Abstract
Free full text

COVID-19–Associated Miller Fisher Syndrome: MRI Findings
SUMMARY:
Miller Fisher syndrome, also known as Miller Fisher variant of Guillain-Barré syndrome, is an acute peripheral neuropathy that can develop after exposure to various viral, bacterial, and fungal pathogens. It is characterized by a triad of ophthalmoplegia, ataxia, and areflexia. Miller Fisher syndrome has recently been described in the clinical setting of the novel coronavirus disease 2019 (COVID-19) without accompanying imaging. In this case, we report the first presumptive case of COVID-19–associated Miller Fisher syndrome with MR imaging findings.
Miller Fisher syndrome (MFS), also known as Miller Fisher variant of Guillain-Barré syndrome, is an acute peripheral neuropathy that can develop after exposure to various viral, bacterial, and fungal pathogens. It is often immune-mediated and associated with anti-GQ1b antibodies, characterized by a triad of ophthalmoplegia, gait ataxia, and areflexia. Ophthalmoplegia is due to involvement of cranial nerves III, IV, or VI. Ataxia is thought to be due to cerebellar involvement, and areflexia is due to lower motor neuron involvement. MFS has recently been described in the clinical setting of the novel coronavirus disease 2019 (COVID-19) without accompanying imaging findings.1 While patients with COVID-19 typically present with fever, shortness of breath, and cough, neurologic manifestations, including headache, ataxia, cognitive impairment, anosmia, and stroke, have been reported.2-4 One retrospective review of 214 patients found neurologic symptoms in 36.4% of patients, with involvement of the CNS (24.8%) greater than the peripheral nervous system (8.9%).4 In this case, we report the first presumptive case of COVID-19–associated Miller Fisher syndrome with imaging.
A 36-year-old man with a remote history of left eye strabismus (asymptomatic for 30 years) was brought to the emergency department by ambulance, presenting with left eye drooping, blurry vision, and reduced sensation and paresthesia in both legs for 2 days. He was in his usual state of health until 4
years) was brought to the emergency department by ambulance, presenting with left eye drooping, blurry vision, and reduced sensation and paresthesia in both legs for 2 days. He was in his usual state of health until 4 days before presentation, when he developed viral symptoms in a COVID-19-endemic region, reporting subjective fevers, chills, and myalgia. Physical examination was notable for a partial left third nerve palsy and decreased sensation below the knees to all modalities. MR imaging of the brain, including high-resolution imaging of the orbits and retro-orbital region, with and without gadolinium, was notable for striking enlargement, prominent enhancement with gadolinium, and T2 hyperintense signal of the left cranial nerve (CN) III (Figure). No other cranial nerves demonstrated abnormal signal or enhancement characteristics. MR imaging of the brain had normal findings. No cerebellar lesions were seen to explain the patient’s ataxia. There were no findings of meningitis, encephalitis, demyelination, or infarction. MR imaging of the spine, which may have provided an imaging correlate for the patient’s areflexia, was not performed.
days before presentation, when he developed viral symptoms in a COVID-19-endemic region, reporting subjective fevers, chills, and myalgia. Physical examination was notable for a partial left third nerve palsy and decreased sensation below the knees to all modalities. MR imaging of the brain, including high-resolution imaging of the orbits and retro-orbital region, with and without gadolinium, was notable for striking enlargement, prominent enhancement with gadolinium, and T2 hyperintense signal of the left cranial nerve (CN) III (Figure). No other cranial nerves demonstrated abnormal signal or enhancement characteristics. MR imaging of the brain had normal findings. No cerebellar lesions were seen to explain the patient’s ataxia. There were no findings of meningitis, encephalitis, demyelination, or infarction. MR imaging of the spine, which may have provided an imaging correlate for the patient’s areflexia, was not performed.
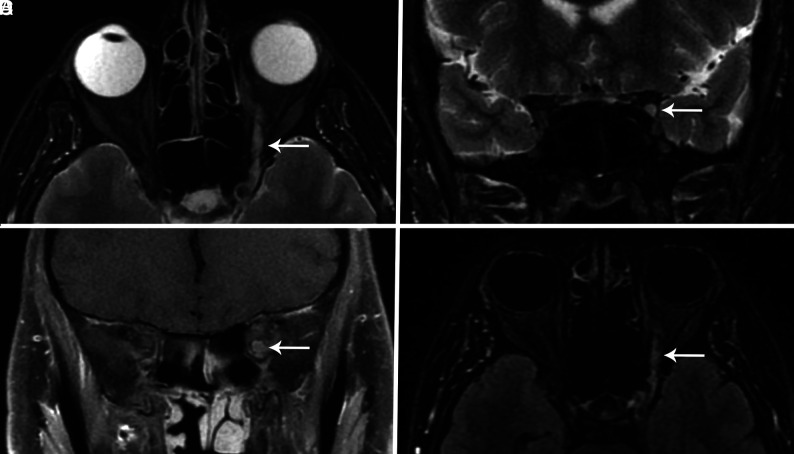
Axial and coronal T2-weighted fat-suppressed images through the orbits (A and B) demonstrate an enlarged CN III (arrow) with increased signal involving the nerve from the cavernous sinus through the orbit. Coronal T1-weighted fat-suppressed postcontrast image (C) also shows an enlarged CN III within the proximal orbit (arrow) and demonstrates marked enhancement of the nerve. The abnormal nerve is also visible on a whole-brain T2 FLAIR fat-suppressed postcontrast image (arrow, D).
The diagnosis of COVID-19 was confirmed by qualitative detection of Severe Acute Respiratory Syndrome coronavirus 2 RNA in a nasopharyngeal swab specimen by real-time reverse transcription polymerase chain reaction amplification and detection using TaqMan fluorescent oligonuecleotide probes (Altona Diagnostics) on the Rotor-Gene Q instrument. Serologic ganglioside antibody testing was performed by semi-quantitative enzyme-linked immunosorbent assay (ARUP Laboratories) and showed Asialo GM1 antibody in the equivocal range, while testing for other antibodies, including anti-GQ1b, was negative. Nonetheless, the patient’s hospital course was characterized by progressive ophthalmoparesis (including initial left CN III and eventual bilateral CN VI palsies), ataxia, and hyporeflexia, and the clinical picture was thought to be consistent with MFS from COVID-19 infection. The patient was treated with intravenous immunoglobulin, with subsequent improvement of neurologic symptoms. The patient also received hydroxychloroquine to treat the underlying COVID-19 infection. No repeat MR imaging was performed, and the patient was discharged after 4 days of hospitalization.
days of hospitalization.
MFS accounts for 1%–5% of cases of Guillain-Barré syndrome in Western countries, affects men twice as often as women, and is preceded by an upper respiratory illness in most patients.5 MFS presents commonly with diplopia (78%), ataxia (48%), or both (34%).6 Our patient presented with symptoms of COVID-19 as well as diplopia, which was found to be due to a CN III palsy noted on the patient’s clinical examination. MR imaging demonstrated corresponding enlargement, T2 hyperintensity, and enhancement of the affected CN III from the cavernous sinus through the orbit. This is the first presumptive case report of MFS associated with COVID-19 infection with imaging findings.
Although testing was negative for anti-GQ1b, the clinical picture was consistent with MFS, and the patient improved with treatment. Negative ganglioside antibody testing is a limitation of this report; however, a review of 123 patients with MFS found that 15% were negative for anti-GQ1b.7 The GQ1b ganglioside is a cell surface component that is concentrated in the paranodal regions, cranial nerves III, IV, and VI. Anti-GQ1b antibodies have been shown to bind to fractions of Campylobacter jejuni and Haemophilus influenzae and are thought to cause the symptoms of MFS through molecular mimicry.8
When antibody testing is negative in patients with MFS, symptoms may be due to viral neurotropism rather than immune-mediated injury.9 The functional receptor for COVID-19 is angiotensin-converting enzyme 2 and is present in neural tissue.4 Access to the CNS may be either hematogenous or via retrograde neural propagation along bipolar cells. Retrograde propagation along the olfactory pathway may account for the occurrence of anosmia in some patients with COVID-19.4 It has been proposed that retrograde propagation could lead to brain stem involvement and contribute to respiratory symptoms by affecting the nuclei that regulate respiratory rhythm such as chemoreceptors that detect changes in oxygen and CO2.10 Understanding of COVID-19 pathophysiology in the CNS and peripheral nervous system and its contribution to morbidity and mortality is still in its infancy. Whether MFS is the result of immune-mediated injury or viral neurotropism, this potential complication should be recognized by clinicians and radiologists so that appropriate treatment can be offered to these symptomatic patients.
ABBREVIATIONS:
| CN | cranial nerve |
| COVID-19 | coronavirus disease 2019 |
| MFS | Miller Fisher syndrome |
| CNS | central nervous system |
| PNS | peripheral nervous system |
References
Articles from AJNR: American Journal of Neuroradiology are provided here courtesy of American Society of Neuroradiology
Full text links
Read article at publisher's site: https://doi.org/10.3174/ajnr.a6609
Read article for free, from open access legal sources, via Unpaywall:
https://www.ajnr.org/content/ajnr/41/7/1184.full.pdf
Citations & impact
Impact metrics
Article citations
Neurological Complications of COVID-19 Infection: A Comprehensive Review.
Cureus, 16(7):e65192, 23 Jul 2024
Cited by: 1 article | PMID: 39176347 | PMCID: PMC11341106
Review Free full text in Europe PMC
Miller Fisher Syndrome Associated With COVID-19: A History of Molecular Mimicry and an Up-to-Date Review of the Literature.
Cureus, 15(8):e43111, 08 Aug 2023
Cited by: 1 article | PMID: 37692684 | PMCID: PMC10484161
Review Free full text in Europe PMC
Miller Fischer syndrome after COVID-19 infection and vaccine: a systematic review.
Acta Neurol Belg, 123(5):1693-1701, 19 Jul 2023
Cited by: 3 articles | PMID: 37468803 | PMCID: PMC10505097
Review Free full text in Europe PMC
Molecular mimicry and autoimmunity in the time of COVID-19.
J Autoimmun, 139:103070, 12 Jun 2023
Cited by: 10 articles | PMID: 37390745 | PMCID: PMC10258587
Review Free full text in Europe PMC
Third cranial nerve palsy due to COVID-19 infection.
BMJ Case Rep, 16(5):e255142, 03 May 2023
Cited by: 2 articles | PMID: 37137545 | PMCID: PMC10163548
Go to all (75) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
COVID-19 presenting with ophthalmoparesis from cranial nerve palsy.
Neurology, 95(5):221-223, 01 May 2020
Cited by: 177 articles | PMID: 32358218
Miller-Fisher-like syndrome related to SARS-CoV-2 infection (COVID 19).
J Neurol, 267(9):2495-2496, 26 May 2020
Cited by: 37 articles | PMID: 32458195 | PMCID: PMC7249969
Miller Fisher syndrome and COVID-19: is there a link?
BMJ Case Rep, 13(8):e236419, 11 Aug 2020
Cited by: 32 articles | PMID: 32784241 | PMCID: PMC7418682
Miller Fisher syndrome developing as a parainfectious manifestation of dengue fever: a case report and review of the literature.
J Med Case Rep, 13(1):120, 02 May 2019
Cited by: 5 articles | PMID: 31043165 | PMCID: PMC6495497
Review Free full text in Europe PMC
 a
a 




