Abstract
Background and aims
Type 1 diabetes (T1D) is associated with other autoimmune diseases (AIDs), but the prevalence and associated predictive factors for these comorbidities of T1D across all age groups have not been fully characterized.Materials and methods
Data obtained from 25 759 participants with T1D enrolled in the T1D Exchange Registry were used to analyze the types and frequency of AIDs as well as their relationships to gender, age, and race/ethnicity. Diagnoses of autoimmune diseases, represented as ordinal categories (0, 1, 2, 3, or more AIDs) were obtained from medical records of Exchange Registry participants.Results
Among the 25 759 T1D Exchange participants, 50% were female, 82% non-Hispanic white, mean age was 23.0 ± 16.9 years and mean duration of diabetes was 11 years. Of these participants, 6876 (27%) were diagnosed with at least one AID. Frequency of two or more AIDs increased from 4.3% in participants aged younger than 13 years to 10.4% in those aged 50 years or older. The most common AIDs were thyroid (6097, 24%), gastrointestinal (1530, 6%), and collagen vascular diseases (432, 2%). Addison's disease was rare (75, 0.3%). The prevalence of one or more AIDs was increased in females and non-Hispanic whites and with older age.Conclusions
In the T1D Exchange Clinic Registry, a diagnosis of one or more AIDs in addition to T1D is common, particularly in women, non-Hispanic whites, and older individuals. Results of this study have implications for both primary care and endocrine practice and will allow clinicians to better anticipate and manage the additional AIDs that develop in patients with T1D.Free full text

Autoimmune Diseases in Children and Adults With Type 1 Diabetes From the T1D Exchange Clinic Registry
Abstract
Background and Aims:
Type 1 diabetes (T1D) is associated with other autoimmune diseases (AIDs), but the prevalence and associated predictive factors for these comorbidities of T1D across all age groups have not been fully characterized.
Materials and Methods:
Data obtained from 25 759 participants with T1D enrolled in the T1D Exchange Registry were used to analyze the types and frequency of AIDs as well as their relationships to gender, age, and race/ethnicity. Diagnoses of autoimmune diseases, represented as ordinal categories (0, 1, 2, 3, or more AIDs) were obtained from medical records of Exchange Registry participants.
Results:
Among the 25 759 T1D Exchange participants, 50% were female, 82% non-Hispanic white, mean age was 23.0 ± 16.9 years and mean duration of diabetes was 11 years. Of these participants, 6876 (27%) were diagnosed with at least one AID. Frequency of two or more AIDs increased from 4.3% in participants aged younger than 13 years to 10.4% in those aged 50 years or older. The most common AIDs were thyroid (6097, 24%), gastrointestinal (1530, 6%), and collagen vascular diseases (432, 2%). Addison’s disease was rare (75, 0.3%). The prevalence of one or more AIDs was increased in females and non-Hispanic whites and with older age.
Conclusions:
In the T1D Exchange Clinic Registry, a diagnosis of one or more AIDs in addition to T1D is common, particularly in women, non-Hispanic whites, and older individuals. Results of this study have implications for both primary care and endocrine practice and will allow clinicians to better anticipate and manage the additional AIDs that develop in patients with T1D.
Type 1 diabetes (T1D) is an autoimmune disease that affects 1.25 million children and adults in the United States and many more worldwide (1, 2). In addition to the vascular and neuropathic complications of diabetes, a significant source of morbidity in T1D is the increased prevalence of other autoimmune diseases (AIDs), including hyper- and hypothyroidism, celiac disease, adrenal failure, inflammatory bowel diseases, collagen vascular diseases, skin diseases, and others (3–8). These diseases either occur sporadically or together in defined syndromes with distinct clinical characteristics (6, 9) and have insidious onsets that might not be readily identified by clinical signs and symptoms. Some, such as hormonal decompensations in adrenal crisis and thyroid storm, are potentially life threatening if not discovered and treated in a timely manner.
These additional AIDs can be mild and easily treated, such as hypothyroidism, or they can have a profound impact on growth (celiac and inflammatory bowel diseases), impair the management of T1D (Addison’s, hyperthyroidism), or cause other systemic problems (collagen vascular disease). Predicting susceptibility to this disparate group of conditions is important for both screening and for making diagnoses early, before the patient suffers harm. The utility of screening for associated AIDs in T1D is still debated (10–15). Adding to this controversy is the fact that the prevalence of these comorbidities of T1D has not been fully characterized, especially in older patients and in racial and ethnic minorities.
The T1D Exchange Clinic Network includes 80 US-based pediatric and adult endocrinology practices. A registry of patients with T1D commenced enrollment in September 2010 that included collection of a comprehensive set of clinical and laboratory data from the participant’s medical record and from participant/parent questionnaires (16). In this report, we analyzed the T1D Exchange Registry data to address the knowledge gap regarding prevalence of individual AIDs in T1D across all age groups. The large size of the database that includes more than 25 000 patients also allowed us to examine differences in the prevalence of these disorders with respect to gender, age, and race/ethnicity.
Materials and Methods
Study population
The study population included 25 759 individuals with T1D across all age groups from 1 to 93 years who participated in the type 1 Diabetes Exchange Clinic Network from 2010 to 2016. Informed consent was obtained from adult participants and parents/guardians of minors, with assent from minors as required. Data were collected for the registry’s central database both from the participant’s medical record and by having the participant or parent complete a comprehensive questionnaire, as previously published (16). The registry study was approved by the institutional review boards and ethics research committees at each of the participating clinics and the Jaeb Center for Health Research.
Data analysis
Analysis methods were defined a priori. Information pertaining to gender and race/ethnicity was obtained from a standard questionnaire completed by the participant or parent/guardian. Age, date of diabetes onset, hemoglobin A1c (HbA1c), and other medical history were collected by chart abstraction, which was standardized for all registry participants. Autoimmune diagnoses were established clinically based on signs and symptoms, available serological markers, other supporting laboratory results, medication use, and patient self-report or by parent report in case of young children. All patients were followed up by endocrinologists and received standard treatment for their AIDs. Mean HbA1c over the year before the registry assessment, calculated from all available for the previous year, was used to represent HbA1c in this analysis.
Statistical analysis
AID frequencies were defined according to the number of autoimmune diagnoses in addition to T1D and classified as 0, 1, 2, and 3 or more. Associations between one or more AIDs and each demographic and clinical characteristic were evaluated using logistic regression models. SAS software version 9.4 (2011; SAS Institute Inc) was used for data analysis. All P values are two sided, and a value of P < .01 was considered statistically significant.
Role of the funding source
The study sponsor had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. J.M. had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
The 25 759 participants ranged in age from 1 to 93 years. Whereas the mean age was 23.0 ± 16.9 years (Table 1), there were 7324 participants (28%) who were 26 years of age or older. Subjects were 50% female, 82% non-Hispanic white, and had a mean duration of diabetes of 10.6 years at the time of data collection. Median HbA1c ± SD was 8.4% ± 1. 6%. Additional cohort characteristics are shown in Table 1. Among the participants, 27% of study participants (n = 6870) had a diagnosis of at least one AID. Of these, 20% (n = 5262) had one; 5% (n = 1374) had two; less than 1% (n = 214) had three; less than 1% (n = 19) had four, and less than 1% (n = 1) had five AIDs.
Table 1
Study Participant Characteristics
| Characteristic | n, % (n = 25 759) |
|---|---|
| Gender, female | 12 895 (50%) |
| Age, y, mean ± SD | 23.0 ± 16.9 |
 <6 <6 | 1275 (5%) |
 6 to <13 6 to <13 | 6962 (27%) |
 13 to <18 13 to <18 | 6321 (25%) |
 18 to <26 18 to <26 | 3877 (15%) |
 26 to <50 26 to <50 | 4479 (17%) |
 50 to <65 50 to <65 | 2145 (8%) |
 ≥65 ≥65 | 700 (3%) |
| Diabetes duration, y, mean ± SD | 10.6 ± 11.8 |
| Age of diagnosis of T1D, y, mean ± SD | 11.6 ± 10.6 |
 <18 <18 | 21 528 (84%) |
 ≥18 ≥18 | 4231 (16%) |
| Race/ethnicity | |
 White non-Hispanic White non-Hispanic | 21 141 (82%) |
 Black non-Hispanic Black non-Hispanic | 1314 (5%) |
 Hispanic or Latino Hispanic or Latino | 2049 (8%) |
 Asian/Pacific Islander Asian/Pacific Islander | 344 (1%) |
 Native American Native American | 91 (<1%) |
 More than one race More than one race | 721 (3%) |
| Participants with coexisting AIDs | 6819 (26%) |
Factors associated with AIDs
Participants with one or more AIDs were more likely to be older (P < .001), female gender (P < .001 after adjusting for age and duration), and of white non-Hispanic race/ethnicity (P < .001 after adjusting for age and duration, Table 2). Although the frequency of one or more AIDs was greatest in non-Hispanic whites (29% of this racial group affected), the likelihood of one or more AIDs was also high in the other ethnic groups: 21% of Hispanic/Latino (n = 420), 12% of black non-Hispanics (n = 156), and 21% of other races (n = 243), illustrating increased autoimmune propensity in T1D-affected individuals of any race, compared with what would be expected in the general population (Table 2).
Table 2
Factors Associated With Additional AID Diagnosis
| Additional AID (Total = 6962), n (row %) | Odds Ratio (95% Confidence Interval)a | P Valuea | |
|---|---|---|---|
| Age, y | <.001 | ||
 <6 <6 | 243 (19%) | Referent | |
 6–<13 6–<13 | 1540 (22%) | 1.20 (1.04, 1.40) | |
 13–<18 13–<18 | 1504 (24%) | 1.32 (1.14, 1.54) | |
 18–<26 18–<26 | 917 (24%) | 1.31 (1.12, 1.54) | |
 26–<50 26–<50 | 1544 (34%) | 2.23 (1.91, 2.60) | |
 50–<65 50–<65 | 884 (41%) | 2.97 (2.52, 3.50) | |
 ≥65 ≥65 | 330 (47%) | 3.80 (3.10, 4.64) | |
| Diabetes duration, y | <.001 | ||
 <1 <1 | 588 (18%) | Referent | |
 1 to <10 1 to <10 | 3009 (23%) | 1.34 (1.22, 1.48) | |
 10 to <20 10 to <20 | 1502 (29%) | 1.84 (1.65, 2.06) | |
 20 to <50 20 to <50 | 1737 (40%) | 3.01 (2.70, 3.35) | |
 ≥50 ≥50 | 126 (48%) | 4.17 (3.22, 5.41) | |
| Genderb | <.001 | ||
 Male Male | 2496 (19%) | Referent | |
 Female Female | 4462 (35%) | 2.18 (2.06, 2.31) | |
| Race/ethnicityc | <.001 | ||
 Black non-Hispanic Black non-Hispanic | 158 (12%) | Referent | |
 White non-Hispanic White non-Hispanic | 6120 (29%) | 2.71 (2.30, 3.21) | |
 Hispanic or Latino Hispanic or Latino | 420 (21%) | 2.04 (1.70, 2.57) | |
 Other Other | 244 (21%) | 2.06 (1.65, 2.57) | |
| Age of Diagnosis of T1D, y | <.001 | ||
 <18 <18 | 5451 (25%) | Referent | |
 ≥18 ≥18 | 1511 (36%) | 1.34 (1.24, 1.44) | |
| HbA1c, %d | .02 | ||
 <7.0 <7.0 | 1097 (29%) | Referent | |
 ≥7.0 ≥7.0 | 5819 (27%) | 1.09 (1.00, 1.18) |
Participants with one or more AIDs were also more likely to have a longer T1D duration (P < .001) and be diagnosed with T1D later in life (P < .001 after adjusting for duration). However, the burden of additional AIDs was not associated with metabolic control after adjusting for confounding from age and T1D duration: frequency of one or more AIDs was 29% among participants meeting American Diabetes Association glycemic target of HbA1c less than 7.0% and 27% among those not meeting the target (P = .02; Table 2).
The distribution of AIDs by age is shown in Figure 1. Most affected participants across all age groups have a single AID (black bars), as opposed to more than one (gray bars). Notably, there is an age-dependent increase in the percentage affected with one AID starting in participants 18 years old and older with no plateau effect. An increase in the participants with two or more AIDs was also observed with older age.
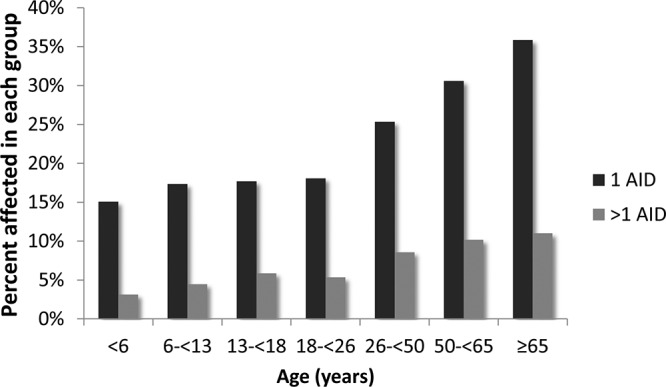
Age-dependent increase of additional AIDs. More study participants are affected by a single AID (black bars) than multiple AIDs (gray bars). Notably, there is an age-dependent increase in individuals affected with AIDs with no plateau effect, with 47% of those aged 65 years or older having one or more AIDs (n = 330 of 700).
Prevalence of AIDs by disease type
The types of AIDs and their frequencies are presented in Table 3. Thyroid diseases were the most common reported AID, affecting 20% (n = 5121) of all study participants. Among these, most had hypothyroidism or Hashimoto’s disease (n = 4895, 96% of all thyroid diseases), whereas fewer had hyperthyroidism or Graves’ disease (n = 400, 8% of all thyroid diseases). Less common were rheumatoid arthritis (RA; n = 244, <1%), vitiligo (n = 169, <1%), psoriasis (n = 170, <1%), and Addison’s disease (n = 76, <1%).
Table 3
AID Diagnosis According to Gender and Race/Ethnicity, n, %
| Overall (n = 25 759) | Gendera | Race/Ethnicityb | |||||
|---|---|---|---|---|---|---|---|
| Male (n = 12 855) | Female (n = 12 895) | Black Non-Hispanic (n = 1315) | White Non-Hispanic (n = 21 141) | Hispanic or Latino (n = 2048) | Other (n = 1156) | ||
| Additional AIDs, n | |||||||
 0 0 | 18 940 (74%) | 10 361 (81%) | 8574 (66%) | 1165 (89%) | 15 134 (72%) | 1643 (80%) | 919 (79%) |
 1 1 | 5225 (20%) | 2017 (16%) | 3204 (25%) | 123 (9%) | 4585 (22%) | 321 (16%) | 180 (16%) |
 2 2 | 1358 (5%) | 413 (3%) | 945 (7%) | 25 (2%) | 1205 (6%) | 74 (4%) | 52 (4%) |
 ≥3 ≥3 | 236 (<1%) | 64 (<1%) | 172 (1%) | 2 (<1%) | 217 (1%) | 10 (<1%) | 5 (<1%) |
| Thyroid disease | 5121 (20%) | 1709 (13%) | 3408 (26%) | 115 (9%) | 4526 (21%) | 288 (14%) | 178 (15%) |
 Hashimoto’s and/or hypothyroidism Hashimoto’s and/or hypothyroidism | 4895 (19%) | 1649 (13%) | 3242 (25%) | 104 (8%) | 4334 (21%) | 272 (13%) | 171 (15%) |
 Graves’ and/or hyperthyroidism Graves’ and/or hyperthyroidism | 400 (2%) | 103 (<1%) | 297 (2%) | 19 (1%) | 343 (2%) | 24 (1%) | 13 (1%) |
| Addison’s disease | 76 (<1%) | 36 (<1%) | 40 (<1%) | 0 | 72 (<1%) | 3 (<1%) | 1 (<1%) |
| GI disease | 1626 (6%) | 701 (5%) | 925 (7%) | 11 (<1%) | 1467 (7%) | 92 (4%) | 48 (4%) |
 Celiac Celiac | 1517 (6%) | 650 (5%) | 867 (7%) | 10 (<1%) | 1370 (6%) | 87 (4%) | 45 (4%) |
 Colitis Colitis | 79 (<1%) | 32 (<1%) | 47 (<1%) | 0 | 72 (<1%) | 4 (<1%) | 2 (<1%) |
 Crohn’s Crohn’s | 39 (<1%) | 21 (<1%) | 18 (<1%) | 2 (<1%) | 33 (<1%) | 1 (<1%) | 1 (<1%) |
| Collagen vascular disease | 456 (2%) | 149 (1%) | 307 (2%) | 10 (1%) | 396 (2%) | 27 (1%) | 14 (1%) |
 RA RA | 244 (<1%) | 62 (<1%) | 182 (1%) | 14 (1%) | 208 (<1%) | 16 (<1%) | 6 (<1%) |
 Psoriasis Psoriasis | 170 (<1%) | 87 (<1%) | 83 (<1%) | 1 (<1%) | 152 (<1%) | 8 (<1%) | 8 (<1%) |
 Lupus Lupus | 43 (<1%) | 3 (<1%) | 40 (<1%) | 2 (<1%) | 37 (<1%) | 3 (<1%) | 1 (<1%) |
 Scleroderma Scleroderma | 11 (<1%) | 1 (<1%) | 10 (<1%) | 1 (<1%) | 10 (<1%) | 0 | 0 |
| Skin disease | 272 (1%) | 117 (<1%) | 155 (1%) | 11 (<1%) | 221 (1%) | 26 (1%) | 13 (1%) |
 Vitiligo Vitiligo | 169 (<1%) | 81 (<1%) | 88 (<1%) | 6 (<1%) | 139 (<1%) | 13 (<1%) | 10 (<1%) |
 Alopecia Alopecia | 93 (<1%) | 34 (<1%) | 59 (<1%) | 4 (<1%) | 74 (<1%) | 11 (<1%) | 4 (<1%) |
 Dermatomyositis Dermatomyositis | 15 (<1%) | 2 (<1%) | 13 (<1%) | 1 (<1%) | 12 (<1%) | 2 (<1%) | 0 |
Abbreviation: GI, gastrointestinal. Table excludes diseases that were rare in our sample: multiple sclerosis (one participant) and Sjogren’s syndrome (one participant).
We observed a strong female predominance across the spectrum of AIDs. Of those with thyroid diseases, 67% (n = 3408) were female, with a similar distribution among those with Hashimoto’s thyroiditis (66% female) and Graves’ disease (74% female). Among collagen vascular diseases, systemic lupus erythematosus has by far the highest female predominance at 93% (n = 40), followed by scleroderma at 91% (n = 10) and RA at 75% (n = 182). Other disease categories showed minor female preponderance, ranging from 57% (n = 867) in celiac disease to 53% (n = 40) in Addison’s disease. As shown in Table 3, females also were more likely to have two or more AIDs compared with males.
Discussion
This is the largest US-based study assessing the frequency of AIDs in individuals with T1D. Although the T1D Exchange is not a population-based registry, our cohort represents the diverse sociodemographic and clinical features in children and adults with T1D throughout the United States. The overall prevalence of additional AIDs in our participants with T1D was 27%, which significantly exceeds the estimated 3%–8% prevalence of AIDs in the general population (17, 18) as well as some of the previously reported AID frequency in T1D populations (6, 19–22). Whereas our registry participants are divided evenly among female and male gender, most of those affected by additional AIDs are female (63%, n = 4321) compared with male (37%, n = 2494), which agrees with the established female predominance in the autoimmune literature.
The prevalence of specific AIDs in T1D differs based on disease type. Autoimmune thyroid disease is well recognized as the most common AID in T1D. We found autoimmune thyroid disease in 24% of our registry participants, a figure that is consistent with prior reports (23). Of note, some studies that relied on autoantibody testing yielded higher prevalence data, including Hanukoglu et al (24), who had shown 27% thyroidperoxidase and/or thyroglobulin antibody positivity in teens with T1D (n = 29 of 109), Barker et al (25), who reported 29% thyroid antibody positivity (n = 236 of 814), and Umpierrez et al (21), who reported 33% as having either autoantibodies or thyroid dysfunction (n = 19 of 58). Because ours was a retrospective observational study, autoantibody data were not available for all patients, which likely led to an underestimation of the true prevalence of thyroid autoimmunity in this population.
In other disease categories, our findings contrasted variably from existing data. Addison’s disease was seen in 0.3% (n = 75 of 25 759) of our T1D participants, lower than previously published 1.7% (n = 11 of 629) (14). RA was seen in less than 1% (n = 228) in our study, whereas its incidence has been reported as higher in other series, particularly in older patients (7, 27). Both these diseases tend to affect individuals after the age of 35–40 years; therefore, it is no surprise that they had lower rates in our cohort (mean age 23.0 y, median age 16.5 y). In contrast, celiac disease, which is often seen in the young, had a prevalence of 6% in our series, which fits within the 4%–11% range that has been previously reported (24–26, 28). The young age of our cohort also made it possible to miss a portion of adult-onset autoimmune diabetes patients, who tend to have a high frequency of associated autoimmunity, particularly of the thyroid (29, 30).
Our cohort demonstrated an age-related increase in AID frequency, with no threshold effect in the very young or a plateau effect in older age groups. Beginning with younger than 6 years of age, 18% of these children already exhibited one or more AIDs in addition to diabetes. The AID frequency remains largely stable through adolescence and early adulthood but increases after the age of 26 years. The older patients in our registry had the highest AID frequency, up to 42.2% of those older than 50 years (n = 1202) and 46.9% of those older than 65 years (n = 328). Without age-of-onset information for each AID diagnosis, we cannot distinguish new-onset AID in each age group from mere accumulation over time. However, there is strong evidence from the literature that certain AIDs cluster in older populations, including Sjogren’s, multiple sclerosis, and RA (27, 31–33), which may be the case in our series. Interestingly, despite what may be higher autoimmunity in the elderly, the clinical manifestations of late-onset disease are often milder, reflecting altered immune regulation and possibly expansion of protective regulatory mechanisms with advancing age (34–36). It is also noteworthy that the increased burden on AIDs did not appear to adversely affect overall metabolic control in our participants.
In addition to cohort size, another advantage of this study is the inclusion of a large number of black and Hispanic participants. For a disease such as T1D that most commonly affects non-Hispanic whites, the large number of black and Hispanic participants in the registry provided a unique opportunity to study the prevalence of AIDs in these minority populations. Whereas non-Hispanic white participants had the highest prevalence of AIDs, our data demonstrate that the prevalence of associated autoimmunity is also substantial in racial and ethnic minorities, including greater than 20% of Hispanic/Latino and 12% of black non-Hispanic T1D individuals. A significant difference likely exists in the prevalence of specific AIDs in each population. In support of this, studies have found an increased risk of systemic lupus erythematosus in Hispanic and black women as well as more prevalent Graves’ disease in non-Hispanic blacks and Asians (37, 38). In both lupus and Graves’ disease, early diagnosis and intervention can reduce end-organ damage and improve overall clinical outcomes; therefore, it is important that T1D providers be cognizant of the possibility of these AIDs in at-risk groups. With additional analyses of our data set in the future, we may be able to identify specific AID prevalence by race and to draw more conclusions about how genetic factors contribute to the development of each AIDs in the context of preexisting T1D.
Our data have several implications for AID screening in individuals with T1D. First, because there is a large increase in AID frequency after adolescence, screening efforts should be reinforced in the early-adult population (age 18–26 y) to identify clinically silent but developing tissue-specific autoimmunity. Screening may need to continue in patients with advanced age because our cohort showed no maximal age cutoff for identifying additional AIDs after an individual has developed T1D. In all age groups, thyroid disease and celiac disease should be a priority for screening, given their high prevalence and potential long-term complications of untreated disease (12, 39, 40). For other AIDs with lower prevalence, our evidence does not support routine screening, unless there are signs and symptoms suggestive of a new autoimmune diagnosis. Screening for Addison’s disease should be done for patients with a positive family history.
Limitations of this study include its retrospective nature and the relative young age of the cohort. Also, because we relied on clinical history rather than autoantibody positivity, AID prevalence was likely underestimated. The fact that the cohort is analyzed at a single time point also limits our ability to extrapolate true AID frequency in older age groups. Future studies should endeavor to identify AID risk at the earliest time point, whether it is autoantibody positivity or DNA analysis for human leukocyte antigen (HLA) genotyping. For example, children carrying the HLA-DRB1 and DQB1 alleles may be at increased susceptibility to celiac disease and RA in addition to T1D (41–43). Non-HLA loci including CTLA4, PD1, and pathways regulating inflammation have now been identified as potential modulators in T1D and may also predict susceptibility to other AIDs (44–48), including CTLA4 as a shared susceptibility gene for T1D and autoimmune thyroid disease (49, 50). These markers may reveal why it is that AIDs tent to cosegregate in certain demographic groups and what are the contributions from genetic vs environmental factors for the development of AIDs. Future studies should also include date of onset for AIDs to show whether the presence of T1D affects the development of other autoimmune diseases, or vice versa, and to better understand disease associations.
Conclusion
Overall, the high prevalence (27%) and wide spectrum of AIDs in our study reinforces the need to identify AIDs during the follow-up of patients with T1D. The most frequent T1D-associated autoimmune diseases include Hashimoto’s thyroiditis, celiac disease, Graves’ disease, RA, and vitiligo. These findings have implications for screening practices and pretest probability of identifying autoimmune disease, suggesting that clinicians should remain vigilant to the possibility of other autoimmune diseases even in older T1D patients.
Acknowledgments
We thank Jeffrey Saunders for coordinating the manuscript preparations and the members of the T1D Exchange Steering Committee who provided comments on the manuscript including William Tamborlane, MD; Roy W. Beck, MD, PhD; and David Maahs, MD, PhD.
Author contributions included the following: J.H. and J.M. designed the study. T.R. and K.M. did the statistical analysis. J.H., T.R., K.M., and J.M. interpreted the findings. J.H. and J.M. performed the literature search, prepared the figures, and wrote the manuscript. All authors contributed to the critical reading and revision of the manuscript.
The study sponsor had no role in the study design, data collection and analysis, or manuscript writing.
This work was supported by the Leona M. and Harry B. Helmsley Charitable Trust.
Disclosure Summary: The authors have nothing to disclose.
Abbreviations
| AID | autoimmune disease |
| HbA1c | hemoglobin A1c |
| HLA | human leukocyte antigen |
| RA | rheumatoid arthritis |
| T1D | type 1 diabetes. |
Reference
Articles from The Journal of Clinical Endocrinology and Metabolism are provided here courtesy of The Endocrine Society
Full text links
Read article at publisher's site: https://doi.org/10.1210/jc.2016-2478
Read article for free, from open access legal sources, via Unpaywall:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7530541
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1210/jc.2016-2478
Article citations
Autoimmune comorbidity in type 1 diabetes and its association with metabolic control and mortality risk in young people: a population-based study.
Diabetologia, 67(4):679-689, 22 Jan 2024
Cited by: 2 articles | PMID: 38252314 | PMCID: PMC10904419
14. Children and Adolescents: Standards of Care in Diabetes-2024.
Diabetes Care, 47(suppl 1):S258-S281, 01 Jan 2024
Cited by: 14 articles | PMID: 38078582
Review
Prevalence of autoimmune diseases in an admixed population of patients with type 1 diabetes: a multicenter study in Brazil.
Diabetol Metab Syndr, 16(1):31, 31 Jan 2024
Cited by: 2 articles | PMID: 38297335 | PMCID: PMC10829295
Diabetes and Multiple Long-term Conditions: A Review of Our Current Global Health Challenge.
Diabetes Care, 46(12):2092-2101, 01 Dec 2023
Cited by: 10 articles | PMID: 38011523
Review
4. Comprehensive Medical Evaluation and Assessment of Comorbidities: Standards of Care in Diabetes-2024.
Diabetes Care, 47(suppl 1):S52-S76, 01 Jan 2024
Cited by: 20 articles | PMID: 38078591
Review
Go to all (37) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Increasing Co-occurrence of Additional Autoimmune Disorders at Diabetes Type 1 Onset Among Children and Adolescents Diagnosed in Years 2010-2018-Single-Center Study.
Front Endocrinol (Lausanne), 11:476, 06 Aug 2020
Cited by: 4 articles | PMID: 32849272 | PMCID: PMC7424019
The T1D Exchange clinic registry.
J Clin Endocrinol Metab, 97(12):4383-4389, 20 Sep 2012
Cited by: 286 articles | PMID: 22996145
Risk for Coexistent Autoimmune Diseases in Familial and Sporadic Type 1 Diabetes is Related to Age at Diabetes Onset.
Endocr Pract, 27(2):110-117, 13 Dec 2020
Cited by: 5 articles | PMID: 33616044
Global epidemiology of type 1 diabetes in young adults and adults: a systematic review.
BMC Public Health, 15:255, 17 Mar 2015
Cited by: 108 articles | PMID: 25849566 | PMCID: PMC4381393
Review Free full text in Europe PMC





