections in pregnancy are summarized in Table 1. Hitherto, COVID-19 outcomes for the mother appear more promising compared to those of SARS and MERS. Pooled data reveal a case fatality rate of 0%, 18%, and 25% for COVID-19, SARS, and MERS, respectively; in the latter 2 disease syndromes, progressive respiratory failure and severe sepsis were the most frequent causes.26 , 27 This is not surprising, given the predisposition to superimposed bacterial infections due to direct mucosal injury, dysregulation of immune responses, and alterations to the respiratory microbiome after viral pneumonia.28 Postnatal maternal deterioration can still occur,29 necessitating continued monitoring.
Fetal complications of COVID-19 include miscarriage (2%), intrauterine growth restriction (IUGR; 10%), and preterm birth (39%). Fever, with a median temperature of 38.1− 39.0oC, is the prevailing symptom7 in COVID-19. Cohort studies in patients with other infections have not shown increased risks of congenital anomalies from maternal pyrexia in the first trimester,30 although childhood inattention disorders are more common, possibly related to hyperthermic injury to fetal neurons.31
Vertical Transmission
There is a theoretical risk of vertical transmission, similar to that seen in SARS, as the ACE2 receptor is widely expressed in the placenta,32 with a similar receptor-binding domain structure between SARS-CoV and SARS-CoV-2. Most recently, 2 neonates from COVID-19−infected mothers are said to have tested positive for SARS-CoV-2 shortly following delivery, casting concerns about the possibility of vertical transmission.33 , 34 However, there have been no confirmed instances of vertical transmission among the 46 other neonates18 , 35, 36, 37, 38, 39, 40 born to COVID-19−infected mothers reported thus far, supported in turn by evidence demonstrating an absence of viral isolates in the amniotic fluid, cord blood, breast milk, and neonatal throat swabs in a subset of these patients.18 It is notable, however, that the overwhelming majority of these women acquired COVID-19 in the third trimester; there are currently no data on perinatal outcomes when the infection is acquired in early pregnancy. Regardless of the risk, it is reassuring that COVID-19 appears to manifest as a mild respiratory disease in the pediatric population.41 , 42
Treatment
Current approach
Symptomatic treatment and pregnancy-specific management of complications such as sepsis and acute respiratory distress syndrome (ARDS) comprise the current standards of care. A high Sequential Organ Failure Assessment (SOFA) score and D-dimer levels of >1 μg/mL on admission predict increased mortality in nonpregnant patients with COVID-19.43 However, D-dimer levels are difficult to interpret, as the values are usually raised in pregnancy, such that only 84%, 33%, and 1% of women in the first, second, and third trimesters, respectively would have normal results based on conventional thresholds.44 The SOFA score should also be adjusted to reflect the influence of pregnancy on hemodynamics and renal blood flow, such as by using a creatinine level of >1.02 mg/dL (instead of >1.20 mg/dL) to signify renal dysfunction.45 In addition, mechanical ventilation requires achieving higher maternal oxygen (target PaO2 >70 mmHg instead of 55–80 mm Hg) and lower carbon dioxide levels (target PaCO2 28–32 mmHg)46 to maintain placental perfusion and prevent fetal hypoxemia and acidosis.
We concur with the WHO recommendation against the routine use of systemic corticosteroids, as it appears to delay viral clearance with no survival benefit.47 Although neither hydrocortisone nor methylprednisolone readily crosses the placenta, prolonged exposure predisposes to maternal hyperglycemia; this is immunosuppressive and sustains the replication of respiratory viruses within pulmonary epithelial cells.48 However, in cases of expedited preterm delivery for obstetric or medical indications, the decision to use corticosteroids to accelerate fetal maturity and to minimize peripartum complications should be individualized. Good obstetric practice should prevail, and urgent delivery should not be delayed.
Options for antiviral therapy
The Monitored Emergency Use of Unregistered Interventions (MEURI) framework from the WHO should guide the ethical use of nonlicensed drugs in pregnancy during pandemics. Recent studies have identified remdesivir and chloroquine49 as strong candidate drugs for the treatment of COVID-19. Remdesivir is a novel, broad-acting antiviral nucleotide prodrug that effectively inhibits replication of SARS-CoV-2 in vitro and that of related coronaviruses including MERS-CoV in nonhuman primates.50 Its use appears to be safe in human pregnancies,51 and phase 3 trials evaluating efficacy in COVID-19 are currently underway in the United States (ClinicalTrials.gov number NCT04280705) and China (ClinicalTrials.gov number NCT04252664 and NCT04257656).
Chloroquine phosphate is a ubiquitous antimalarial quinolone compound with broad spectrum antiviral and immunomodulating activity. It has been shown to block coronavirus infection by increasing the endosomal pH required for cell fusion and by interrupting the glycosylation of cellular receptors of SARS-CoV in cell culture.49 Unpublished data from multicenter clinical trials across China52 have demonstrated that the drug appears to be effective in accelerating the clinical, radiological, and serological resolution of COVID-19. Although chloroquine and its metabolites cross the placenta, it may be safely used in all trimesters of pregnancy, with no increased risk of adverse perinatal outcomes. However, it is worth noting that chloroquine is a drug with a large volume of distribution, and pharmacokinetic studies53 have shown significantly lower plasma drug concentrations in pregnancy, which suggests the need for a higher dose in COVID-19 (at least 500 mg twice daily).52 A relevant side effect of high-dose chloroquine, however, is systolic hypotension, which may exacerbate the hemodynamic changes from supine aortocaval compression by a gravid uterus.
In addition, as all betacoronaviruses including SARS-CoV, SARS-CoV-2 and MERS-CoV contain 2 cysteine proteases that process the viral polypeptides necessary for their replication,54 , 55 viral protease inhibitors such as lopinavir-ritonavir (LPV/r) have shown some benefit in the adjunct management of COVID-19.56 Although not studied specifically in pregnant women with respiratory infections, LPV/r is known to be safe: an analysis of population-based surveillance data of LPV/r exposure in HIV-positive pregnancies found no increase in the risk of fetal anomalies, preterm birth, or low-birthweight infants.57
Conversely, ribavirin, an antiviral guanosine analogue commonly used in coronavirus treatment cocktails,1 , 29 is teratogenic: it induces miscarriage as well as craniofacial and limb defects in the embryos of pregnant mice exposed to doses >25 mg/kg,58 and should be avoided, especially in early pregnancy. Similarly, baricitinib, a Janus kinase inhibitor, has been identified through machine learning59 as a potential drug for the treatment of COVID-19 by inhibiting the endocytosis of SARS-CoV-2 into pulmonary cells. However, we opine that baricitinib is contraindicated in pregnancy, as animal studies have demonstrated embryotoxicity.60
Currently, there no approved vaccines for the prevention of COVID-19, although several are under development but will not be available for some time. An open-label, phase 1 clinical trial in nonpregnant women and men evaluating a candidate vaccine, mRNA-1273, led by the U.S. National Institutes of Health (NIH), commenced recruitment on March 16, 2020 (ClinicalTrials.gov number NCT 04283461). The safety and immunogenicity of this lipid nanoparticle (LNP)−encapsulated mRNA-based vaccine in pregnancy is, at present, unknown.
Obstetric Management
Antenatal care
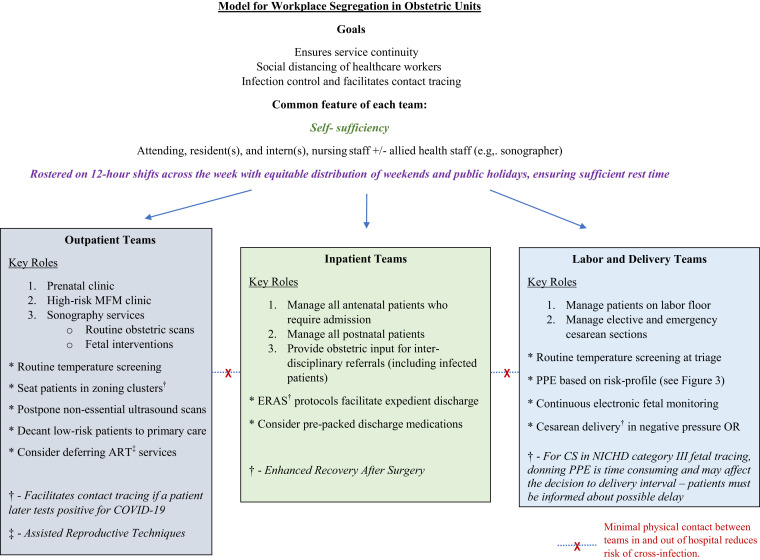
In a pandemic, social distancing measures have proven to be effective in reducing disease transmission.61 Obstetric care can be served by this model, as our own experience attests, by streamlining medical care providers into self-sufficient groups, each minimally comprising the attending, resident, intern, and nursing or midwifery staff (Figure 2 ). The individual teams function independently and provide inpatient labor and delivery services, outpatient antenatal care, or surgical services, including treating women with suspected or confirmed COVID-19 infection with full personal protective equipment (PPE) compliance. If a team member is exposed to or infected with COVID-19, that individual’s team will be quarantined for at least 2 weeks; workforce segregation thus ensures adequate clinical coverage by nonaffected teams in this event. Although inter-hospital movement of doctors and patients is restricted, approved urgent inter-hospital transfer of prenatal patients to tertiary maternity units takes place with full adherence to infection control measures, including isolation when necessary. Ambulatory clinical care is increasingly conducted on Health Insurance Portability and Accountability Act (HIPAA)−compliant telemedicine video conferencing platforms (Zoom Video Communications Inc, San Jose, CA), which allow joint management decisions to be made with primary care providers in real time.
Fetal surveillance
Protracted respiratory compromise increases the risk of fetal growth restriction due to maternal hypoxia, which drives the release of potent vasoconstrictors such as endothelin-1 and hypoxia-inducible factor, resulting in placental hypoperfusion and reduced oxygen delivery to the fetus.62 Given that intrauterine growth restriction (IUGR) complicates approximately 10% of pregnancies with COVID-19 (Table 1), we would monitor the fetus with at least 1 ultrasound assessment of growth following maternal recovery. After sonographic evaluation in high-risk patients, the ultrasound transducers should be disinfected according to the manufacturer’s recommendations.63
Labor, delivery, and breastfeeding
Women who arrive at the labor ward must be stratified, based on local case definitions, into low, moderate, or high risk for COVID-19 infection, to determine the disposition of the patient and type of infection control precautions required of the healthcare staff (Figure 3 ).
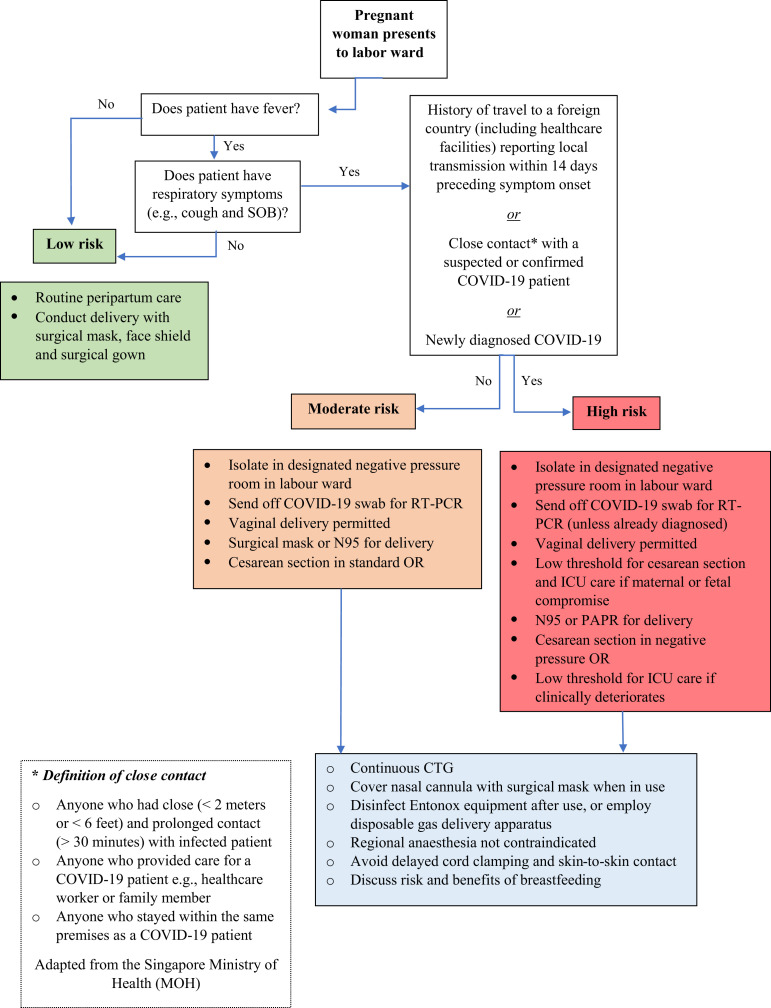
Labor ward triage
Schematic representation demonstrating a model for stratifying risk in obstetric patients presenting to the labor floor.
Dashraath. COVID-19 pandemic and pregnancy. Am J Obstet Gynecol 2020.
The mode of delivery is directed by obstetric factors and clinical urgency. As there is no convincing evidence of vertical transmission,18 vaginal delivery is not contraindicated in patients with COVID-19. When emergent delivery is required in a critically ill parturient, a cesarean delivery is most appropriate; these indications include rapid maternal deterioration, difficulty with mechanical ventilation due to the gravid uterus, and fetal compromise. Delivery, including cesarean delivery, should be carried out with respiratory precautions using full personal protective equipment (PPE) and in rooms with negative pressure ventilation.64
Patient self-administered inhalation of nitrous oxide and oxygen (Entonox) is a widely used labor analgesic. However, respiratory viruses contaminating the gas delivery apparatus may be a neglected source of cross-infection, and birth attendants should be aware of decontamination guidelines, which include the cleaning of the expiratory valve between patients, and the use of a microbiological filter (pore size <0.05μm) between the mouthpiece or facemask.65 Similarly, in a woman with suspected or confirmed COVID-19 requiring supplemental oxygen in labor, a surgical mask should be worn over the nasal cannula, as humidifying oxygen results in the aerosolization (or spray) of infectious particles to a radius of about 0.4 meters, with a resultant risk of nosocomial droplet infection.66 , 67
Although the data do not suggest a risk of vertical transmission, delayed clamping of the umbilical cord and skin-to-skin contact should be avoided following delivery, extrapolating from recommendations by the Canadian Society of Obstetricians and Gynecologists guidelines for SARS in pregnancy.64
Breastfeeding is not contraindicated, based on current published guidelines68 , 69; a retrospective analysis of COVID-19 in pregnancy showed that none of the women had detectable viral loads of SARS-CoV-2 in breastmilk.18 Regardless, if the patient chooses to breastfeed, she should wear a face mask because of the close proximity between mother and child, to reduce the risk of droplet transmission. The presence of coronavirus antibodies in breastmilk depends on the gestation at which maternal infection occurred and if there was any preceding use of high-dose corticosteroids which could suppress maternal antibody responses.70
Personal Protective Equipment
The safety of healthcare providers is of the utmost importance in any pandemic, and the type of personal protective equipment (PPE) necessary depends on the degree of perceived risk (Table 2 ). Surgical face masks are appropriate for general clinical duties, as randomized trial data have shown them to be as effective as N95 respirators in preventing droplet transmission in influenza.71
Table 2
Personal protective equipment (PPE)a for healthcare workers caring for a patient with COVID-19 in pregnancy
| Risk | Examples of clinical encounters in obstetrics | Recommended PPEa for staff attending to patient with COVID-19 |
|---|---|---|
| Low risk |
|
|
| Moderate risk |
|
|
| High risk |
|
|
COVID-19, coronavirus disease 2019; HEPA, high-efficiency particulate air; PAPR, powered air-purifying respirator.
Dashraath. COVID-19 pandemic and pregnancy. Am J Obstet Gynecol 2020.
N95 respirators in pregnancy
The use of N95 respirators (also known as FFP2 masks) is recommended by the CDC for healthcare providers with high-risk exposure to patients with suspected or proven COVID-19.72 However, these filtering facepiece respirators are associated with resistance to airflow and increased static dead space volumes, which may affect maternal cardiorespiratory function and fetal oxygenation when worn for prolonged periods.
Controlled clinical studies73 , 74 of nurses wearing N95 respirators during an hour of physical activity in their second and third trimesters of pregnancy demonstrated reduced tidal volume (23%) and minute ventilation (26%), resulting in lower oxygen uptake (14%) and increased carbon dioxide production (9%) due to labored breathing. Although there were no changes in fetal heart rate, maternal capillary lactate levels, or oxygen saturations, we caution against the use of N95 respirators in pregnant healthcare workers with growth-restricted fetuses, and recommend that they be exempted from frontline duty during the COVID-19 outbreak. Powered air-purifying respirators (PAPR) with high-efficiency particulate air (HEPA) filters, with less airway resistance, are a reasonable alternative.
Conclusion
Pregnant women represent a uniquely vulnerable group in any infectious disease outbreak because of their altered physiology, susceptibility to infections, and compromised mechanical and immunological functions. The need to safeguard the fetus adds to the challenge of managing their health. Special precautions are required to minimize cross-infection of healthcare providers while performing procedures that require close physical contact and promote droplet exposure, such as vaginal delivery. Much of the obstetric management is based on consensus and best practice recommendations, as clinical efficacy data regarding antiviral therapy and corticosteroid use is evolving. This narrative represents an integrated framework to provide an appropriate level of care for these patients and hospital staff during the COVID-19 pandemic.
Acknowledgments
We thank See Kay Choong, MBBS, MPH, FRCP, FCCP, from the Division of Respiratory and Critical Care Medicine, National University Hospital, Singapore, and Shaun Tan Shi Yan, MBChB, MS, from the Department of Laboratory Medicine, National University Hospital, Singapore, for their input during the preparation of this manuscript. This work did not receive any sources of funding or grants.
Useful resources
U.S. CDC COVID-19 Resource Page: https://www.cdc.gov/coronavirus/2019-ncov/index.html
JAMA COVID-19 Resource Page: https://jamanetwork.com/journals/jama/pages/coronavirus-alert
Report of WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19): https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf
Practical Advice for Healthcare Workers: COVID-19 and Pregnancy – Gianluigi Pilu, MD, University of Bologna: https://m.facebook.com/watch/?v=1118006391865743&_rdr
How to use PPE: https://www.cdc.gov/hai/pdfs/ppe/PPEslides6-29-04.pdf
Footnotes
The authors report no conflict of interest.
P.D. and J.L.J.W. contributed substantially and equally. All authors were involved in the writing and revision of the manuscript. All authors read and approved the final version.
References
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.ajog.2020.03.021
Read article for free, from open access legal sources, via Unpaywall:
http://www.ajog.org/article/S0002937820303434/pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1016/j.ajog.2020.03.021
Article citations
Mapping vulnerability for increased COVID-19 susceptibility and worse outcomes: a scoping review.
Front Public Health, 12:1432370, 10 Oct 2024
Cited by: 0 articles | PMID: 39450391 | PMCID: PMC11499102
Review Free full text in Europe PMC
Maternal and Fetal Outcome of COVID-19 Infection among Pregnant Women.
Medicina (Kaunas), 60(10):1676, 12 Oct 2024
Cited by: 0 articles | PMID: 39459463 | PMCID: PMC11509149
Analysis of Fetal Growth and Pregnancy Outcome in Pregnant Women Infected with Novel Coronavirus in Mid-Pregnancy.
Int J Womens Health, 16:1625-1631, 01 Oct 2024
Cited by: 0 articles | PMID: 39372668 | PMCID: PMC11453137
T-cell responses to ancestral SARS-CoV-2 and Omicron variant among unvaccinated pregnant and postpartum women living with and without HIV in South Africa.
Sci Rep, 14(1):20348, 02 Sep 2024
Cited by: 0 articles | PMID: 39223211 | PMCID: PMC11369237
Prevalence of Thrombocytopenia in Pregnant Women with COVID-19: A Systematic Review and Meta-Analysis.
J Clin Med, 13(17):4980, 23 Aug 2024
Cited by: 0 articles | PMID: 39274209 | PMCID: PMC11396774
Review Free full text in Europe PMC
Go to all (517) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials (3)
- (1 citation) ClinicalTrials.gov - NCT04280705
- (1 citation) ClinicalTrials.gov - NCT04252664
- (1 citation) ClinicalTrials.gov - NCT04257656
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The effect of coronavirus infection (SARS-CoV-2, MERS-CoV, and SARS-CoV) during pregnancy and the possibility of vertical maternal-fetal transmission: a systematic review and meta-analysis.
Eur J Med Res, 25(1):39, 04 Sep 2020
Cited by: 89 articles | PMID: 32887660 | PMCID: PMC7471638
Review Free full text in Europe PMC
Care of the pregnant woman with coronavirus disease 2019 in labor and delivery: anesthesia, emergency cesarean delivery, differential diagnosis in the acutely ill parturient, care of the newborn, and protection of the healthcare personnel.
Am J Obstet Gynecol, 223(1):66-74.e3, 10 Apr 2020
Cited by: 47 articles | PMID: 32283073 | PMCID: PMC7151436
Effect of coronavirus disease 2019 (COVID-19) on maternal, perinatal and neonatal outcome: systematic review.
Ultrasound Obstet Gynecol, 56(1):15-27, 01 Jul 2020
Cited by: 288 articles | PMID: 32430957 | PMCID: PMC7276742
Review Free full text in Europe PMC
An Analysis of 38 Pregnant Women With COVID-19, Their Newborn Infants, and Maternal-Fetal Transmission of SARS-CoV-2: Maternal Coronavirus Infections and Pregnancy Outcomes.
Arch Pathol Lab Med, 144(7):799-805, 01 Jul 2020
Cited by: 408 articles | PMID: 32180426
Review