Abstract
Free full text
Guillain–Barré syndrome spectrum associated with COVID-19: an up-to-date systematic review of 73 cases
Abstract
Since coronavirus disease-2019 (COVID-19) outbreak in January 2020, several pieces of evidence suggested an association between the spectrum of Guillain–Barré syndrome (GBS) and severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). Most findings were reported in the form of case reports or case series, whereas a comprehensive overview is still lacking. We conducted a systematic review and searched for all published cases until July 20th 2020. We included 73 patients reported in 52 publications. A broad age range was affected (mean 55, min 11–max 94 years) with male predominance (68.5%). Most patients showed respiratory and/or systemic symptoms, and developed GBS manifestations after COVID-19. However, asymptomatic cases for COVID-19 were also described. The distributions of clinical variants and electrophysiological subtypes resemble those of classic GBS, with a higher prevalence of the classic sensorimotor form and the acute inflammatory demyelinating polyneuropathy, although rare variants like Miller Fisher syndrome were also reported. Cerebrospinal fluid (CSF) albuminocytological dissociation was present in around 71% cases, and CSF SARS-CoV-2 RNA was absent in all tested cases. More than 70% of patients showed a good prognosis, mostly after treatment with intravenous immunoglobulin. Patients with less favorable outcome were associated with a significantly older age in accordance with previous findings regarding both classic GBS and COVID-19. COVID-19-associated GBS seems to share most features of classic post-infectious GBS and possibly the same immune-mediated pathogenetic mechanisms. Nevertheless, more extensive epidemiological studies are needed to clarify these issues.
Introduction
Coronavirus disease 2019 (COVID-19) pandemic has rapidly spread around the world from Jan-2020, with more than 14,000,000 cases confirmed so far [1]. Although primary affecting the respiratory system, central and peripheral neurological manifestations associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection have been increasingly reported [2–4]. In detail, several pieces of evidence suggested an association between SARS-CoV-2 infection and the development of Guillain–Barré Syndrome (GBS) [5–56].
GBS represents the most common cause of acute flaccid paralysis [57]. The classic form is an immune-mediated acute-onset demyelinating polyradiculoneuropathy (acute inflammatory demyelinating polyneuropathy—AIDP) typically presenting with ascending weakness, loss of deep tendon reflexes, and sensory deficits. Diagnosis of GBS relies on the results of clinical, electrophysiological, and cerebrospinal fluid (CSF) examinations (classically albuminocytological dissociation) [57–59]. The clinical spectrum of GBS encompasses a classic sensorimotor form, Miller Fisher syndrome (MFS), bilateral facial palsy with paraesthesia, pure motor, pure sensory, paraparetic, pharyngeal–cervical–brachial variants, polyneuritis cranialis (GBS–MFS overlap), and Bickerstaff brainstem encephalitis [57–60]. As regard electrophysiological features, three main subtypes are recognized: AIDP, acute motor axonal neuropathy (AMAN), and acute motor sensory axonal neuropathy (AMSAN) [57, 58, 61]. Peripheral nerve damage is thought to be provoked by an aberrant immune response to infections, in some cases driven by the production of autoreactive antibodies (anti-ganglioside antibodies) [57–59]. Potential triggering pathogens include both viruses [e.g., cytomegalovirus (CMV), Epstein–Barr virus (EBV), influenza virus, hepatitis E virus, and Zika virus] and bacteria (e.g., Campylobacter Jejuni, Mycoplasma Pneumoniae) [57, 58, 62]. However, a relationship with other events has been also described (e.g., vaccinations, surgery, administration of checkpoint inhibitors, and malignancy) [57, 58]. Given that a potential causal association with beta-coronaviruses [Middle East Respiratory Syndrome (MERS-CoV)] has already been speculated, the relationship between COVID-19 and GBS deserves undoubtedly further attention [63, 64].
With this background, our systematic review aimed to provide a comprehensive and updated overview of all case reports and series of COVID-19-related GBS to identify predominant clinical, laboratory, and neurophysiological patterns and to discuss the possible underlying pathophysiology.
Methods
We performed a systematic review according to the SALSA (Search, Appraisal, Synthesis, and Analysis) analytic framework [65]. We screened in PubMed and Google Scholar databases for all case descriptions of GBS associated with COVID-19 that were published from January 1st 2020 up to July 20th 2020. Keywords (including all commonly used abbreviations of these terms) used in the search strategy were as follows: [“acute autoimmune neuropathy” OR “acute inflammatory demyelinating polyneuropathy” OR “acute inflammatory demyelinating polyradiculoneuropathy,” OR “acute inflammatory polyneuropathy” OR “Demyelinating Polyradiculoneuropathy” OR “Guillain–Barre Syndrome” OR “Guillain–Barre” OR ““Miller–Fisher” OR “Bickerstaff encephalitis” OR “AIDP” OR “AMAN” OR “AMSAN” OR polyneuritis cranialis] AND [“COVID-19” OR “Wuhan coronavirus” OR “novel coronavirus” OR “novel coronavirus 2019” OR “SARS” OR “SARS-CoV-2”]. Suitable references were also identified in the authors’ archives of scientific literature on GBS. We restricted our search to studies published in English, Spanish, or Italian. Publications that were not peer-reviewed were excluded from this study. PRISMA criteria were applied. For each case, we extracted data concerning demographic and clinical variables, results of diagnostic investigations, and outcome. If the GBS clinical variant [57] or the electrophysiological subtype [61] was not explicitly reported in the paper, we reconstructed it, when possible, from reported details. We also classified the diagnostic certainty of all cases according to the Brighton Criteria [66]. Searches were performed by SAR, AA, and MF. The selection of relevant articles was shared with all authors.
For statistical analysis, we used IBM SPSS Statistics version 21 (IBM, Armonk, NY, USA). Based on the distribution of values, continuous data were expressed as mean ± standard deviation or as median and interquartile range (IQR). Depending on the number of groups and data distribution, we applied the t test, the Mann–Whitney U test or the Kruskal–Wallis test (followed by Dunn–Bonferroni post hoc test). All reported p values were adjusted for multiple comparisons. We adopted the Chi-square test for categorical variables. Differences were considered statistically significant at p < 0.05.
For the present study, no authorization to an Ethics Committee was asked, because the original reports, nor this work, provided any personal information of the patients.
Results
Our literature search identified 101 papers, including 37 case reports, 12 case series, 3 reviews with case reports, 42 reviews, 4 letters, 1 original article, 1 point of view, and 1 brief report. Four and one patients were excluded from the analysis because of a missing laboratory-proven SARS-CoV-2 infection or an ambiguous GBS diagnosis [disease course resembling chronic inflammatory demyelinating neuropathy (CIDP)], respectively. A total of 52 studies were included in the final analysis (total patients =
= 73) [5–56]. All data concerning the analyzed patients are reported in Table 1. For one case [20], most clinical and diagnostic details were not reported; therefore, many of our analyses were limited to 72 patients.
73) [5–56]. All data concerning the analyzed patients are reported in Table 1. For one case [20], most clinical and diagnostic details were not reported; therefore, many of our analyses were limited to 72 patients.
Table 1
Summary of clinical findings, results of diagnostic investigations, and outcome in 73 GBS cases
| Article | Country | Age | Sex | GBS clinical picture | COVID-19 clinical picture | Previous comorbidities | GBS diagnosis | Level of diagnostic certaintyb | GBS variant | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Days between COVID-19 symptoms and GBS onset | Onset | Disease course | Autonomic disturbances | Respiratory symptoms/failure | Time to Nadira | |||||||||
| Agosti et al. [5] | Italy | 68 | M | 5 days after | LL weakness | Bilateral facial palsy, progressive symmetric ascending flaccid tetraparesis, achilles tendon areflexia | NA | No | NA | Dry cough associated with fever, dysgeusia, and hyposmia | Dyslipidemia, benign prostatic hypertrophy, hypertension, abdominal aortic aneurysm | Clinical + CSF + electrophysiology | 1 | Pure motor |
| Alberti et al. [6] | Italy | 71 | M | 4 days after (no resolution of pneumonia) | LL paraesthesia | Ascendant weakness, flaccid tetraparesis, hypoesthesia and paraesthesia in the 4 limbs, generalized areflexia, dyspnea | None | Yes (concurrent pneumonia) | 4 days after symptoms onset (24 h after the admission) | Fever (low grade), dyspnea, pneumonia | Hypertension, treated abdominal aortic aneurysm, treated lung cancer | Clinical + + CSF + electrophysiology CSF + electrophysiology | 1 | Classic sensorimotor |
| Arnaud et al. [7] | France | 64 | M | 23 days after | Fast progressive LL weakness | Generalized areflexia, severe flaccid proximal paraparesis, decreased proprioceptive length-dependent sensitivity and LL pinprick and light touch hypoesthesia | None | No | 4 days after symptoms onset | Fever, cough, diarrhea, dyspnea, severe interstitial pneumonia | DM type 2 | Clinical + + CSF + electrophysiology CSF + electrophysiology | 1 | Classic sensorimotor |
| Assini et al. [8] | Italy | 55 | M | 20 days after | Bilateral eyelid ptosis, dysphagia, dysphonia | Masseter weakness, tongue protusion (bilateral hypoglossal nerve paralysis), UL and LL hyporeflexia without muscle weakness, soft palate elevation defect | None | Yes (concurrent pneumonia) | NA | Fever, anosmia, ageusia, cough, pneumonia | NA | Clinical + electrophysiology | 2 | Classic sensorimotor overlapping with Miller-Fisher |
| Assini et al. [8] | Italy | 60 | M | 20 days after | Distal tetraparesis with right foot drop, autonomic disturbances | UL and LL distal weakness, right foot drop, generalized areflexia | Gastroplegia, paralytic ileus, loss of blood pressure control | Yes (concurrent pneumonia) | NA | Fever, severe interstitial pneumonia | NA | Clinical + electrophysiology | 2 | Pure motor |
| Bigaut et al. [9] | France | 43 | M | 21 days after | UL and LL paraesthesia, distal LL weakness | Extension to midthigh and tips of the finger with ataxia, right peripheral facial nerve palsy, generalized areflexia | None | No | 2 days after symptoms onset | Cough, asthenia, myalgia in legs, followed by acute anosmia and ageusia with diarrhea, mild interstitial pneumonia | NA | Clinical + + CSF + electrophysiology CSF + electrophysiology | 1 | Classic sensorimotor |
| Bigaut et al. [9] | France | 70 | F | 10 days after | Acute proximal tetraparesis, distal forelimb and perioral paraesthesia | Respiratory weakness, loss of ambulation | None | Yes | 3 days after symptoms onset | Anosmia, ageusia, diarrhea, asthenia, myalgia, moderate interstitial pneumonia | Obesity | Clinical + + CSF + electrophysiology CSF + electrophysiology | 1 | Classic sensorimotor |
| Bracaglia et al. [10] | Italy | 66 | F | Unknown (due to asymptomatic infection) | Acute proximal and distal tetraparesis, lumbar pain and distal tingling sensation | Loss of ambulation, difficulty in speeching and swallowing, generalized areflexia | None | No | NA | Asymptomatic | None | Clinical + electrophysiology | 2 | Classic sensorimotor |
| Camdessanche et al. [11] | France | 64 | M | 11 days after | UL and LL paraesthesia | Ascendent weakness, flaccid tetraparesis, generalized areflexia, dysphagia | None | Yes | 3 days after symptoms onset | Fever (high grade), cough, pneumonia | None | Clinical + + CSF + electrophysiology CSF + electrophysiology | 1 | Classic sensorimotor |
| Chan et al. [12] | Canada | 58 | M | 20 days after home isolation for suspected contact | Bilateral facial weakness, dysarthria, feet paraesthesia, LL areflexia | NA | None | No | NA | Asymptomatic, interstitial pneumonia | None | Clinical + + CSF + electrophysiology CSF + electrophysiology | 1 | Bilateral facial palsy with paraesthesia |
| Chan et al. [13] | USA | 68 | M | 18 days after | Gait disturbance, hands and feet paraesthesia | LL proximal weakness, absent vibratory and proprioceptive sense at the toes, UL hyporeflexia, LL areflexia, unsteady gait with inability to toe or heel walk, bilateral facial weakness, dysphagia, dysarthria, neck flexion weakness | None | No | 8 days after the onset of symptoms | Fever and upper respiratory symptoms | NA | Clinical + + CSF CSF | 2 | Classic sensorimotor |
| Chan et al. [13] | USA | 84 | M | 16 days after | Hands and feet paraesthesia, progressive gait disturbance | Bilateral facial weakness, progressive arm weakness, neuromuscular respiratory failure | Yes (not specified autonomic dysfunction) | Yes | 25 days after the onset of symptoms | Fever | NA | Clinical + + CSF CSF | 2 | Classic sensorimotor |
| Coen et al. [14] | Switzerland | 70 | M | 6 days after | Paraparesis, distal allodynia | Generalized areflexia | Difficulties in voiding and constipation | No | NA | Dry cough, myalgia, fatigue | None | Clinical + + CSF + 0electrophysiology CSF + 0electrophysiology | 1 | Classic sensorimotor |
| Ebrahimzadeh et al. [15] | Iran | 46 | M | 18 days after | Pain and numbness in distal LL and UL extremities, ascending weakness in legs | Mild peripheral right facial nerve palsy, generalized areflexia | None | No | 7 days after symptoms onset | Low-grade fever, sore thorat, dry cough and mild dyspnea, bilateral interstitial pneumonia (concurrent with neurological symptoms) | None | Clinical + + CSF + electrophysiology CSF + electrophysiology | 1 | Classic sensorimotor |
| Ebrahimzadeh et al. [15] | Iran | 65 | M | 10 days after | Progressive ascending LL and UL extremities weakness and paraesthesia | Proximal and distal UL and LL weakness, UL hyporeflexia and LL areflexia | None | No | 14 days after symptoms onset | History of COVID-19 (symptoms not specified), fine crackles in both lungs (concurrent with neurological symptoms) | Hypertension | Clinical + electrophysiology | 2 | Classic sensorimotor |
| El Otmani et al. [16] | Morocco | 70 | F | 3 days after | Weakness and paraesthesia in the 4 limbs | Tetraparesis, hypotonia, generalized areflexia, bilateral positive Lasègue sign | None | No | NA | Dry cough, pneumonia | Rheumatoid arthritis | Clinical + + CSF + electrophysiology CSF + electrophysiology | 1 | Classic sensorimotor |
| Esteban Molina et al. [17] | Spain | 55 | F | 14 days after | Paraesthesia and weakness in the 4 limbs | Lumbar pain, dysphagia, tetraplegia, general areflexia, bilateral facial palsy, lingual and perioral paraesthesia | None | Yes | 3 days after symptoms onset (48 h after the admission) | Fever, dry cough and dyspnoea, pneumonia | Dyslipidemia | Clinical + + CSF + electrophysiology CSF + electrophysiology | 1 | Classic sensorimotor |
| Farzi et al. [18] | Iran | 41 | M | 10 days after | Paraesthesia of the feet | Tetraparesis, areflexia at the LL and hyporeflexia at the UL, stocking-and-glove hypesthesia and reduced sense of vibration and position | None | No | 7 days after symptoms onset | Cough, dyspnea and fever | DM type II | Clinical + + electrophysiology electrophysiology | 2 | Classic sensorimotor |
| Fernández–Domínguez et al. [19] | Spain | 74 | F | 15 days after | Gait ataxia and generalized areflexia | NA | NA | No | NA | Respiratory symptoms (not further detailed) | Hypertension and follicular lymphoma | Clinical + + CSF CSF | 2 | Miller Fisher variant |
| Finsterer et al. [20] | India | 20 | M | 5 days after | NA | NA | NA | NA | NA | NA | NA | Clinical + electrophysiology | 2 | NA |
| Frank et al. [21] | Brazil | 15 | M | > 5 days after | Paraparesis, pain in the LL | Rapidly progressive ascending tetraparesis, areflexia | NA | No | NA | Fever, intense sweating | NA | Clinical + + electrophysiology electrophysiology | 2 | Classic sensorimotor |
| Gigli et al. [22] | Italy | 53 | M | NA | Paraesthesia, gait ataxia | NA | NA | NA | NA | Fever, diarrhea | NA | Clinical + + CSF CSF + + electrophysiology electrophysiology | 1 | NA |
| Gutiérrez-Ortiz et al. [23] | Spain | 50 | M | 3 days after | Vertical diplopia, perioral paraesthesia, gait ataxia | Right internuclear ophthalmoparesis and right fascicular oculomotor palsy, ataxia, generalized areflexia | None | No | NA | Fever, cough, malaise, headache, low back pain, anosmia, ageusia | Bronchial asthma | Clinical + + CSF CSF | 2 | Miller Fisher variant |
| Gutiérrez-Ortiz et al. [23] | Spain | 39 | M | 3 days after | Diplopia (bilateral abducens palsy) | Generalized areflexia | None | No | NA | Diarrhea, low-grade fever | None | Clinical + + CSF CSF | 2 | Polyneuritis cranialis (GBS–Miller Fisher Interface) |
| Helbok et al. [24] | Austria | 68 | M | 14 days after | Hypoaesthesia and paraesthesia in the LL, proximal weakness, areflexia, stand ataxia | Ascending weakness, flaccid tetraparesis, generalized areflexia | NA | Yes | 2 days after symptoms onset (24 h after the admission) | Fever, dry cough, myalgia, anosmia and ageusia. | None | Clinical + + CSF CSF + + electrophysiology electrophysiology | 1 | Classic sensorimotor |
| Hutchins et al. [25] | USA | 21 | M | 16 days after | Right-sided facial numbness and weakness | Bilateral facial palsy, severe dysarthria, bilateral LL weakness , bilateral UL paraesthesia, areflexia | NA | No | 3 days after symptoms onset | Fever, cough, dyspnoea, diarrhea, nausea, headache | Hypertension, prediabetes, and class I obesity | Clinical + CSF + electrophysiology | 1 | Bilateral facial palsy with paraesthesia |
| Juliao Caamaño et al. [26] | Spain | 61 | M | 10 days after | Facial diplegia | No progression | None | No | 1 day after symptoms onset | Fever and cough | None | Clinical + + electrophysiology electrophysiology | 3 | Bilateral facial nerve palsy |
| Khalifa et al. [27] | Kingdom of Saudi Arabia | 11 | M | 20 days after | Gait ataxia, areflexia and paraesthesia in the LL | Gradual motor improvement, persistent hyporeflexia | NA | No | NA | Acute upper respiratory tract infection, low-grade fever, dry cough. | NA | Clinical + CSF + electrophysiology | 1 | Classic sensorimotor |
| Kilinc et al. [28] | The Netherlands | 50 | M | 24 days after | Facial diplegia, symmetrical proximal weakness, paraesthesia of distal extremities, gait ataxia, areflexia | Progression of limb weakness and inability to walk | NA | No | 11 days after symptoms onset | Dry cough | None | Clinical + + electrophysiology electrophysiology | 2 | Classic sensorimotor |
| Lampe et al. [29] | Germany | 65 | M | 2 days after | Acute right UL and LL weakness causing recurrent falls | Right UL paresis, slight paraparesis more pronounced on the right side, generalized hyporeflexia | None | No | 3 days after symptoms onset | Fever and dry cough | None | Clinical + + CSF CSF + + electrophysiology electrophysiology | 1 | Pure motor |
| Lantos et al. [30] | USA | 36 | M | 4 days after | Opthalmoparesisa and hypoesthesia below knee | Progressive ophthalmoparesis (including initial left III cranial nerve and eventual bilateral VI cranial nerve palsies), ataxia, and hyporeflexia | None | No | NA | Fever, chills, and myalgia | None | Clinical | 3 | Miller Fisher variant |
| Lascano et al. [31] | Switzerland | 52 | F | 15 days after (no resolution of pneumonia) | Back pain, diarrhea, rapidly progressive tetraparesis, distal paraesthesia | Worsening of proximal weakness (tetraplegia), generalized areflexia, ataxia | Constipation, abdominal pain | Yes | 4 days after symptoms onset | Dry cough, dysgeusia, cacosmia | None | Clinical + + CSF + electrophysiology CSF + electrophysiology | 1 | Classic sensorimotor |
| Lascano et al. [31] | Switzerland | 63 | F | 7 days after (no resolution of pneumonia) | Limb weakness, pain on the left calf | Moderate tetraparesis, LL and left UL areflexia, distal hypoesthesia and paraesthesia | None | No | 5 days after symptoms onset | Dry cough, shivering, breathing difficulties, chest pain, odynophagia | DM type 2 | Clinical + electrophysiology | 2 | Classic sensorimotor |
| Lascano et al. [31] | Switzerland | 61 | F | 22 days after | LL weakness, dizziness, dysphagia | Moderate tetraparesis, bilateral facial palsy, lower limb allodynia, severe hypopallesthesia, areflexia (except for bicipital tendon reflexes) | None | Yes | 4 days after symptoms onset | Productive cough, headaches, fever, myalgia, diarrhea, nausea, vomiting, weight loss, recurrent episodes of transient loss of consciousness | None | Clinical + + CSF + electrophysiology CSF + electrophysiology | 1 | Classic sensorimotor |
| Manganotti et al. [32] | Italy | 50 | F | 16 days after | Diplopia and facial paraesthesia | Ataxia, diplopia in vertical and lateral gaze, left upper arm dysmetria, generalized areflexia, mild lower facial defects, and mild hypoesthesia in the left mandibular and maxillary branch | None | Yes (concurrent pneumonia) | NA | Fever, cough, ageusia, bilateral pneumonia | None | Clinical + + CSF CSF | 2 | Miller Fisher variant |
| Manganotti et al. [33] | Italy | 72 | M | 18 days after | Tetraparesis UL > > LL, LL paraesthesia , generalized areflexia, facial weakness on the right side LL, LL paraesthesia , generalized areflexia, facial weakness on the right side | NA | NA | No | NA | Fever, dyspnea, hyposmia and ageusia | NA | Clinical + CSF + electrophysiology | 1 | Classic sensorimotor |
| Manganotti et al. [33] | Italy | 72 | M | 30 days after | Tetraparesis LL > > UL, paraesthesia, global areflexia UL, paraesthesia, global areflexia | NA | NA | No | NA | Fever, cough, dyspnea, hyposmia and ageusia | NA | Clinical + electrophysiology | 1 | Classic sensorimotor |
| Manganotti et al. [33] | Italy | 49 | F | 14 days after | Ophthalmoplegia, limb ataxia, generalized areflexia, diplopia, facial hypoesthesia, facial weakness | NA | NA | No | NA | Fever, cough, dyspnea, hyposmia and ageusia | NA | Clinical + CSF + electrophysiology | 1 | Miller Fisher variant |
| Manganotti et al. [33] | Italy | 94 | M | 33 days after | LL weakness, generalized hyporeflexia | NA | NA | No | NA | Fever, cough, gastrointestinal symptoms | NA | Clinical + electrophysiology | 2 | Classic sensorimotor |
| Manganotti et al. [33] | Italy | 76 | M | 22 days after | Quadriparesis UL > > LL, generalized areflexia, facial weakness, transient diplopia LL, generalized areflexia, facial weakness, transient diplopia | NA | NA | No | NA | Fever, cough, dysuria, hyposmia, ageusia | NA | Clinical + CSF + electrophysiology | 1 | Pure motor |
| Marta-Enguita et al. [34] | Spain | 76 | F | 8 days after | Back pain and progressive tetraparesis with distal-onset paraesthesia | Progressive with dysphagia and cranial nerves involvement, generalized areflexia | NA | Yes | 10 days after symptom onset | Cough and fever without dyspnea | None | Clinical | 3 | NA |
| Mozhdehipanah et al. [35] | Iran | 38 | M | 16 days after | Progressive LL paraesthesia, facial diplegia, lobal areflexia | Mild LL weakness , bulbar symptoms developed | Blood pressure instability, tachycardia | No | 8 days after symptoms onset | Upper respiratory infection (no further details) | NA | Clinical + CSF + electrophysiology | 1 | Bilateral facial palsy with paraesthesia |
| Mozhdehipanah et al. [35] | Iran | 14 | F | NA | Ascending quadriparesis, UL hyporeflexia, LL areflexia, distal hypoesthesia, ataxia | NA | NA | No | NA | Upper respiratory infection (no further details) | NA | Clinical + CSF | 2 | Classic sensorimotor |
| Mozhdehipanah et al. [35] | Iran | 44 | F | 26 days after | Weakness of LL | Tetraparesis, generalized areflexia, symmetrical hypoesthesia | NA | Yes | NA | Dry cough, fever, myalgia, progressive dyspnea | COPD | Clinical + CSF + electrophysiology | 1 | Classic sensorimotor |
| Mozhdehipanah et al. [35] | Iran | 66 | F | 30 days after | Progressive UL and LL weakness, generalized areflexia, symmetrical hypoesthesia | NA | No | No | NA | Fever, dry cough, severe myalgia | DM, hypertension, and rheumatoid arthritis | Clinical + CSF + electrophysiology | 1 | Classic sensorimotor |
| Naddaf et al. [36] | USA | 58 | F | 17 days after | Progressive paraparesis, imbalance, severe lower thoracic pain without radiation | Mild neck flexion weakness, mild/moderate distal UL and proximal and distal LL weakness, UL hyporeflexia, LL areflexia, moderately severe length-dependent sensory loss in the feet, ataxic gait | None | No | NA | Fever, dysgeusia without anosmia, bilateral interstitial pneumonia | None | Clinical + CSF + electrophysiology | 1 | Classic sensorimotor |
| Oguz-Akarsu et al. [37] | Turkey | 53 | F | Concurrent pneumonia | Dysarthria, progressive LL weakness and numbness | Ataxia, generalized areflexia | None | No | NA | Mild fever (37.5 °C), pneumonia | None | Clinical + electrophysiology | 2 | Classic sensorimotor |
| Ottaviani et al. [38] | Italy | 66 | F | 7 days after (concurrent pneumonia) | Flaccid paraparesis, no sensory symptoms | Progressively developed proximal weakness in all limbs, dysesthesia, and unilateral facial palsy, generalized areflexia | NA | Yes | 13 days after symptoms onset | Fever and cough, pneumonia | NA | Clinical + + CSF CSF + + electrophysiology electrophysiology | 1 | Classic sensorimotor |
| Padroni et al. [39] | Italy | 70 | F | 23 days after | UL and LL paraesthesia, gait difficulties, asthenia | Ascendant weakness, tetraparesis, generalized areflexia | None | Yes | 6 days after symptoms onset | Fever (38.5 °C), dry cough, pneumonia | None | Clinical + + CSF CSF + + Electrophysiology Electrophysiology | 1 | Classic sensorimotor |
| Paterson et al. [40] | UK | 42 | M | 13 day after | Distal limb numbness and weakness, dysphagia | Tetraparesis, generalized areflexia, sensory loss | NA | Yes | 16 days after symptom onset | Cough, fever dyspnea, diarrhea, anosmia | None | Clinical + CSF + electrophysiology | 1 | Classic sensorimotor |
| Paterson et al. [40] | UK | 60 | M | 1 day before | Distal limb numbness and weakness | Tetraparesis, generalized areflexia, sensory loss, dysautonomia, facial and bulbar weakness | Yes | Yes | 5 days after symptom onset | Headache, ageusia, anosmia | NA | Clinical + CSF + electrophysiology | 1 | Classic sensorimotor |
| Paterson et al. [40] | UK | 38 | M | 21 day after | Distal limb numbness, weakness, clumsiness | Mild distal weakness, sensory ataxia | None | No | NA | Cough, diarrhea | NA | Clinical + CSF + electrophysiology | 1 | Classic sensorimotor |
| Paybast et al. [41] | Iran | 38 | M | 21 days after | Acute progressive ascending paraesthesia of distal LL | Quadriparesthesia, bilateral facial droop with drooling of saliva and slurred speech, generalized areflexia, swallowing inability, bilaterally absent gag reflex | Tachycardia and blood pressure instability | No | 3 days after symptoms onset | Symptoms of upper respiratory tract infection | Hypertension | Clinical + + CSF + electrophysiology CSF + electrophysiology | 1 | Classic sensorimotor |
| Paybast et al. [41] | Iran | 14 | F | 21 days after | Progressive ascending quadriparesthesia, mild LL weakness | Mild proximal and distal LL weakness, hypoactive deep tendon reflexes in UL and absent in LL, decreased light touch, position, and vibration sensation in all distal limbs up to ankle and elbow joints, gait ataxia | None | No | 2 days after symptoms onset | Symptoms of upper respiratory tract infection | None | Clinical + + CSF CSF | 2 | Classic sensorimotor |
| Pfefferkorn et al. [42] | Germany | 51 | M | 14 days after | UL and LL weakness, acral paraesthesia | Tetraparesis, generalized areflexia, deterioration to an almost complete peripheral locked-in syndrome with tetraplegia, complete sensory loss at 4 limbs, bilateral facial and hypoglossal paresis | None | Yes | 15 days after symptoms onset | Fluctuating fever, flu-like symptoms with marked fatigue and dry cough, pneumonia | NA | Clinical + + CSF + electrophysiology CSF + electrophysiology | 1 | Classic sensorimotor |
| Rana et al. [43] | USA | 54 | M | 14 days after | LL paresthesias of LL | Ascending tetraparesis, general areflexia, burning sensation diplopia, facial diplegia, mild ophthalmoparesis | Resting tachycardia and urinary retention | Yes | NA | Rhinorrhea, odynophagia, fever, chills, and night sweats | Hypertension, hyperlipidemia, restless leg syndrome, and chronic back pain, concurrent C. Difficile infection | Clinical + + electrophysiology electrophysiology | 2 | Miller Fisher variant |
| Reyes-Bueno et al. [44] | Spain | 50 | F | 15 days after | Root-type pain in all four limbs, dorsal and lumbar back pain | LL Weakness, ataxia, diplopia, bilateral facial palsy, generalized areflexia | Dry mouth, diarrhea and unstable blood pressure | No | 12 days after symptoms onset | Diarrhea, odynophagia and cough | NA | Clinical + + CSF CSF + + electrophysiology electrophysiology | 1 | Miller Fisher variant |
| Riva et al. [45] | Italy | 60+ | M | 17 days after | Progressive limb weakness and distal paresthesia at four limbs | Ascending paraparesis with involvement of the cranial nerves (facial diplegia), generalized areflexia | None | No | 10 days after symptoms onset | Fever, headache, myalgia, anosmia and ageusia | NA | Clinical + + electrophysiology electrophysiology | 2 | Classic sensorimotor |
| Sancho-Saldaña et al. [46] | Spain | 56 | F | 15 days after | Unsteadiness and paraesthesia in both hands | Lumbar pain and ascending weakness, global areflexia, bilateral facial nerve palsy, oropharyngeal weakness and severe proximal tetraparesis | No | Yes | 3 days after symptoms onset | Fever, dry cough and dyspnea, pneumonia | NA | Clinical + + CSF CSF + + electrophysiology electrophysiology | 1 | Classic sensorimotor |
| Scheidl et al. [47] | Germany | 54 | F | 11 days after | Proximal weakness of LL, numbness of 4 limbs | Initial worsening of the paraparesis with rapid improvement upon initiation of the treatment, areflexia | None | No | 12 days after symptoms onset | Temporary ageusia, | None | Clinical + + CSF CSF + + electrophysiology electrophysiology | 1 | Paraparetic variant |
| Sedaghat et al. [48] | Iran | 65 | M | 14 days after | LL distal weakness | Ascending weakness, tetraparesis, facial bilateral palsy, generalized areflexia, LL distal hypoesthesia and hypopallesthesia | None | No | 4 days after symptoms onset | Fever, cough and sometimes dyspnea, pneumonia | DM type 2 | Clinical + electrophysiology | 2 | Classic sensorimotor |
| Sidig et al. [49] | Sudan | 65 | M | 5 days after | Numbness and weakness in both UL and LL | Ascending weakness, bilateral facial paraesthesia and palsy, clumsiness of UL, tetraparesis, slight palatal muscle weakness, areflexia | Urinary incontinence | Yes | NA | Low-grade fever, sore throat, dry cough, headache and generalized fatigability | DM and Hypertension | Clinical + electrophysiology | 2 | Classic sensorimotor |
| Su et al. [50] | USA | 72 | M | 6 days after | Proximal UL and LL weakness | Progression with worsening of the paresis, areflexia, hypoesthesia | Hypotension alternating with hypertension and tachycardia | Yes | 8 days after symptoms onset | Mild diarrhea, anorexia and chills without fever or respiratory symptoms | Coronary artery disease, hypertension and alcohol abuse | Clinical + + CSF CSF + + electrophysiology electrophysiology | 1 | Classic sensorimotor |
| Tiet et al. [51] | United Kingdom | 49 | M | 21 days after | Distal LL paraesthesia | LL and UL weakness, facial diplegia, distal reduced sensation to pinprick and vibration sense, LL dysesthesia, generalized areflexia | None | No | 4 days after symptoms onset | Shortness of breath, headache and cough | Sinusitis | Clinical + CSF + electrophysiology | 1 | Classic sensorimotor |
| Toscano et al. [52] | Italy | 77 | F | 7 days after | UL and LL paraesthesia | Flaccid tetraplegia, areflexia, facial weakness, dysphagie, tongue weakness | None | Yes | NA | Fever, cough, ageusia, pneumonia | Previous ischemic stroke, diverticulosis, arterial hypertension, atrial fibrillation | Clinical + + CSF + electrophysiology CSF + electrophysiology | 1 | Classic sensorimotor |
| Toscano et al. [52] | Italy | 23 | M | 10 days after | Facial diplegia | LL paraesthesia, generalized areflexia, sensory ataxia | None | No | 2 days after symptoms onset | Fever, pharyngitis | NA | Clinical + + CSF + electrophysiology CSF + electrophysiology | 1 | Bilateral facial palsy with paraesthesia |
| Toscano et al. [52] | Italy | 55 | M | 10 days after | Neck pain, Paresthesias in the 4 limbs, LL weakness | Flaccid tetraparesis, areflexia, facial weakness | None | Yes | NA | Fever, cough, pneumonia | NA | Clinical + + CSF + electrophysiology CSF + electrophysiology | 1 | Classic sensorimotor |
| Toscano et al. [52] | Italy | 76 | M | 5 days after | Lumbar pain, LL weakness | Flaccid tetraparesis, generalized areflexia, ataxia | None | No | 4 days after symptoms onset | Cough and hyposmia | NA | Clinical Electrophysiology | 1 | Classic sensorimotor |
| Toscano et al. [52] | Italy | 61 | M | 7 days after | LL weakness and paraesthesia | Ascending weakness, tetraplegia, facial weakness, areflexia, dysphagia | None | Yes | NA | Cough, ageusia and anosmia, pneumonia | NA | Clinical + + CSF+ electrophysiology CSF+ electrophysiology | 1 | Classic sensorimotor |
| Velayos Galán et al. [53] | Spain | 43 | M | 10 days after | Distal weakness and numbness of the 4 limbs, gait ataxia | Progression of the weakness with bilateral facial paresis and dysphagia, generalized areflexia | NA | No | 2 days after admission | Cough, pneumonia | NA | Clinical + electrophysiology | 2 | Classic sensorimotor |
| Virani et al. [54] | USA | 54 | M | 8 days after | LL weakness, numbness | Ascending weakness, tetraparesis, areflexia | Urinary retention | Yes | Shortly after presentation in the outpatient clinic (after 2 days of symptoms onset) | Fever (102 F), dry cough, pneumonia | Clostridium difficile colitis 2 days before GBS onset | Clinical | 3 | Classic sensorimotor |
| Webb et al. [55] | United Kingdom | 57 | 6 days after | Ataxia, progressive limb weakness and foot dysaesthesia, | Tetraparesis, generalized areflexia, hypoesthesia in the 4 limbs, hypopallesthesia in LL, dysphagia | None | Yes | 3 days after symptoms onset | Mild cough and headache, myalgia and malaise, slight fever, diarrhea, pneumonia | Untreated hypertension and psoriasis | Clinical + CSF + electrophysiology | 1 | Classic sensorimotor | |
| Zhao et al. [56] | China | 61 | F | 8 days before | LL weakness | Ascending weakness, tetraparesis, areflexia, LL distal hypoesthesia | None | No | 4 days after symptoms onset | Fever (38·2 °C), dry cough pneumonia | NA | Clinical + CSF + electrophysiology | 1 | Classic sensorimotor |
| Article | COVID-19 diagnosis | Blood findings | Auto-antibodies and screening for most common GBS causes | CSF findings | Electrophysiology: Neuropathy type and GBS electrophysiologic subtype | MRI (brain and spinal) | Management and therapy | Outcome | |
|---|---|---|---|---|---|---|---|---|---|
| GBS | COVID-19 | ||||||||
| Agosti et al. [5] | RT-PCR + + chest CT chest CT | Thrombocytopenia (101 × × 109 /L, reference value: 125–300 109 /L, reference value: 125–300 × × 109 /L), lymphocytopenia (0.48 109 /L), lymphocytopenia (0.48 × × 109 /L, reference value: 1.1–3.2 × 109 /L) 109 /L, reference value: 1.1–3.2 × 109 /L) | Negative ANA, anti-DNA, c-ANCA, p-ANCA, negative screening for Campylobacter jejuni, Mycoplasma pneumoniae, Salmonella enterica, CMV, HSV 1 and 2, VZV, influenza virus A and B, HIV, normal B12 and serum protein electrophoresis | Increased total protein (98 mg/dl), cell count: 2/106 L | Demyelinating AIDP | NA | IVIG 400 mg/kg/day (5 days) | Antiviral drugs (not specifically mentioned) | Improvement, discharged home after 30 days |
| Alberti et al. [6] | RT-PCR + + chest CT chest CT | NA | NA | Increased total protein (54 mg/dl), 9 cells/µl, negative SARS-CoV-2 PCR | Demyelinating AIDP | NA | IVIG 400 mg/kg (5 days) + + mechanical invasive ventilation mechanical invasive ventilation | Lopinavir/ritonavir, hydroxychloroquine | 24 h after admission, death because of respiratory failure |
| Arnaud et al. [7] | RT-PCR + + chest CT chest CT | NA | Negative anti-ganglioside and antineural antibodies, negative Campylobacter Jejuni, HIV, syphilis, CMV, EBV serology | Increased total protein (1.65 g/L), no pleyocitosis, negative oligoclonal bands, negative SARS-CoV-2 PCR, negative EBV and CMV RT-PCR | Demyelinating AIDP | NA | IVIG 400 mg/kg (5 days) | Hydroxychloroquin, cefotaxime, azithromycine | Progressive improvement |
| Assini et al. [8] | RT-PCR | Lymphocytopenia, increased LDH and inflammation markers; low serum albumin (2.9 mg/dL) | NA | Normal total protein level, increased IgG/albumin ratio (233), negative SARS-CoV-2 PCR, presence of oligoclonal bands (both in serum and CSF) | Demyelinating with sural sparing AIDP | Brain: no pathological findings | IVIG 400 mg/kg (5 days) | Hydroxychloroquine, arbidol, ritonavir and lopinavir + + mechanical invasive ventilation mechanical invasive ventilation | 5 days after IVIG, improvement of swallowing, speech, tongue motility, eyelid ptosis and strength |
| Assini et al. [8] | RT-PCR + + chest CT chest CT | Lymphocytopenia, increased LDH and GGT, leucocytosis, low serum albumin (2.6 mg/dL) | Negative anti-ganglioside antibodies | Normal total protein level, increased IgG/albumin ratio (170), negative SARS-CoV-2 PCR, presence of oligoclonal bands (both in serum and CSF) | Motor sensory axonal, muscular neurogenic changes AMSAN | NA | IVIG 400 mg/kg (5 days) | Hydroxychloroquine, antiretroviral therapy, tocilizumab + + tracheostomy and assisted ventilation tracheostomy and assisted ventilation | 5 days after IVIG, improvement of vegetative symptoms, persistence of hyporeflexia and right foot drop |
| Bigaut et al. [9] | RT-PCR + + chest CT chest CT | Normal blood count, negative CRP | Negative anti-ganglioside antibodies, negative HIV, Lyme and syphilis serology | Increased total protein (0.95 g/L), cell count: 1 × × 106/L, negative SARS-CoV-2 PCR 106/L, negative SARS-CoV-2 PCR | Demyelinating AIDP | Spinal: Radiculitis and plexitis on both brachial and lumbar plexus; multiple cranial neuritis (in III, VI, VII, and VIII nerves) | IVIG 400 mg/kg (5 days) + + non-invasive ventilation non-invasive ventilation | NA | Progressive improvement |
| Bigaut et al. [9] | RT-PCR + + chest CT chest CT | Increased CRP | Negative anti-ganglioside antibodies | Increased total protein (1.6 g/L), cell count: 6 × × 106/L, negative SARS-CoV-2 PCR 106/L, negative SARS-CoV-2 PCR | Demyelinating AIDP | NA | IVIG 400 mg/kg (5 days) | NA | Slow progressive improvement |
| Bracaglia et al. [10] | RT-PCR (normal chest CT) | Elevated CPK (461 U/L, normal < < 145), CRP 5,65 mg/dL (normal 145), CRP 5,65 mg/dL (normal < < 0.5), lymphocyto- penia (0·68 0.5), lymphocyto- penia (0·68 × × 109/L, normal 1·10–4), mild increase of LDH (284 U/L, normal 109/L, normal 1·10–4), mild increase of LDH (284 U/L, normal < < 248), GOT and GPT (549 and 547 U/L, normal 248), GOT and GPT (549 and 547 U/L, normal < < 35), elevation of IL-6 (11 pg/mL, normal 35), elevation of IL-6 (11 pg/mL, normal < < 5.9) 5.9) | Negative anti-ganglioside antibodies; negative microbiologic testing on CSF and serum for HSV1-2, EBV, VZV, CMV, HIV, Mycoplasma Pneumoniae and Borrelia. | Increased total protein (245 mg/dL) and increased cell count: 13 cells/mm3, polymorphonucleate 61.5% | Demyelinating AIDP | NA | IVIG 400 mg/kg (5 days) | Hydroxychloroquine, ritonavir, darunavir | Improvement of UL and LL weakness, development of facial diplegia |
| Camdessanche et al. [11] | RT-PCR + + chest CT chest CT | NA | Negative anti-gangliosides antibodies; negative screening for Campylobacter jejuni, Mycoplasma pneumoniae, Salmonella enterica, CMV, EBV, HSV1-2, VZV, Influenza virus A & B, HIV, and hepatitis E | Increased total protein (1.66 g/L), normal cell count | Demyelinating AIDP | NA | IVIG 400 mg/kg (5 days) + + mechanical invasive ventilation mechanical invasive ventilation | Oxygen therapy, paracetamol, low molecular weight heparin, lopinavir/ritonavir 400/100 mg twice a day for 10 days | NA |
| Chan et al. [12] | RT-PCR + + chest CT chest CT | Persistent thrombocytosis (maximum PC 688 ×109/L), elevated d-dimer (1.47 mg/L) | NA | Increased total protein (1.00 g/L), cell count: 4 × 106/L (normal), negative SARS-CoV-2 PCR | Demyelinating AIDP | Brain: bilateral intracranial facial nerve enhancement | IVIG 400 mg/kg (5 days) | Empiric azithromycin and ceftriaxone | Slight improvement of facial weakness, unchanged paraesthesia |
| Chan et al. [13] | RT-PCR | NA | Negative anti-gangliosides antibodies | Increased total protein (226 mg/dL), leucocytes: 3 cells/mm3, glucose: 56 mg/dL, negative SARS-CoV-2 PCR | NA | Lumbosacral spine: no pathological findings | 5 sessions of plasmapheresis | NA | Resolution of dysphagia, ambulation with minimal assistance 28 days after symptoms onset |
| Chan et al. [13] | RT-PCR | NA | Elevated GM2 IgG/IgM antibodies | Increased total protein (67 mg/dL), leucocytes: 1 cells/mm3, glucose 58 mg/dL, negative SARS-CoV-2 PCR | NA | NA | Mechanical invasive ventilation + + 5 sessions of plasmapheresis (without benefit on ventilation) 5 sessions of plasmapheresis (without benefit on ventilation) + + IVIG IVIG | NA | Persistence of quadriparesis with intermittent autonomic dysfunction, slowly weaned from the ventilator |
| Coen et al. [14] | RT-PCR + + serology serology | Normal (not specified) | Negative anti-gangliosides antibodies; negative meningitis/encephalitis panel | Albuminocytological dissociation, no intrathecal IgG synthesis, negative SARS-CoV-2 PCR | Demyelinating with sural sparing AIDP | Brain: NA Spinal: no pathological findings | IVIG 400 mg/kg (5 days) | NA | Rapid improvement. From day 11 from hospitalisation Rehabilitation |
| Ebrahimzadeh et al. [15] | RT-PCR + + chest CT chest CT | Normal CRP (5 mg/L), normal serum protein immunoelectrophoresis | Negative anti-GQ1b antibodies, negative screening for Campylobacter jejuni, HIV, EBV, CMV, influenza virus (type A and B), HCV, non-reactive VDRL | Increased total protein (78 mg/dL), normal cell count (erythrocyte = = 0/mm3, leukocyte 0/mm3, leukocyte = = 4/mm3), normal glucose (70 mg/dL) 4/mm3), normal glucose (70 mg/dL) | Demyelinating AIDP | Brain: no pathological findings Spinal: no pathological findings | None | Hydroxychloroquine for 5 days | Improvement of muscle strength to near normal after 16 days |
| Ebrahimzadeh et al. [15] | RT-PCR + + chest CT chest CT | Slightly elevated CRP (34 mg/L), normal serum protein immunoelectrophoresis | Negative anti-GQ1b antibodies, negative screening for Campylobacter jejuni, HIV, EBV, CMV, influenza virus (type A and B), HCV, non-reactive VDRL | NA | Demyelinating AIDP | NA | IVIG | NA | Improvement of muscle strength in all extremities after 14 days |
| El Otmani et al. [16] | RT-PCR + + chest CT chest CT | Lymphocytopenia (520/ml) | NA | Increased total protein (1 g/L), normal cell count, negative PCR assay for SARS-CoV-2 | Motor sensory axonal AMSAN | NA | IVIG 400 mg/kg/day (5 days) | Hydroxychloroquine 600 mg/day; azithromycin 500 mg at the first day, then 250 mg per day | At week 1 from admission no significant neurological improvement |
| Esteban Molina et al. [17] | RT-PCR + + chest X-ray chest X-ray | Leucocyte 7400/mm3, lymphocyte 2400/mm3. Hb 14 g/dl. PC 408,000/mm3, d-Dimer 556 ng/ml. Ferritin 544 ng/ml, CRP 2.04 mg/dl, Fibrinogen 6.8 g/dl | Negative bacteriological and viral tests | Increased total protein (86 mg/dL), cell count: 3x106/L | Demyelinating AIDP | Brain: leptomeningeal enhancement in midbrain and cervical spine | IVIG 400 mg/kg/day (5 days) | Hydroxychloroquine, azithromycin, ceftriaxon | Motor improvement but persistence of paraesthesia |
| Farzi et al. [18] | RT-PCR + + chest CT chest CT | Lymphopenia (WBC:5.9 × × 109/L, neutrophils: 85%, lymphocyte:15%), elevated levels of CRP, ESR 69 mm/h 109/L, neutrophils: 85%, lymphocyte:15%), elevated levels of CRP, ESR 69 mm/h | NA | NA | Demyelinating AIDP | NA | IVIG (2 g/kg over 5 days) | Lopinavir/ritonavir and hydroxychloroquine | Improvement after 3 days, favorable outcome |
| Fernández–Domínguez et al. [19] | RT-PCR | NA | Negative anti-GD1b antibodies, negative other anti-ganglioside antibodies | Increased total protein (110 mg/dL), albuminocytological dissociation | Demyelinating NA | Brain: no pathological findings | IVIG 20 g/day (5 days) | Hydroxychloroquine, lopinavir/ritonavir | NA |
| Finsterer et al. [20] | NA | NA | NA | NA | Axonal AMAN | NA | IVIG | NA | Recovery |
| Frank et al. [21] | RT-PCR, + + serology (IgG and IgM) serology (IgG and IgM) | WBC and CRP normal | Negative hepatitis B and C, HIV and VDRL tests | Two CSF analysis 2 weeks apart, both showing normal cell count and CSF biochemistry, negative SARS-CoV-2 PCR, negative PCR for HSV1, HSV2, CMV, EBV, VZV; Zika virus; Dengue virus and Chikungunya virus | Axonal AMAN | Brain: no pathological findings Spinal: no pathological findings | IVIG 400 mg/kg/day (5 days) | Methylprednisolone, azithromycin, albendazole | Some improvement, weakness persisted |
| Gigli et al. [22] | Chest CT + + serology (negative RT-PCR) serology (negative RT-PCR) | NA | Negative anti-ganglioside antibodies, negative PCR for influenza A and B viruses (nasal swab) | Increased total protein (192.8 mg/L), leucocytes: 2.6 cells/µL, positive Ig for SARS-CoV-2, negative SARS-CoV-2 PCR | Demyelinating AIDP | NA | NA | NA | NA |
| Gutiérrez-Ortiz et al. [23] | RT-PCR | Lymphocytes 1000 cells/UI, CRP 2.8 mg/dl | Positive anti-GD1b antibodies, other anti-ganglioside antibodies negative | Increased total protein (80 mg/dl), no leucocytes, glucose 62 mg/dl, negative SARS-CoV-2 PCR | NA | NA | IVIG 400 mg/kg (5 days) | NA | After 2 weeks from admission complete resolution except anosmia, ageusia |
| Gutiérrez-Ortiz et al. [23] | RT-PCR | Leucopenia (3100 cells/µl) | NA | Increased total protein (62 mg/dl), WBC: 2/μl (all monocytes), glucose: 50 mg/dl, negative SARS-CoV-2 PCR | NA | NA | None | Paracetamol | 2 weeks later complete neurological recovery with no ageusia, complete eye movements, and normal deep tendon reflexes |
| Helbok et al. [24] | Chest CT + + serology (repeated negative RT-PCR) serology (repeated negative RT-PCR) | WBC 8.1G/L (normal: 4.0–10.0G/L), CRP 2.3 mg/dL, (normal: 0.0–0.5 mg/dL), fibrinogen level 650 mg/dL (normal: 210–400 mg/dL), LDH 276 U/L (normal: 100–250 U/L), erythrocyte sedimentation rate 55 mm/1 h | Negative PCR for CMV, EBV, influenza virus A/B, Respiratory Syncytial Virus and IgM antibodies for Chlamydia pneumoniae and Mycoplasma pneumoniae | Increased total protein (64 mg/dl), cell count: 2 cells/mm3, serum/ CSF glucose ratio of 0.83, negative SARS-CoV-2 PCR, positive anti-SARS-CoV-2 antibodies (not determined if intrathecal synthesis or passive transfer from blood) | Demyelinating with sural sparing AIDP | Spinal: no pathological findings | IVIG 30 g + + plasma exchange (4 cycles) plasma exchange (4 cycles) + + mechanical invasive ventilation mechanical invasive ventilation | None | Improvement of muscle forces with recovery of mobility without significant help after 8 weeks |
| Hutchins et al. [25] | RT-PCR + + chest CT chest CT | Lymphopenia (absolute lymphocyte count of 0.7 K/mm3) | Serum HSV IgG and IgM. Respiratory viral panel PCR negative Negative GM1, GD1b, and GQ1b IgG and IgM), aquaporin-4 receptor (IgG), HIV 1/2, HSV 1/2 (IgG and IgM), CMV (IgM), Mycoplasma pneumoniae (IgG and IgM), Borrelia burgdorferi (IgG and IgM), Bartonella species (IgG and IgM), and syphilis (Venereal Disease Research Laboratory test) | Increased total protein (49 mg/dL), normal glucose levels (65 mg/dL), no leukocytes mg/dL), normal glucose levels (65 mg/dL), no leukocytes | Mixed demyelinating and axonal EMG subtype unknown | Brain: enhancement of the facial and abducens nerves bilaterally, as well as the right oculomotor nerve Spinal: no pathological findings | Plasma exchange (5 cycles) | NA | Discharged to inpatient rehabilitation |
| Juliao Caamaño et al. [26] | RT-PCR | NA | NA | Normal total protein (44 mg/dL), no pleocytosis | Absent blink-reflex EMG subtype unknown | Brain: no pathological findings | Oral prednisolone | Hydroxychloroquine and lopinavir/ritonavir for 14 days | Minimal improvement of muscle weakness after 2 weeks |
| Khalifa et al. [27] | RT-PCR + + chest X-ray chest X-ray + + chest CT chest CT | WBC 5.5 × × 103, PC 356 103, PC 356 × × 103, CRP 0.5 mg/dL (normal 0.0–0.5), serum ferritin 87.3 ng/ml (normal 12.0–150.0), elevated d-Dimer levels 0.72 mg/L (0.00–0.49) 103, CRP 0.5 mg/dL (normal 0.0–0.5), serum ferritin 87.3 ng/ml (normal 12.0–150.0), elevated d-Dimer levels 0.72 mg/L (0.00–0.49) | Negative screening for: influenza A and B viruses; influenza A virus subtypes H1, H3, and H5 including subtype H5N1 of the Asian lineage; parainfluenza virus types 1, 2, 3, and 4; respiratory syncytial virus types A and B; adenovirus; metapneumovirus; rhinovirus; enterovirus; Coronavirus 229E, HKU1, NL63, and OC43 | Cell count: 5 mm3, increased total protein (316.7 mg/dL) | Demyelinating AIDP | Brain: no pathological findings Spinal: enhancement of the cauda equina nerve roots | IVIG 1 g/kg (2 days) | Paracetamol, azithromycin, hydroxychloroquine | Discharge to home after 15 days with clinical and electrophysiological improvement |
| Kilinc et al. [28] | Fecal PCR + + serology serology | NA | Negative anti-GQ1b antibodies, serologic tests on Borrelia burgdorferi, syphilis, Campylobacter jejuni, CMV, hepatitis E, Mycoplasma pneumoniae and CMV | Normal cell count, normal proteins | Predominantly demyelinating AIDP | Brain: no pathological findings | IVIG 2 g/kg (5 days) | None | Persistence of mild symptoms at the discharge (after 14 days) |
| Lampe et al. [29] | RT-PCR (negative chest X-ray) | Slightly increased CRP (1.92 mg/dL) | Negative anti-ganglioside antibodies; negative influenza and respiratory syncytial virus | Increased total protein (56 mg/dL), normal cell count (2 cells/μL) | Demyelinating AIDP | NA | IVIG 400 mg/kg (5 days) | None | Improvement of GBS symptoms with persistence of generalized areflexia except for left biceps reflex, discharge after 12 days |
| Lantos et al. [30] | RT-PCR | NA | GM1 antibodies in the equivocal range | NA | NA | Brain: enlargement, prominent enhancement with gadolinium, and T2 hyperintense signal of the left cranial nerve III | IVIG | Hydroxychloroquine | Improvement, discharge after 4 days |
| Lascano et al. [31] | RT-PCR + + chest X-ray chest X-ray + + positive IgM (IgG positivity 2 weeks later) positive IgM (IgG positivity 2 weeks later) | WBC 8900 cells/mm3; lymphocytes 1200 cells/mm3; PC 45,500 cells/mm3 | Negative anti-ganglioside antibodies | Increased total protein (60 mg/dL), leucocytes: 3 cells/μL, negative SARS-CoV-2 PCR | Demyelinating AIDP | Spinal: no nerve root gadolinium enhancement | IVIG 400 mg/kg (5 days) invasive ventilation | Azithromycin | Improvement of tetraparesis. Able to stand up with assistance. |
| Lascano et al. [31] | RT-PCR + + chest X-ray chest X-ray | WBC 3300 cells/mm3; lymphocytes 800 cells/mm3; PC 119,000 cells/mm3 | NA | Normal total protein (40 mg/dl), cell count: 2 cells/μL | Mixed demyelinating (conduction blocks) and axonal with sural sparing pattern Predominantly AIDP | NA | IVIG 400 mg/kg (5 days) | Amoxicillin, clarithromycin | Dismissal with full motor recovery. Persistence of LL areflexia and distal paraesthesia |
| Lascano et al. [31] | RT-PCR + + chest X-ray chest X-ray | WBC 4000 cells/mm3; lymphocytes 600 cells/mm3; PC 322,000 cells/mm3 | NA | Increased total protein (140 mg/dL), cell count: 4 cells/μL, negative SARS-CoV-2 PCR | Demyelinating with sural sparing pattern AIDP | Brain: no pathological findings Spinal cord: lumbosacral nerve root enhancement | IVIG 400 mg/kg (5 days) | Amoxicillin | Improvement of tetraparesis and ability to walk with assistance. Persistence of neuropathic pain and distal paraesthesia |
| Manganotti et al. [32] | RT-PCR + + chest CT chest CT | NA | Negative anti-ganglioside antibodies negative serum anti-HIV, anti-HBV, anti-HCV antibodies | Increased total protein (74.9 mg/dL), negative CSF PCR for bacteria, fungi, Mycobacterium tuberculosis, Herpes viruses, Enteroviruses, Japanese B virus and Dengue viruses | NA | Brain: no pathological findings | IVIG 400 mg/kg (5 days) | Lopinavir/ritonavir, hydroxychloroquine, antibiotic therapy, oxygen support (35%) | Resolution of all symptoms except for minor hyporeflexia at the LL |
| Manganotti et al. [33] | RT-PCR | IL-1: 0.2 pg/ml (< 0.001 pg/ml), IL-6: 113.0 pg/ml (0.8–6.4 pg/ml), IL-8: 20.0 pg/ml (6.7–16.2 pg/ml), TNF-α: 16.0 pg/ml (7.8–12.2 pg/ml) 0.001 pg/ml), IL-6: 113.0 pg/ml (0.8–6.4 pg/ml), IL-8: 20.0 pg/ml (6.7–16.2 pg/ml), TNF-α: 16.0 pg/ml (7.8–12.2 pg/ml) | Negative anti-ganglioside antibodies, negative HIV, HBV, HCV negative serological tests for autoimmune disorders | Increased total protein (52 mg/dl), leucocytes: 1 cell/mm3, negative SARS-CoV-2 PCR | Demyelinating AIDP | NA | IVIG 400 mg/kg/day (5 days) | Hydroxychloroquine, oseltamivir, darunavir, methylprednisolone and tocilizumab + + mechanical invasive ventilation mechanical invasive ventilation | Improvement of motor symptoms |
| Manganotti et al. [33] | RT-PCR | IL-1: 0.5 pg/ml (< 0.001 pg/ml), IL-6: 9.8 pg/ml (0.8–6.4 pg/ml), IL-8: 55.0 pg/ml (6.7–16.2 pg/ml), TNF- α: 16.0 pg/ml (7.8–12.2 pg/ml) 0.001 pg/ml), IL-6: 9.8 pg/ml (0.8–6.4 pg/ml), IL-8: 55.0 pg/ml (6.7–16.2 pg/ml), TNF- α: 16.0 pg/ml (7.8–12.2 pg/ml) | Negative anti-ganglioside antibodies, negative HIV, HBV, HCV negative serological tests for autoimmune disorders | Normal total protein (40 mg/dl), leucocytes: 1 cell/mm3, negative SARS-CoV-2 PCR | Mixed demyelinating and axonal EMG subtype unknown | Brain: no pathological findings | IVIG 400 mg/kg/day (5 days) | Hydroxychloroquine, lopinavir/ritonavir, methylprednisolone + + mechanical invasive ventilation mechanical invasive ventilation | Improvement of motor symptoms |
| Manganotti et al. [33] | RT-PCR | NA | Negative anti-ganglioside antibodies, negative HIV, HBV, HCV negative serological tests for autoimmune disordes | Increased total protein (72 mg/dL), leucocytes: 5 cell/mm3, negative SARS-CoV-2 PCR | Mainly demyelinating Predominantly AIDP | Brain: no pathological findings | IVIG 400 mg/kg/day (5 days) | Hydroxychloroquine, lopinavir/ritonavir, methylprednisolone | Improvement |
| Manganotti et al. [33] | RT-PCR | NA | NA | NA | Mixed demyelinating and axonal EMG subtype unknown | NA | Methylprednisolone 60 mg for 5 days | Methylprednisolone | Stationary |
| Manganotti et al. [33] | RT-PCR | IL-1: 0.2 pg/ml (< 0.001 pg/ml), IL-6: 32.7 pg/ml (0.8–6.4 pg/ml), IL-8: 17.8 pg/ml (6.7–16.2 pg/ml), TNF- α : 11.1 pg/ml (7.8–12.2 pg/ml), IL-2R: 1203.0 pg/ml (440.0–1435.0 pg/ml), IL-10: 4.6 (1.8–3.8 pg/ml) 0.001 pg/ml), IL-6: 32.7 pg/ml (0.8–6.4 pg/ml), IL-8: 17.8 pg/ml (6.7–16.2 pg/ml), TNF- α : 11.1 pg/ml (7.8–12.2 pg/ml), IL-2R: 1203.0 pg/ml (440.0–1435.0 pg/ml), IL-10: 4.6 (1.8–3.8 pg/ml) | Negative anti-ganglioside antibodies, negative HIV, HBV, HCV negative serological tests for autoimmune disordes | Increased total protein (53 mg/dL), leucocytes: 2 cell/mm3, negative SARS-CoV-2 PCR | Mixed demyelinating and axonal EMG subtype unknown | NA | IVIG 400 mg/kg/day (5 days) | Hydroxychloroquine, lopinavir/ritonavir, methylprednisolone, meropenem, linezolid, clarithromycin, fluconazole, doxycycline + + mechanical invasive ventilation mechanical invasive ventilation | Improvement |
| Marta-Enguita et al. [34] | RT-PCR + + chest CT chest CT | Thrombocytopenia, d-Dimer elevation | NA | NA | NA | NA | NA | NA | Death after 10 days |
| Mozhdehipanah et al. [35] | RT-PCR (negative chest CT) | Normal WBC, CRP and ESR | NA | Increased total protein (139 mg/dL), normal cell count, negative CSF HSV serology and gram stain and culture | Demyelinating AIDP | NA | Plasma exchange (5 cycles) | NA | Significant improvement of muscle weakness after 3 weeks, persistence of mild bifacial paresis |
| Mozhdehipanah et al. [35] | RT-PCR | Normal WBC, CRP and ESR | NA | Albuminocytological dissociation | NA | NA | IVIG 400 mg/kg/day (5 days) | NA | Complete recovery, except for the persistence of hyporeflexia |
| Mozhdehipanah et al. [35] | RT-PCR + + chest CT chest CT | Leucocytosis lymphopenia, elevated ESR and CRP | NA | Increased total protein (57 mg/dL), normal cell count and glucose (not further specified) | Axonal AMSAN | NA | IVIG 400 mg/kg/day (3 days) | Hydroxy chloroquine, lopinavir/ ritonavir | Death after 3 days from starting treatment with IVIG |
| Mozhdehipanah et al. [35] | RT-PCR + + chest CT chest CT | Leucocytosis, lymphopenia, elevated ESR and CRP | NA | Increased total protein (89 mg/dL), normal cell count and glucose (not further specified) | Demyelinating AIDP | NA | IVIG 400 mg/kg/day (5 days) | Hydroxy chloroquine, lopinavir/ ritonavir | No significant clinical improvement |
| Naddaf et al. [36] | Positive SARS-CoV-2 IgG (index value: 8.2, normal < < 0.8) and IgA 0.8) and IgA + + chest CT (negative RT-PCR) chest CT (negative RT-PCR) | Normal completed blood count, elevated d-dimer (690 ng/mL), ferritin (575 mcg/L), ESR (26 mm/h), alanine aminotransferase (73 U/L) | Negative anti-ganglioside antibodies negative HIV, syphilis, West Nile virus, Lyme disease testing, EBV and CMV serology consistent with remote infection, negative paraneoplastic evaluation | Increased total protein (273 mg/dL), total cells count: 2/mm3, negative CSF SARS-CoV-2 RT-PCR, negative meningitis/encephalitis panel, negative oligoclonal bands and IgG index | Demyelinating AIDP | Spine: smooth enhancement of the cauda equine roots | Plasma exchange (5 sessions) | Hydroxy chloroquine, zinc, methylprednisolone 40 mg bid for 5 days | Improvement of motor and gait examination. Persistence of slight ataxia without requiring gait aid |
| Oguz-Akarsu et al. [37] | RT-PCR + + chest MRT chest MRT + + chest CT chest CT | Mild neutropenia (1.49 cells/µL) and a high monocyte percentage (19.77) | HIV test negative | Normal total protein (32.6 mg/dL) with no leucocytes | Demyelinating with sural sparing pattern AIDP | Cervical and lumbar and spine: asymmetrical thickening and hyperintensity of post-ganglionic roots supplying the brachial and lumbar plexuses in STIR sequences | Plasma exchange (five sessions, one every other day) | Hydroxychloroquine, azithromycin | Marked neurological improvement after 2 weeks and she was able to walk without assistance |
| Ottaviani et al. [38] | RT-PCR + + chest CT chest CT | Lymphopenia, increased d-dimer, CRP and CK | Negative anti-ganglioside antibodies | Increased total protein (108 mg/dL), cell count: 0 cells/μL | Mainly demyelinating Predominantly AIDP | NA | IVIG 400 mg/kg (5 days) | Lopinavir/ritonavir, hydroxychloroquine | Progressive worsening with multi-organ failure |
| Padroni et al. [39] | RT-PCR + + chest CT chest CT | WBC 10.41 × 109/L (neutrophils 8.15 × 109/L), normal d-dimer | Negative screening for Mycoplasma pneumonia, CMV, Legionella pneumophila, Streptococcus pneumoniae, HSV, VZV, EBV, HIV-1, Borrelia burgdorferi; auto-antibodies not performed | Increased total protein (48 mg/dl), cell count: 1 × 106/L | Motor sensory axonal AMSAN | NA | IVIG 400 mg/kg (5 days) + + mechanical invasive ventilation mechanical invasive ventilation | NA | At day 6 from admission: ICU with mechanical invasive ventilation |
| Paterson et al. [40] | Definite diagnosis (not specified) (normal chest CT) | Increased neutrophils and CRP | NA | Increased total protein (0.5 g/L), leucocytes: 3 cells/μL (0–5), | Demyelinating AIDP | NA | IVIG + + mechanical invasive ventilation mechanical invasive ventilation | None | 17 days of hospitalisation, at discharge able to walk 5 m (across an open space) but incapable of manual work/running |
| Paterson et al. [40] | Definite diagnosis (not specified) (normal chest CT) | Increased CRP and fibrinogen | NA | Increased total protein (0.6 g/L) leucocytes: 2 cells/μL (0-5), Glucose 3.4 (mmol/L; 2.2-4.2) | Demyelinating AIDP | Brain: no pathological findings | IVIG | Mechanical invasive ventilation | 46 days (ongoing) of hospitalisation, still critical and requiring ventilation |
| Paterson et al. [40] | Definite diagnosis (not specified) (normal chest CT) | Not significant findings | NA | Increased total protein (0.9 g/L) leucocytes: | Demyelinating AIDP | Brain: no pathological findings | IVIG | NA | 7 days (ongoing) of hospitalisation, able to walk 5 m (across an open space) but incapable of manual work/running |
| Paybast et al. [41] | RT-PCR | NA | NA | Increased total protein (139 mg/dL), normal glucose and cell count, normal CSF viral serology, negative gram stain and culture | Mixed demyelinating and axonal EMG subtype unknown | NA | 5 sessions of therapeutic plasma exchange, intravenous bolus of labetalol to control sympathetic nervous system over-reactivity | Hydroxychloroquine sulphate 200 mg two times per day for a week | Persistence of generalized hyporeflexia, decreased light touch sensation in distal limbs, mild bilateral facial paresis, sympathetic over-reactivity successfully controlled with labetalol, |
| Paybast et al. [41] | RT-PCR | NA | NA | Albuminocytological dissociation | NA | NA | IVIG 20 g (5 days) | Hydroxychloroquine sulphate 200 mg two times per day for a week | Persistence of generalized hyporeflexia and decreased light touch sensation in distal limbs |
| Pfefferkorn et al. [42] | RT-PCR + + chest CT chest CT | NA | Negative anti-gangliosides antibodies | At admission: Normal total protein, cell count: 9/µL, negative SARS-CoV-2 PCR At day 13th: increased total protein (10.231 mg/L), normal cell count | Demyelinating AIDP | Spinal: massive symmetrical contrast enhancement of the spinal nerve roots at all levels of the spine including the cauda equina. Anterior and posterior nerve roots were equally affected | IVIG 30 g (5 days) + + mechanical invasive ventilation mechanical invasive ventilation + + plasma exchange plasma exchange | NA | At day 31 from admission: motor improvement with regression of facial and hypoglossal paresis but still needed mechanical ventilation |
| Rana et al. [43] | RT-PCR | NA | NA | NA | Demyelinating with sural sparing AIDP | Thoracic and lumbar spine: no evidence of myelopathy or radiculopathy | IVIG 400 mg/kg (5 days) | Hydroxychloroquine and azithromycin | On day 4 respiratory improvement, on day 7 rehabilitation |
| Reyes-Bueno et al. [44] | Serology (negative RT-PCR) | NA | Negative anti-ganglioside antibodies | Increased total protein (70 mg/dl), cell count: 5 cells/µl, albuminocytological dissociation | Demyelinating with alteration of the Blink-Reflex. Further EMG: polyradiculoneuropathy with proximal and brainstem involvement AIDP | NA | IVIG 400 mg/kg (5 days) + Gabapentin | NA | After the 18th day progressive improvement of facial and limb paresis, diplopia and pain. Consequent neurological rehabilitation |
| Riva et al. [45] | Chest CT + + serology (negative RT-PCR) serology (negative RT-PCR) | No pathological findings | Negative anti-ganglioside antibodies | Normal total protein and cells; negative PCR for SARS-CoV2, EBV, CMV, VZV, HSV 1–2, HIV | Demyelinating with sural sparing AIDP | Brain: NA Spinal: no pathological findings | IVIG 400 mg/kg (5 days) | None | Slowly improvement after the 10th day |
| Sancho-Saldaña et al. [46] | RT-PCR + + chest X-Ray chest X-Ray | NA | Negative anti-ganglioside antibodies | Increased total protein (0.86 g/L), cell count: 3 leucocytes | Demyelinating AIDP | Whole spine: brainstem and cervical meningeal enhancement | IVIG 400 mg/kg (5 days) | Hydroxychloroquine, azithromycin | Recovering by day 7 after the onset of weakness. |
| Scheidl et al. [47] | RT-PCR | No pathological findings | Negative Campylobacter Jejuni and Borrelia serology, negative ANA, anti-DNA, c-ANCA,p-ANCA | Increased total protein (140 g/L), albuminocytological dissociation | Demyelinating AIDP | Brain: NA Cervical spine: no pathological findings | IVIG 400 mg/kg (5 days) | None | Complete recovery |
| Sedaghat et al. [48] | RT-PCR + + chest CT chest CT | Increased WBC 14.6 × 103 (neutrophils 82.7%, lymphocytes 10.4%) and CRP | NA | NA | Motor sensory Axonal AMSAN | Brain: no pathological findings Spinal: two cervical intervertebral disc herniations | IVIG 400 mg/kg (5 days) | Hydroxychloroquine, lopinavir/ritonavir, azithromycin | Not reported |
| Sidig et al. [49] | RT-PCR + + chest CT chest CT | NA | NA | None | Demyelinating AIDP | Brain: no pathological findings | NA | NA | Death after 7 days; because of progressive respiratory failure |
| Su et al. [50] | RT-PCR + + chest X-ray chest X-ray | WBC 12,000 cells/µl | Negative anti- ganglioside GM1, GD1b and GQ1b antibodies, acetylcholine receptor binding, voltage-gated calcium channel, antinuclear and ANCA | Increased total protein (313 mg/dL), WBC: 1 cell | Demyelinating AIDP | NA | IVIG 2gm/kg (for 4 days) | None | On day 28 persistence of severe weakness |
| Tiet et al. [51] | RT-PCR | Elevated lactate on venous blood gas (3.3 mmo/L), mildly elevated CRP (20 mg/L). Normal WBC, sodium, potassium and renal function. | NA | Increased total protein (> 1.25 g/L), cell count 1x106/L 1.25 g/L), cell count 1x106/L | Demyelinating AIDP | NA | IVIG 400 mg/kg/day (5 days) | None | Resolution of facial diplegia, improved upper and lower limbs weakness; able to mobilize unassisted 11 weaks after neurorehabilitation |
| Toscano et al. [52] | RT-PCR + + Chest CT Chest CT + + serology serology | Lymphocytopenia, increased CRP, LDH, ketonuria | Negative anti-ganglioside antibodies | Day 2: normal total protein, no cells, negative SARS-CoV-2 PCR Day 10: increased total protein (101) mg/dl, cell count: 4/mm3, negative SARS-CoV-2 PCR | Axonal with sural sparing AMSAN | Brain: no pathological findings Spinal: Enhancement of caudal nerve roots | IVIG 400 mg/kg (2 cycles) + + temporary mechanical non-invasive ventilation temporary mechanical non-invasive ventilation | Paracetamol | At week 4 persistence of severe UL weakness, dysphagia, and LL paraplegia |
| Toscano et al. [52] | RT-PCR (negative chest CT) | Lymphocytopenia; increased ferritin, CRP, LDH | NA | Increased total protein (123 mg/dl), no cells, negative SARS-CoV-2 PCR | Motor sensory axonal with sural sparing AMSAN | Brain: enhancement of facial nerve bilaterally Spinal: no pathological findings | IVIG 400 mg/kg | Amoxycillin | At week 4 improvement of ataxia and mild improvement of facial weakness |
| Toscano et al. [52] | RT-PCR + + chest CT chest CT | Lymphocytopenia; increased CRP, LDH, ketonuria | Negative anti-ganglioside antibodies | Increased total protein (193 mg/dl), no cells, negative SARS-CoV-2 PCR | Motor axonal AMAN | Brain: no pathological findings Spinal: enhancement of caudal nerve roots | IVIG 400 mg/kg (2 cycles) + mechanical invasive ventilation | Azythromicin | ICU admission due to respiratory failure and tetraplegia. At week 4 still critical |
| Toscano et al. [52] | RT-PCR + serology (negative chest CT) | Lymphocytopenia; increased CRP, ketonuria | NA | Normal protein, no cells, negative SARS-CoV-2 PCR | Demyelinating AIDP | Brain: no pathological findings Spinal: no pathological findings | IVIG 400 mg/kg | None | At week 4 mild improvement in UL but unable to stand |
| Toscano et al. [52] | Chest CT + serology (negative RT-PCR in nasopharyngeal swab and BAL) | Lymphocytopenia; increased CRP, LDH | Negative anti-ganglioside antibodies; negative screening for Campylobacter jejuni, EBV, CMV, HSV, VZV, influenza, HIV | Normal total protein (40 mg/dL), white cell count 3/mm3; negative SARS-CoV-2 PCR | Demyelinating AIDP | Brain: NA Spinal: no pathological findings | IVIG 400 mg/kg + + plasma exchange plasma exchange + + mechanical invasive ventilation mechanical invasive ventilation + + enteral nutrition enteral nutrition | None | At week 4 flaccid tetraplegia, dysphagia, ventilation dependent |
| Velayos Galán et al. [53] | RT-PCR + + chest X-ray chest X-ray | NA | NA | NA | Demyelinating AIDP | NA | IVIG 400 mg/kg (5 days) | Hydroxychloroquine, lopinavir/ritonavir, amoxicillin, corticosteroids + + low-flow oxygen therapy low-flow oxygen therapy | NA |
| Virani et al. [54] | rt-pcr + + chest mrt chest mrt | WBC 8.6 × 103; Hb 15.4 g/dl; PC 211 × 103; procalcitonin: 0.15 ng/ml | NA | NA | NA | Brain: NA Spinal: no pathological findings | IVIG 400 mg/kg (5 days) + + mechanical invasive ventilation (4 days) mechanical invasive ventilation (4 days) | Hydroxychloroquine 400 mg bid for first 2 doses, then 200 mg bid for 8 doses | At day 4 of IVIG: liberation from mechanical ventilation, resolution of UL symptoms, persistence of LL weakness. Sent to a rehabilitation facility |
| Webb et al. [55] | RT-PCR + + chest X-ray chest X-ray + + chest CT chest CT | Lymphopenia (0.9 × × 109/L), thrombocytosis (490 109/L), thrombocytosis (490 × × 109/L) raised CRP (25 mg/L) 109/L) raised CRP (25 mg/L) | Negative ANA, ANCA, anti-ganglioside antibodies, syphilis serology HIV, hepatitis B and hepatitis C | Increased total protein (0.51 g/L), normal glucose and cell count, negative SARS-CoV-2 PCR, negative viral PCR | Demyelinating AIDP | NA | IVIG 400 mg/kg/day (5 days) ventilation | Co-amoxiclav | After 1 week in ICU: no oxygen requirement and ventilation |
| Zhao et al. [56] | RT-PCR + + chest CT chest CT | WBC 0.52 × 109; PC 113 × 109/L | NA | Increased total protein (124 mg/dL), cell count 5 × 106/L | Demyelinating AIDP | NA | IVIG (dosing not reported) | Arbidol, lopinavir/ ritonavir | At day 30 resolution of neurological and respiratory symptoms |
AIDP, acute inflammatory demyelinating polyneuropathy; AMAN, acute motor axonal neuropathy; AMSAN, acute motor sensory axonal neuropathy; ANA, antinuclear antibodies; ANCA, anti-neutrophil cytoplasmic antibodies; BAL, bronchoalveolar lavage; CK, creatine kinase; CMV, cytomegalovirus; COPD, chronic obstructive pulmonary disease, COVID-19, coronavirus disease 2019; CRP, C-reactive protein; CSF, cerebrospinal fluid; CT, computed tomography; DM, diabetes mellitus; EBV, Epstein–Barr virus; ESR, erythrocyte sedimentation rate; F, female; GBS, Guillain–Barré syndrome; GGT, gamma-glutamyl transferase; GOT, glutamic oxaloacetic transaminase; GPT, glutamate pyruvate transaminase; Hb, haemoglobin; HIV, human immunodeficiency virus; HSV, herpex simplex virus; ICU, intensive-care unit; IL, interleukin; IVIG, intravenous immunoglobulins; IL, interleukin; LDH, lactate dehydrogenase; LL, lower limbs; M, male; MRI, magnetic resonance imaging; NA, not available; PC, platelet count; PCR, Polymerase Chain Reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; TNF, tumor necrosis factor; UL, upper limbs; VDRL, Veneral Disease Research Laboratory; VZV, varicella-zoster virus; WBC, white blood cells; X-ray: radiography
aTime to Nadir refers to days elapsed between the onset of neurological symptoms and the development of the worst clinical picture when no progression was reported nadir was considered concomitant with GBS symptoms onset
bAccording to Brighton diagnostic criteria [66]
Epidemiological distribution and demographic characteristics of the patients
To date, GBS cases (n =
= 73) were reported from all continents except Australia. In details, patients were originally from Italy (n
73) were reported from all continents except Australia. In details, patients were originally from Italy (n =
= 20), Iran (n
20), Iran (n =
= 10), Spain (n
10), Spain (n =
= 9), USA (n
9), USA (n =
= 8), United Kingdom (n
8), United Kingdom (n =
= 5), France (n
5), France (n =
= 4), Switzerland (n
4), Switzerland (n =
= 4), Germany (n
4), Germany (n =
= 3), Austria (n
3), Austria (n =
= 1), Brazil (n
1), Brazil (n =
= 1), Canada (n
1), Canada (n =
= 1), China (n
1), China (n =
= 1), India (n
1), India (n =
= 1), Morocco (n
1), Morocco (n =
= 1), Saudi Arabia (n
1), Saudi Arabia (n =
= 1), Sudan (n
1), Sudan (n =
= 1), The Netherlands (n
1), The Netherlands (n =
= 1), and Turkey (n
1), and Turkey (n =
= 1) (Table 1, Fig. 1). The mean age at onset was 55
1) (Table 1, Fig. 1). The mean age at onset was 55 ±
± 17 years (min 11–max 94), including four pediatric cases [21, 27, 35, 41]. A significative prevalence of men compared to women was noticed (50 vs. 23 cases: 68.5% vs. 31.5%) with no significant difference in age at onset between men and women (mean: 55
17 years (min 11–max 94), including four pediatric cases [21, 27, 35, 41]. A significative prevalence of men compared to women was noticed (50 vs. 23 cases: 68.5% vs. 31.5%) with no significant difference in age at onset between men and women (mean: 55 ±
± 18 vs. 56
18 vs. 56 ±
± 16 years, p
16 years, p =
= 0.643). Comorbidities were variably reported with no prevalence of a particular disease.
0.643). Comorbidities were variably reported with no prevalence of a particular disease.
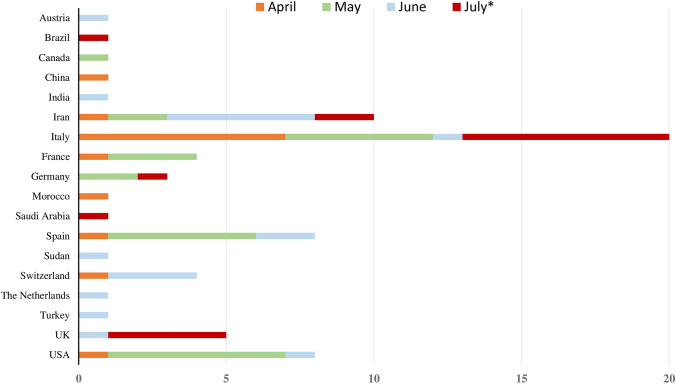
Temporal and spatial distribution of reported cases with COVID-19-associated Guillain–Barré syndrome in literature from 1st January until 20th July 2020. The x-axis shows the number of described patients. The y-axis illustrates the countries of provenience of the cases. In each line, different colours represent the months of April, May, June, and July (* until 20th July) 2020, in which the cases were published. Abbreviations: UK, United Kingdom, USA, United States of America
Clinical picture, diagnosis, and therapy of COVID-19
All reported GBS cases (n =
= 72) except two were symptomatic for COVID-19 with various severity. Most common manifestations of COVID-19 included fever (73.6%, 53/72), cough (72.2%, 52/72), dyspnea and/or pneumonia (63.8%, 46/72), hypo-/ageusia (22.2%, 16/72), hypo-/anosmia (20.8%, 15/72), and diarrhea (18.1%, 13/72). One of the two asymptomatic subjects never developed fever, respiratory symptoms, or pneumonia [10], whereas the other patient showed an asymptomatic pneumonia at chest computed tomography (CT) [12]. In all but six patients with available data [22, 24, 36, 44, 45, 52], SARS-CoV-2 RT-PCR with naso- or oropharyngeal swab or fecal exam was positive at first or following tests. Nevertheless, these six patients tested positive at SARS-CoV-2 serology. In four patients, the laboratory exam for the diagnostic confirmation was not specified [20, 40]. Typical “ground glass” aspects at chest-CT or similar findings at CT, Magnetic Resonance Imaging (MRI) or X-ray compatible with COVID-19 interstitial pneumonia were reported in 40 cases. The detailed therapies for COVID-19 are described in Table 1.
72) except two were symptomatic for COVID-19 with various severity. Most common manifestations of COVID-19 included fever (73.6%, 53/72), cough (72.2%, 52/72), dyspnea and/or pneumonia (63.8%, 46/72), hypo-/ageusia (22.2%, 16/72), hypo-/anosmia (20.8%, 15/72), and diarrhea (18.1%, 13/72). One of the two asymptomatic subjects never developed fever, respiratory symptoms, or pneumonia [10], whereas the other patient showed an asymptomatic pneumonia at chest computed tomography (CT) [12]. In all but six patients with available data [22, 24, 36, 44, 45, 52], SARS-CoV-2 RT-PCR with naso- or oropharyngeal swab or fecal exam was positive at first or following tests. Nevertheless, these six patients tested positive at SARS-CoV-2 serology. In four patients, the laboratory exam for the diagnostic confirmation was not specified [20, 40]. Typical “ground glass” aspects at chest-CT or similar findings at CT, Magnetic Resonance Imaging (MRI) or X-ray compatible with COVID-19 interstitial pneumonia were reported in 40 cases. The detailed therapies for COVID-19 are described in Table 1.
Clinical features of GBS spectrum
In all (n =
= 72) but four patients [10, 37, 40, 56], GBS manifestations developed after those of COVID-19 [median (IQR): 14 (7–20), min 2–max 33 days]. Differently, COVID-19 symptoms began concurrent in one case [37], 1 day [40] and 8 days [55] after GBS onset in two other cases and never developed in another one [10] (Table 1). Common clinical manifestations at onset included sensory symptoms (72.2%, 52/72) alone or in combination with paraparesis or tetraparesis (65.2%, 47/72, respectively). Cranial nerve involvement (e.g., facial, oculomotor nerves) was less frequently described at onset (16.7%, 12/72). Moreover, all cases but one [26] showed lower limbs or generalized areflexia, whereas in 37.5% (27/72) of the cases, gait ataxia was reported at onset or during the disease course. Even if ascending weakness evolving into flaccid tetraparesis (76.4%, 55/72) and spreading/persistence of sensory symptoms (84.7%, 61/72) represented the most common clinical evolutions, 50.0% (36/72) and 23.6% (17/72) patients showed cranial nerve deficits and dysphagia, respectively, during disease course (Table 1). Moreover, 36.1% (26/72) of the patients developed respiratory symptoms, and some of them evolved to respiratory failure (Table 1). Autonomic disturbances were rarely reported (16.7%, 12/72). In cases with MFS/MFS-GBS overlap, areflexia, oculomotor disturbances, and ataxia were present in 100% (9/9), 66.7% (6/9) and 66.7% (6/9), respectively [8, 19, 23, 30, 32, 33, 43, 44]. The median of time to nadir was calculated in 40 patients with available data and resulted 4 days (IQR 3–9) (Table (Table11).
72) but four patients [10, 37, 40, 56], GBS manifestations developed after those of COVID-19 [median (IQR): 14 (7–20), min 2–max 33 days]. Differently, COVID-19 symptoms began concurrent in one case [37], 1 day [40] and 8 days [55] after GBS onset in two other cases and never developed in another one [10] (Table 1). Common clinical manifestations at onset included sensory symptoms (72.2%, 52/72) alone or in combination with paraparesis or tetraparesis (65.2%, 47/72, respectively). Cranial nerve involvement (e.g., facial, oculomotor nerves) was less frequently described at onset (16.7%, 12/72). Moreover, all cases but one [26] showed lower limbs or generalized areflexia, whereas in 37.5% (27/72) of the cases, gait ataxia was reported at onset or during the disease course. Even if ascending weakness evolving into flaccid tetraparesis (76.4%, 55/72) and spreading/persistence of sensory symptoms (84.7%, 61/72) represented the most common clinical evolutions, 50.0% (36/72) and 23.6% (17/72) patients showed cranial nerve deficits and dysphagia, respectively, during disease course (Table 1). Moreover, 36.1% (26/72) of the patients developed respiratory symptoms, and some of them evolved to respiratory failure (Table 1). Autonomic disturbances were rarely reported (16.7%, 12/72). In cases with MFS/MFS-GBS overlap, areflexia, oculomotor disturbances, and ataxia were present in 100% (9/9), 66.7% (6/9) and 66.7% (6/9), respectively [8, 19, 23, 30, 32, 33, 43, 44]. The median of time to nadir was calculated in 40 patients with available data and resulted 4 days (IQR 3–9) (Table (Table11).
Results of electrophysiological, CSF, biochemical, and neuroimaging investigations
Detailed electroneurography results were reported in 84.9% (62/73) of the cases. Specifically, 77.4% (48/62) cases showed a pattern compatible with a demyelinating polyradiculoneuropathy. In contrast, axonal damage was prominent in 14.5% (9/62). In a minority of the patients (8.1%), a mixed pattern was reported (5/62). Regarding CSF analysis (full results were available in 59 out of 73 cases), the classical albuminocytological dissociation (cell count <
< 5/µl with elevated CSF proteins) was detected in 71.2% of the cases (42/59) with a median CSF protein of 100.0 mg/dl (min: 49, max: 317 mg/dl). Mild pleocytosis (i.e., cell count
5/µl with elevated CSF proteins) was detected in 71.2% of the cases (42/59) with a median CSF protein of 100.0 mg/dl (min: 49, max: 317 mg/dl). Mild pleocytosis (i.e., cell count ≥ 5/µl), with a maximum cell count of 13/µl, was evident in 5/59 cases (8.5%). Furthermore, CSF SARS-CoV-2 RNA was undetectable in all tested patients (n
≥ 5/µl), with a maximum cell count of 13/µl, was evident in 5/59 cases (8.5%). Furthermore, CSF SARS-CoV-2 RNA was undetectable in all tested patients (n =
= 31) (Table (Table11).
31) (Table (Table11).
Detailed blood haematological and biochemical examinations showed variably leucocytosis (n =
= 4), leucopenia (n
4), leucopenia (n =
= 17), thrombocytosis (n
17), thrombocytosis (n =
= 3), thrombocytopenia (n
3), thrombocytopenia (n =
= 5), and increased levels of C-reactive protein (CRP) (n
5), and increased levels of C-reactive protein (CRP) (n =
= 22), erythrocyte sedimentation rate (n
22), erythrocyte sedimentation rate (n =
= 4), d-Dimer (n
4), d-Dimer (n =
= 5), fibrinogen (n
5), fibrinogen (n =
= 3), ferritin (n
3), ferritin (n =
= 3), LDH (n
3), LDH (n =
= 7), IL-6 (n
7), IL-6 (n =
= 4), IL-1 (n
4), IL-1 (n =
= 3), IL-8 (n
3), IL-8 (n =
= 3), and TNF-α (n
3), and TNF-α (n =
= 3) (Table (Table11).
3) (Table (Table11).
Furthermore, anti-GD1b and anti-GM1 antibodies were positive in one patient with MFS [23] and in one with classic sensorimotor GBS [13], respectively, whereas 33 cases tested negative (one in equivocal range) for anti-ganglioside antibodies.
Cranial and spinal MRI scans were performed in a minority of the patients (23/73, 31.5%). Five patients (three cases with AIDP [9, 12, 25], one case with MFS [30], and one case with bilateral facial palsy with paresthesia [52]) showed cranial nerve contrast enhancement in the context of correspondent cranial nerve palsies. Moreover, brainstem leptomeningeal enhancement was described in two cases with AIDP, both with clinical cranial nerve involvement [18, 46]. On the other hand, spinal nerve roots and leptomeningeal enhancement were reported in eight [9, 27, 31, 36, 37, 42, 52] and two cases [17, 46], respectively (Table (Table11).
Distribution of clinical and electrophysiological variants and diagnosis of GBS
From the clinical point of view, most examined patients presented with a classic sensorimotor variant (70.0%, 51/73), whereas Miller Fisher syndrome, GBS/MFS overlap variants (including polyneuritis cranialis), bilateral facial palsy with paresthesia, pure motor, and paraparetic were described in seven, two, five, four, and one patients, respectively. In three cases, no clinical variant could be established using the reported details (Table (Table1).1). In the examined population, 81.8% subjects fulfilled electrophysiological criteria for AIDP (45/55), 12.7% (7/55) for AMSAN, and 5.4% (3/55) for AMAN subtypes. Finally, a specific electrophysiological subtype was not attributable in 18 patients due to the lack of detailed information. The diagnosis of GBS was established based on clinical, CSF, and electrophysiological findings in 44/73 (60.3%) patients, clinical, and electrophysiological data in 18/73 (24.7%) cases, clinical, and CSF data in 8/73 (11.0%), and only clinical findings in 3/73 (4.1%) patients. Indeed, the highest level of diagnostic certainty (level one) was confirmed in 44/73 cases (60.3%). Level two and three were obtained in 24/73 cases (32.9%) and 5/73 (6.8%), respectively (Table (Table11).
Management of GBS and patient outcomes
All cases with available therapy data (n =
= 70) except ten [13, 15, 23, 25, 26, 33, 35–37, 41] were treated with intravenous immunoglobulin (IVIG) (Table 1). Conversely, plasma exchange and steroid therapy were performed in ten (four of them received also IVIG) and two cases, respectively. In two patients, no therapy was given. Mechanical or non-invasive ventilation was implemented in 21.4% (15/70) and 7.1% (5/70) patients due to worsening of GBS or COVID-19, respectively. At further observation (n
70) except ten [13, 15, 23, 25, 26, 33, 35–37, 41] were treated with intravenous immunoglobulin (IVIG) (Table 1). Conversely, plasma exchange and steroid therapy were performed in ten (four of them received also IVIG) and two cases, respectively. In two patients, no therapy was given. Mechanical or non-invasive ventilation was implemented in 21.4% (15/70) and 7.1% (5/70) patients due to worsening of GBS or COVID-19, respectively. At further observation (n =
= 68), 72.1% (49/68) patients demonstrated clinical improvement with partial or complete remission, 10.3% (7/68) cases showed no improvement, 11.8% (8/68) still required critical care treatment, and 5.8% (4/68) died (Table 1).
68), 72.1% (49/68) patients demonstrated clinical improvement with partial or complete remission, 10.3% (7/68) cases showed no improvement, 11.8% (8/68) still required critical care treatment, and 5.8% (4/68) died (Table 1).
Interestingly, patients with no improvement or poor outcome (n =
= 19) showed a slightly higher (but not significant) frequency of clinical history and/or a radiological picture of COVID-19 pneumonia (14/19, 73.7%) compared to those with a favorable prognosis (29/48, 60.4%, p
19) showed a slightly higher (but not significant) frequency of clinical history and/or a radiological picture of COVID-19 pneumonia (14/19, 73.7%) compared to those with a favorable prognosis (29/48, 60.4%, p =
= 0.541). Moreover, the former group of patients was significantly older (mean 62.7
0.541). Moreover, the former group of patients was significantly older (mean 62.7 ±
± 17.8 years, p
17.8 years, p =
= 0.011), but with comparable distribution of sex (p
0.011), but with comparable distribution of sex (p =
= 0.622) and electrophysiological subtypes (p
0.622) and electrophysiological subtypes (p =
= 0.144) and similar latency between COVID-19 and GBS (p
0.144) and similar latency between COVID-19 and GBS (p =
= 0.588) and nadir (p
0.588) and nadir (p =
= 0.825), compared to the latter (mean age 51.8
0.825), compared to the latter (mean age 51.8 ±
± 16.6 years). The same findings were confirmed even after excluding cases with no improvement from the analysis (to prevent a possible bias related to the short follow-up time).
16.6 years). The same findings were confirmed even after excluding cases with no improvement from the analysis (to prevent a possible bias related to the short follow-up time).
Discussion
COVID-19 pandemic prompts all efforts for the early recognition and treatment of its manifestations. In analogy to other viruses, belonging or not to the coronavirus family [63, 67], neurologic complications in COVID-19 are emerging as one of the most significant clinical chapters of this pandemic. In this regard, peripheral and central nervous system damage in COVID-19 has been postulated to be the consequence of two different mechanisms: 1) hematogenous (infection of endothelial cells or leucocytes) or trans-neuronal (via olfactory tract or other cranial nerves) dissemination to central nervous system in relation with viral neurotropism, and 2) abnormal immune-mediated response causing secondary neurological involvement [62, 68, 69]. The first mechanism is supposed to be responsible for the most common neurological symptoms developed by patients with COVID-19 (e.g., hypogeusia, hyposmia, headache, vertigo, and dizziness). In contrast, the second can lead to severe complications during or after the course of the illness, either dysimmune (e.g., myelitis, encephalitis, GBS) or induced by cytokine overproduction (hypercoagulable state and cerebrovascular events) [68, 69].
In the present systematic review, we reviewed clinical features, results of diagnostic investigations, and outcome in 73 cases of COVID-19-associated GBS spectrum [5–56].
In the present study, mean age at onset in patients with GBS largely overlapped that of classic COVID-19 subjects [70, 71]. However, pediatric cases with GBS have been increasingly reported in the literature [21, 27, 35, 41], suggesting that, with the spreading of the pandemic, a broader age range might be affected. Moreover, we found a higher prevalence of GBS in males compared to females, as previously reported for Zika virus–GBS [72]. This finding may also reflect the gender epidemiology of SARS-CoV-2. In this regard, males typically show a worse COVID-19 outcome compared to the females [70, 71], possibly due to a generally shorter life expectancy or to higher circulating Angiotensin-Converting-Enzyme 2 (ACE2) levels, the cellular receptor for SARS-CoV-2, in the former compared to the latter [71]. Moreover, given that GBS is a rare disease [57] the epidemiological distribution of the reported cases seems to reflect current worldwide outbreaks, with Europe being the “hottest” spot in March–May 2020 and USA together with Asia in the following period [73, 74]. On another issue, despite a few GBS cases seemed to have a para-infectious profile [10, 37, 38, 40, 55, 56] as described for Zika virus [75], all other reported patients developed neurological symptoms with a typical latency after COVID-19 (median time 14 days). This feature, together with the frequently reported negative nasopharyngeal swab at GBS onset [22, 24, 36, 44, 45, 52] and clinical improvement after IVIG therapy, seems to support the notion of a prominent post-infectious immune-mediated mechanism. However, in this context, the massive release of cytokines in COVID-19 may also contribute to the amplification of the dysimmune process underlying GBS [76, 77]. In this regard, the increase of blood inflammatory markers (e.g., CRP, IL-6, TNF-α, IL-1, etc.) in GBS tested cases may reinforce the hypothesis of a systemic inflammatory storm in COVID-19 [76, 77]. However, given the limited data, we could not perform an accurate analysis of the distribution and, eventually, prognostic value of inflammatory markers in COVID-19-associated GBS. Moreover, we cannot exclude that in cases with GBS developing before or together with COVID-19 symptoms, the disease might have progressed sub-clinically in the early phase to manifest afterwards with its typical systemic clinical picture. Indeed, two cases [10, 12], who tested positive for SARS-CoV-2, never developed COVID-19 respiratory or systemic symptoms and one of them showed an asymptomatic pneumonia at chest-CT [12]. However, only more extensive epidemiological and translational studies, with the aim to compare the characteristics of GBS associated or not with COVID-19, could clarify these issues.
In our population, most common clinical manifestations and distribution of clinical variants resemble those of classic GBS confirming the predominance of the sensorimotor syndrome compared to MFS and other rare variants [57–59, 66]. Similarly, the results of CSF analysis reflected typical neurochemical findings in non-COVID-19 GBS. In the latter, elevated CSF proteins and pleocytosis were described in about 50–80% [57, 78] and 11–15% cases, respectively [58, 79, 80], largely overlapping with the percentages in our cohort. In this regard, the mostly normal cell count, together with the absence of SARS-CoV-2 RNA in all tested CSF samples [6–9, 12–14, 16, 21–24, 31, 33, 36, 42, 44, 52, 55], makes the possibility of a direct invasion from SARS-CoV-2 into the nerve roots with intrathecal viral replication less probable. However, a possible bias might rely on the lack of systematic data concerning the latency between symptom onset and CSF sampling in COVID-19 GBS cases. On another issue, in a further case of MFS associated withCOVID-19, who came to our attention, we observed the absence of intrathecal synthesis of SARS-CoV-2 antibodies together with a massive increase of CSF phosphorylated neurofilament heavy chain (pNfH) and serum neurofilament light chain (NfL) proteins, supporting the role of neurochemical markers as easily implementable tools for the detection of nervous system affection in COVID-19-related diseases [81, 82].
At variance with CSF findings, we found a discrepancy concerning MRI findings between classic GBS and COVID-19-related GBS. Specifically, while most cases of the former group showed typically spinal root enhancement at MRI [83], in the latter group, in analogy with Zika-associated GBS, the same finding was less frequently reported [84]. However, caution should be warranted in the interpretation of these results, given that MRI findings might have been underestimated, due to lack of a sufficient number of exams in the context of pandemic-imposed restrictions in the routine clinical setting.
Regarding the distribution of GBS electrophysiological variants, our analysis showed that COVID-19-associated GBS manifests prevalently with AIDP and, to a lesser extent, with AMSAN and AMAN, in line with classic GBS in Western countries [66, 85]. Conversely, the observation of positive anti-GD1b antibodies in one COVID-19-related MFS patient and negative anti-ganglioside antibodies in other five cases appear in discordance with the high prevalence (≈ 90%) of anti-GQ1b antibodies among non-COVID-19 MFS cases [86], and may suggest different immune-mediated mechanisms. However, these results could not be generalized until a wider population would be tested.
90%) of anti-GQ1b antibodies among non-COVID-19 MFS cases [86], and may suggest different immune-mediated mechanisms. However, these results could not be generalized until a wider population would be tested.
In analogy to classic GBS, approximately one-fifth of COVID-19-associated GBS subjects required mechanical ventilation during hospitalisation [87]. In this regard, cases with no improvement or unfavorable outcome showed, in comparison to those with a good prognosis, an older age, confirming similar findings both in classic GBS [58, 88] and in COVID-19 [89], and a slightly higher frequency (without reaching a statistical significance) of past or concurrent COVID-19 pneumonia. However, given the short follow-up time in most cases, we could not reach a definite conclusion on the impact of past or concurrent COVID-19 restrictive syndrome due to pneumonia on the prognosis of GBS patients. Future prospective studies are needed to clarify this issue. Moreover, given that also preceding diarrhea (mostly caused by Campylobacter Jejuni infection) is a strong negative prognostic factor in classic GBS [57, 88], further prospective studies are needed to compare the severity of GBS related to COVID-19 to that associated with C. jejuni. Finally, in the context of respiratory failure and ventilation associated with COVID-19, the differential diagnosis should always take into consideration critical illness neuropathy and myopathy, which tend to develop later during the critical course [90]. Despite these findings, approximately one-third of COVID-19-related GBS patients showed no clinical and/or radiological evidence of pneumonia, providing evidence that GBS may also develop in the context of a paucisymptomatic or even asymptomatic COVID-19. However, given that among the GBS population only two asymptomatic COVID-19 patients were reported to date, we may speculate that, in most cases, a certain degree of lung injury (even minimal) or at least hematic dissemination (e.g., fever underlying significant viral load) is necessary to trigger the immuno-mediated process through lymphocytic recognition of self-antigens or molecular mimicry.
Major strengths of our review are the inclusion of a high number of patients, together with an in-depth analysis of the clinical and diagnostic features of COVID-19-associated GBS. We are aware that selection bias might have occurred, given that most reported cases to date have been described mostly in Europe (47 out of 73) and during COVID-19 highest spreading. Therefore, future extensive epidemiological studies are necessary to ascertain the nature of the association between COVID-19 and GBS (causal or coincidental). Moreover, we cannot exclude the possibility that at least some of the cases represent instances of CIDP, given the frequent absence of a follow-up longer than 2 months. On another issue, the low but possible evidence of an epidemiological link between vaccines and GBS development [57, 58] should aware the clinicians of the possible occurrence of GBS after COVID-19 vaccination in the long-term future.
In conclusion, based on the systematic review of 73 cases, we showed that the clinical picture of COVID-19-associated GBS seems to resemble that of classic GBS or Zika-associated GBS. Moreover, the chronological evolution, the response to IVIG, and the absence of SARS-CoV-2 RNA in CSF may suggest a prominent post-infectious immune-mediated mechanism rather than a para-infectious one. Although most cases were symptomatic for COVID-19, the preliminary report of a few patients without respiratory or systemic symptoms raises a significant healthcare issue, namely the importance of SARS-CoV-2 testing in all patients with suspected GBS during the pandemic, with the aim to provide an eventual rapid case isolation. Nevertheless, only further analyses on more comprehensive cohorts could help in clarifying better all these issues.
Acknowledgements
Open Access funding provided by Projekt DEAL. This work was in part supported by a COVID-19 grant from the state Baden-Württemberg.
Authors’ contributions
Conceptualization: all authors; methodology, formal analysis, and investigation: Samir Abu-Rumeileh, Ahmed Abdelhak, and Matteo Foschi; writing—original draft preparation: all authors; figure preparation: Matteo Foschi; writing—review and editing: all authors; supervision: Markus Otto and Hayrettin Tumani.
Compliance with ethical standards
The authors declare that they have no conflict of interest related to the content of this article.
For the present study, no authorization to an Ethics Committee was asked, because the original reports, nor this work, provided any personal information of the patients.
References
Full text links
Read article at publisher's site: https://doi.org/10.1007/s00415-020-10124-x
Read article for free, from open access legal sources, via Unpaywall:
https://link.springer.com/content/pdf/10.1007/s00415-020-10124-x.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1007/s00415-020-10124-x
Article citations
Explore genetic susceptibility association between viral infections and Guillain-Barré syndrome risk using two-sample Mendelian randomization.
J Transl Med, 22(1):890, 02 Oct 2024
Cited by: 0 articles | PMID: 39358724 | PMCID: PMC11446148
Circulating miRNAs in the Plasma of Post-COVID-19 Patients with Typical Recovery and Those with Long-COVID Symptoms: Regulation of Immune Response-Associated Pathways.
Noncoding RNA, 10(5):48, 02 Sep 2024
Cited by: 0 articles | PMID: 39311385 | PMCID: PMC11417918
Complex Neurological Sequelae: Axonal Guillain-Barré Syndrome Post COVID-19 in a Young Patient.
Cureus, 16(8):e67213, 19 Aug 2024
Cited by: 0 articles | PMID: 39295668 | PMCID: PMC11410110
Bilateral Facial Paralysis in an Atypical Clinical Onset of COVID-Associated Guillain-Barre Syndrome.
Cureus, 16(6):e63498, 30 Jun 2024
Cited by: 0 articles | PMID: 39081416 | PMCID: PMC11287485
Neurological Complications of COVID-19 Infection: A Comprehensive Review.
Cureus, 16(7):e65192, 23 Jul 2024
Cited by: 1 article | PMID: 39176347 | PMCID: PMC11341106
Review Free full text in Europe PMC
Go to all (227) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Guillain-Barré syndrome associated with SARS-CoV-2 infection: A systematic review and individual participant data meta-analysis.
J Peripher Nerv Syst, 25(4):335-343, 05 Nov 2020
Cited by: 39 articles | PMID: 33112450
Review
Concomitant Guillain-Barré Syndrome and COVID-19: A Meta-Analysis of Cases.
Medicina (Kaunas), 58(12):1835, 13 Dec 2022
Cited by: 7 articles | PMID: 36557036 | PMCID: PMC9788175
Review Free full text in Europe PMC
Guillain-Barré syndrome after SARS-CoV-2 infection in an international prospective cohort study.
Brain, 144(11):3392-3404, 01 Dec 2021
Cited by: 26 articles | PMID: 34553216 | PMCID: PMC8677532
[Clinical cases of Guillain-Barré and Miller-Fisher syndromes linked to COVID-19].
Rev Med Inst Mex Seguro Soc, 60(1):91-95, 01 Feb 2022
Cited by: 0 articles | PMID: 35274917 | PMCID: PMC10395868

 1
1



