Abstract
Free full text

The Impact of COVID-19 Disease on Platelets and Coagulation
Abstract
Coronavirus disease 2019 (COVID-19) causes a spectrum of disease; some patients develop a severe proinflammatory state which can be associated with a unique coagulopathy and procoagulant endothelial phenotype. Initially, COVID-19 infection produces a prominent elevation of fibrinogen and D-dimer/fibrin(ogen) degradation products. This is associated with systemic hypercoagulability and frequent venous thromboembolic events. The degree of D-dimer elevation positively correlates with mortality in COVID-19 patients. COVID-19 also leads to arterial thrombotic events (including strokes and ischemic limbs) as well as microvascular thrombotic disorders (as frequently documented at autopsy in the pulmonary vascular beds). COVID-19 patients often have mild thrombocytopenia and appear to have increased platelet consumption, together with a corresponding increase in platelet production. Disseminated intravascular coagulopathy (DIC) and severe bleeding events are uncommon in COVID-19 patients. Here, we review the current state of knowledge of COVID-19 and hemostasis.
Introduction
The Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) that has swept the globe in 2019 and 2020 causes Coronavirus Disease 2019 (COVID-19), a predominantly respiratory illness with 11.5–13% mortality among hospitalized patients [1, 2, 3]. 80% of patients infected by SARS-CoV-2 may be asymptomatic or only mildly symptomatic, but around 10% develop severe respiratory symptoms that evolve to acute respiratory distress syndrome (ARDS) [4].
SARS-CoV-1 and SARS-CoV-2, the enveloped single-stranded RNA viruses responsible for the 2002–2004 SARS epidemic and the more recent COVID-19 pandemic, respectively, bind angiotensin-converting enzyme 2 (ACE2), an intrinsic membrane protein with enzymatic activity that physiologically counters the activation of the renin-angiotensin-aldosterone system. ACE2 is expressed broadly, including in lung alveolar pneumocytes, as well as endothelial cells, the heart, and the kidneys [5, 6]. SARS-CoV-2 has an at least 10-fold-greater affinity for human ACE2 than SARS-CoV-1 has [7].
SARS-CoV-2 causes lung inflammation which progresses to cytokine storm in the most severe cases. The lungs of patients with COVID-19 show extensive alveolar and interstitial inflammation [8]. COVID-19 causes a spectrum of disease, with frequent involvement of the hemostatic system [9, 10]. Severe pulmonary inflammation causes activation and damage of the pulmonary vasculature and may trigger pulmonary thrombosis early in the disease course [11]. There is a high incidence of venous thromboembolism (VTE) in hospitalized COVID-19 patients, particularly those with severe illness. The incidence of thrombotic complications is 16–69% in patients with COVID-19 admitted to intensive care [3, 10, 12, 13]; the incidence was highest in Llitjos et al. (69%) due to active ultrasound surveillance for deep-vein thrombosis (DVT). The incidence of venous and possibly arterial thrombosis remains high in COVID-19 patients despite administering standard thromboprophylaxis [3]. In one Italian COVID-19 study, the incidence of VTE (despite thromboprophylaxis) was 27.6% in the ICU and 6.6% in the general ward. The rate of ischemic stroke and acute coronary syndrome was 2.5 and 1.1%, respectively [14].
Hypercoagulability due to severe viral pneumonia is not novel. This increased VTE incidence in COVID-19 patients is similar to that seen in patients with other epidemic coronavirus pneumonias, including severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS-CoV) [15, 16]. H1N1 influenza carried an 18-fold increased risk of developing VTE when compared to critically ill patients with ARDS with no H1N1 influenza infection [17].
The SARS-CoV-2 virus does not appear to have intrinsic procoagulant effects itself; rather, the coagulopathy is most likely the result of the profound COVID-19 inflammatory response and endothelial activation/damage [18]. Recent COVID-19 autopsy reports demonstrate pulmonary endothelial viral inclusions and apoptosis, increased angiogenesis, and increased capillary microthrombi [19, 20].
Patients with COVID-19 pneumonia exhibit coagulation abnormalities, most commonly elevated levels of fibrinogen and D-dimer, often with mild thrombocytopenia [18, 21]. Elevated D-dimer has been associated with a higher mortality rate. A subset of COVID-19 patients can have abnormally short PT and aPTT [15]. The shortened aPTT is often related to elevated Factor VIII (FVIII) [22] as an acute-phase response. In more severely affected patients, a disseminated intravascular coagulopathy (DIC)-like state can develop with relatively mild prolongation of the PT and aPTT (while fibrinogen tends to remain normal/elevated) [18]. However, D-dimer levels are elevated far out of proportion to any abnormalities detected in the PT/INR, aPTT, fibrinogen level, or platelet count; these findings are unusual for DIC, as defined by the criteria of the International Society of Thrombosis and Hemostasis (ISTH) [23]. Unlike the pattern seen in classic DIC from bacterial sepsis or trauma, in COVID-19 prolongation of the aPTT and/or PT is minimal [24], thrombocytopenia is mild (a platelet count of 100–150 ×109/L), hypofibrinogenemia is rare, and laboratory results supporting hyperfibrinolysis are uncommon [25]. COVID-19-associated coagulopathy is the term used to describe this spectrum of coagulation changes. Three stages of COVID-19-associated coagulopathy have been proposed: stage 1 showing elevated D-dimer, stage 2 showing elevated D-dimer together with mildly prolonged PT/INR and aPTT and mild thrombocytopenia, and stage 3 with critical illness and laboratory studies progressing towards classic DIC [11].
Here, we will discuss what is known about COVID-19-associated changes in platelet count, activation states, and production; we will review the association of these platelet parameters with COVID-19 outcomes. Additionally, we will review the predominantly procoagulant changes seen in the coagulation system during COVID-19 infection and their association with COVID-19 mortality.
Platelets
Of the patients affected by the 2003 SARS epidemic, 20–55% had thrombocytopenia [26]. Subsequent rebound thrombocytosis was also reported [16, 27]. The patients who developed thrombocytopenia during the epidemic experienced greater morbidity/mortality [28]. MERS was also associated with thrombocytopenia [16].
Thrombocytopenia is detected in 5–41.7% of COVID-19 patients (the incidence varies according to disease severity) [1, 29, 30, 31], and it is typically mild (counts are generally 100–150 ×109/L). Mild thrombocytopenia has been detected in 58–95% of severe cases of COVID-19 [21, 30, 32]; on average, patients with severe disease have a platelet count only 23 ×109/L to 31 ×109/L lower than those with non-severe disease [33, 34]. The fact that such severely ill patients with systemic immune and coagulation activation maintain reasonable platelet counts implies a marked compensatory platelet production response. Severe thrombocytopenia is only rarely reported in COVID-19 patients, for instance, in association with an immune thrombocytopenic purpura-like state [35].
A meta-analysis of 7,613 COVID-19 patients revealed that patients with severe disease had a lower platelet count than those with non-severe disease. Additionally, the non-survivors had a much lower platelet count than the survivors [25, 36]. However, not all studies have found platelet counts to be a predictor of COVID-19 mortality [37]. Compared to patients with severe pneumonia but without COVID-19, those with COVID-19 disease actually had higher platelet counts, according to Yin et al. [38]. The pediatric COVID-19 syndrome of Kawasaki-like illness has been associated with a lower average platelet count than that seen in classical Kawasaki disease, although the platelet counts did not fall below the lower limit of normal [39].
Thrombocytopenia at admission in COVID-19 patients was associated with a 4.24-fold increased risk of inpatient mortality in a study from Wuhan [1]. Patients with thrombocytopenia (median 105 ×109/L) were more likely to be older, male, have a higher APACHE II score, lower absolute neutrophil and lymphocyte counts, higher C-reactive protein (CRP), and a lower PaO2/FiO2 ratio than those without thrombocytopenia (median 186 ×109/L). The hemoglobin and D-dimer levels did not differ in these two groups.
A temporal trend of dropping platelet counts in patients with COVID-19 could suggest a worsening thrombotic state [40]; lower nadir platelet counts are associated with increased mortality. In Yang et al. [31], compared to the reference group (i.e., a platelet count ≥150 ×109/L), nadir platelet counts of 100–150, 50–100, and 0–50 ×109/L had a relative risk for in-hospital mortality of 3.4 (95% confidence interval [CI] 2.4–5.0), 10.0 (95% CI 7.2–14.0), and 13.7 (95% CI 9.9–18.9), respectively. Conversely, improvement of thrombocytopenia in COVID-19 patients can signify imminent clinical improvement [1].
Viral infection can be associated with thrombocytopenia due to a variety of causes [41]. While hypoproliferative thrombocytopenia is observed at later stages of viral infection, the rapid development of thrombocytopenia in response to viral infections is generally mediated via enhanced platelet clearance/destruction [42]. Platelets can be activated by viral antigen-antibody complexes or host inflammatory responses; activated platelets are more readily cleared from the circulation by the reticuloendothelial system [42]. Viruses can also interact with megakaryocytes and reduce platelet synthesis [43].
Platelets play an important role in inflammatory signaling as well as in infectious response [44]. By combining thrombotic and immune recruitment functions, platelets may help focus hemostasis and immune responses against potential infectious agents to prevent microbial invasion [45]. Platelets interact directly with viruses via a variety of receptors [42], including Toll-like receptors [46, 47]. While platelets are capable of engulfing and aggregating pathogens, their microbial killing potential is limited [45]. Platelets and their released products have been variably reported to suppress viral infection and support virus persistence, depending on the particular infection [42]. Platelets also appear to play a role in recruiting and activating circulating leukocytes to the endothelial surface, leading to white blood cell diapedesis [48, 49]. The interactions between endothelial cells, platelets, and leukocytes play a critical role in the procoagulant effect of viral infections [16]. Thrombocytopenia, platelet secretion, and interactions with leukocytes may have either injurious or protective immune consequences in viral infections [47].
While platelets contribute to the basal barrier integrity of the alveolar capillaries, they may also contribute to lung injury in a variety of pulmonary disorders and syndromes [50]. Platelet-leukocyte aggregates [51, 52] and platelet-endothelial interactions [53] appear to play a role in the pathogenesis of acute lung injury in animal models due to physico/chemical damage and influenza infection, respectively. For example, in dengue fever, platelet-derived IL-1β causes increased endothelial permeability [54].
Several mechanisms of COVID-19-associated thrombocytopenia have been posited (Table (Table1).1). This could be purely consumptive, particularly related to endothelial damage and platelet aggregate formation in the lung, but marrow suppression and immune clearance are also possible contributors [29]. Thachil [32] suggests that platelets are being consumed to form pulmonary thrombi, with a possible anti-infective effect, to prevent viremic spread via the bloodstream. In preliminary data from our institution, McMullen et al. [American Journal of Clinical Pathology, in press] have shown there are significant intravascular platelet aggregates in COVID-19 autopsy lung specimens, located primarily in the interalveolar capillaries and smaller vessels; the degree of platelet deposition is not more than is seen in other fatal pulmonary infections, however. Of note, none of the COVID-19 patients assessed in their study were thrombocytopenic.
Table 1
Possible mechanisms of COVID-19-associated thrombocytopenia (modified from [103])
| Platelet activation and subsequent clearance by reticuloendothelial system − Activation by increased thrombin generation and consumptive coagulopathy − Direct viral-platelet interaction activation −associated with formation of platelet-leukocyte aggregates − FcR-mediated interaction with immune complexes |
| Platelet clearance due to increased endothelial damage − Pulmonary vasculature-specific − Widespread damage |
| Platelet autoantibody formation, with subsequent platelet clearance |
| Splenic/hepatic sequestration Marrow/megakaryocyte suppression − Due to inflammatory response − Due to direct viral infection − Due to reduced thrombopoietin |
Liu et al. [1] showed that COVID-19 patients with thrombocytopenia had a statistically significantly larger mean platelet volume (MPV, median 10.3 fL) than COVID-19 patients with retained platelet counts (median 9.9 fL). Outside of congenital platelet disorders, an increased mean platelet volume implies an increase in circulating young platelets and is the body's response to thrombocytopenia [55]. In healthy adults with normal platelet counts, the normal MPV range is 9.0–12.4 fL [56]. Platelet size positively correlates with surface receptor number and platelet ATP content. The number of ribosomes is higher in large platelets and they incorporate more amino acids, suggesting a higher potential for protein synthesis [57]. Larger platelets have increased hemostatic potential, bind more fibrinogen, and have greater levels of phosphorylation after thrombin stimulation than smaller platelets [57].
In preliminary data, COVID-19 patients at our hospital were found to have significantly larger MPVs than critically ill non-COVID-19 patients matched for platelet count (11.6 vs. 10.5 fL) (Table (Table2).2). We compared MPV in 10 patient pairs matched for platelet count: 10 adult COVID-19-positive patients admitted to ICU (either the COVID-19 cohort ICU or the cardiac ICU) and 10 adult patients testing negative for SARS-CoV-2 and admitted to the surgical, medical, or neurological ICU.
Table 2
Platelet parameters in COVID-19 patients
| COVID-19 patients | Non-COVID-19 ICU patients | Normal range | |||
|---|---|---|---|---|---|
| mean | SD | mean | SD | ||
| Platelet count, ×109/L | (n = 10) 234.1 | (n = 10) 151.1 | (n = 10) 250.30 | (n = 10) 152.7 | 150–450 |
| MPV, fL | (n = 9) 11.58* | (n = 9) 1.04 | (n = 9) 10.49 | (n = 9) 1.00 | 9.0–12.4 |
| median | range | median | range | ||
|---|---|---|---|---|---|
| IPF, ×109/L IPF, % | (n = 3) 42.57 14.63 | (n = 3) 12.2–99.5 4.2–25.0 | (n = 2) 4.25 7.05 | (n = 2) 3.3–5.2 6.4–7.7 | 1.25–7.02 3.3–8.6 |
N = 20, i.e., 10 pairs of patients matched for platelet count. * p = 0.013, paired Student's t test (compared to non-COVID-19 patients). MPV, mean platelet volume; IPF, immature platelet fraction.
This trend for higher MPV in COVID-19 patients persisted in a larger dataset of hematologic values from the same 20 patients when no longer matched for platelet count (Student's t test p < 5 ×10−7) (Fig. (Fig.1).1). This trend towards greater MPV persists even in COVID-19 patients with normal platelet counts.
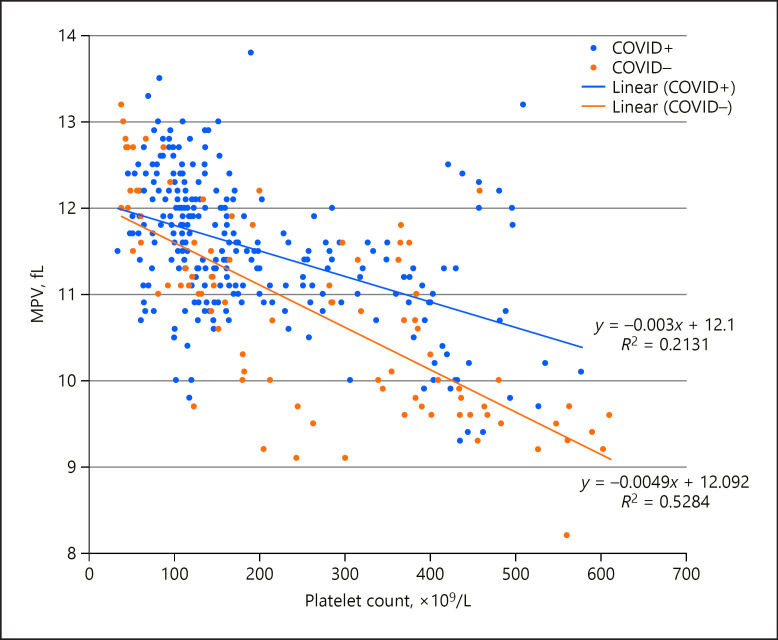
Relationship between platelet count and mean platelet volume (MPV) in a sample of ICU patients affected by COVID-19 (COVID+; n = 10, with 266 measurements) or not (COVID–; n = 10, with 91 measurements). These populations were significantly different from each other (Student's t test, p < 5 ×10−7).
Reticulated platelets are immature platelets with a high granule content, residual mRNA, and increased mean volume compared with older circulating platelets [58, 59, 60]. In healthy adults with normal platelet counts, the relative immature platelet fraction (IPF, also known as reticulated platelets) ranges from 3.3 to 8.6% [56]. Younger platelets show higher levels of activation in response to agonists (as assessed by P-selectin exposure), and thus more readily promote the formation of platelet aggregates [56]. The reticulated platelet count is positively associated with cardiovascular risk and mortality [58, 61].
In our hospital, there is a significant trend of elevated IPF in COVID-19 patients (Fig. (Fig.2).2). We investigated four COVID-19-positive and four COVID-19-negative thrombocytopenic patients from our original dataset described above. COVID-19 positivity was highly predictive of an absolute IPF of 7.5 ×109/L or higher (Fig. (Fig.2a).2a). In the non-COVID-19 patients, a relative IPF of ≥8% was restricted to those with platelet counts less than 70 ×109/L (Fig. (Fig.2b).2b). In contrast, COVID-19-positive patients had relative IPF ≥8% at platelet counts up to 251 ×109/L (data not shown).
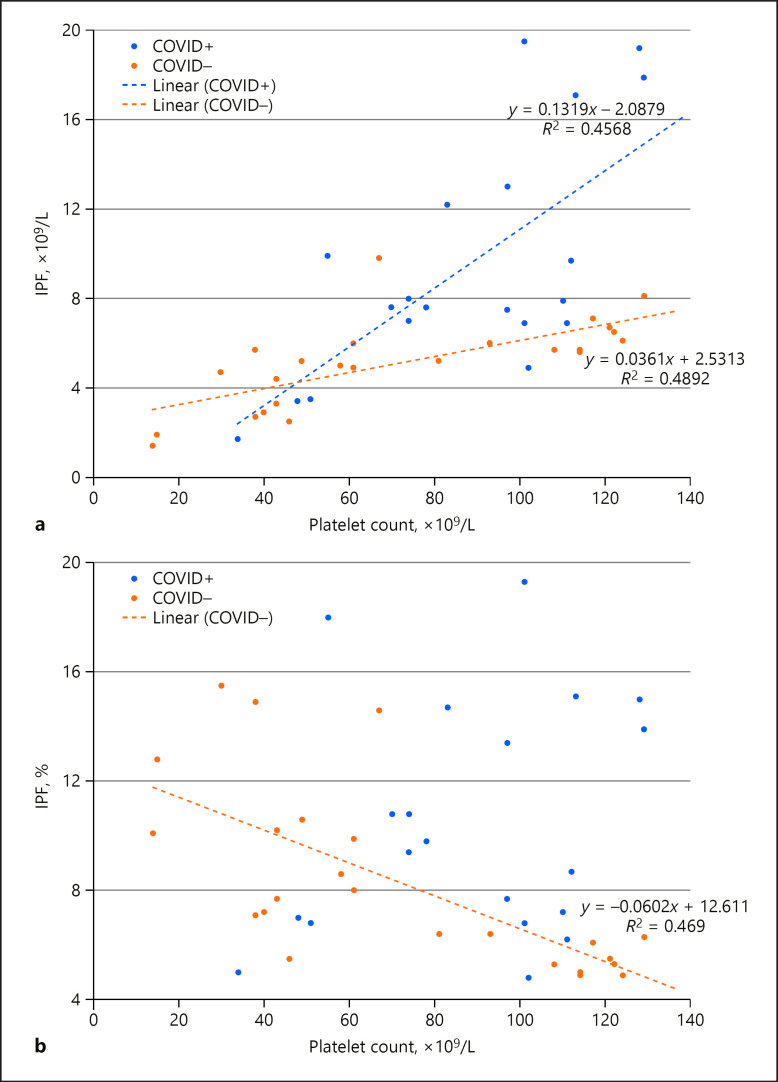
Relationship between platelet count and immature platelet fraction (IPF) in a sample of thrombocytopenic ICU patients affected by COVID-19 (COVID+; n = 4, with 23 measurements) or not (COVID–; n = 4, with 23 measurements). a Absolute IPF counts. These populations were significantly different from each other (Student's t test, p < 0.005). b Relative IPF counts. Of note, the relative IPF counts for COVID+ patients did not form a strong enough relationship with platelet count to display a relevant trendline. These populations were significantly different from each other (Student's t test, p < 0.05).
These findings suggest that COVID-19 is associated with the increased production of large immature platelets, as megakaryocytes respond to increased platelet consumption. Interestingly, COVID-19 is associated with increased numbers of immature platelets even at normal platelet counts. As immature platelets are known to be more functional [56, 58], this could be another mechanism for increased clotting events in COVID-19.
In keeping with these findings that COVID-19 patients have larger and less mature platelets (parameters associated with increased platelet reactivity), Ranucci et al. [62]showed that, upon admission to the ICU, a group of intubated COVID-19 patients with normal platelet counts had an increased platelet contribution to clot strength according to a viscoelastic analysis.
In addition to having increased numbers of immature platelets, COVID-19 patients may have increased levels of circulating activated platelets. Such activated platelets are normally only a small proportion of the total circulating population. Hille et al. [56] reported that reticulated (younger) and nonreticulated (older) platelets from healthy patients show a low percentage of P-selectin positivity in unstimulated samples (median in all groups <5%). Similarly, Handtke et al. [55] reported that small and large platelets have similar basal surface levels of P-selectin. Manne et al. [63] recently reported that circulating platelets from at least some of the COVID-19 patients they studied had a higher level of P-selectin detectable on their surface membranes than did normal controls. Although there was a considerable overlap of results between patients and normal donors, a statistically higher level of surface P-selectin was found in the patient group following in vitro exposure to low-concentration (2.5 µM) thrombin receptor-activating peptide (TRAP). At the higher stimulus intensity of 25 µM TRAP, a difference in response between patients and normal donors was no longer seen. The enhancement of platelet aggregation in the patients was similarly observed to be greater in response to lower concentrations than higher concentrations of several platelet agonists [63]. Zhang et al. [64] have very recently taken such analysis still further, reporting their detection of ACE2 expression by platelets, the direct stimulatory effects of SARS-CoV-2 or its Spike protein on platelets, and the ability of recombinant human ACE2 protein or anti-Spike monoclonal antibody to inhibit Spike protein-induced platelet activation. Hottz et al. [104]have further recently demonstrated increased platelet-monocyte interaction and associated tissue factor expression by monocytes in severe COVID-19 patients.
Antiplatelet Agents
A role for antiplatelet agents in COVID-19 disease has been considered, but there is no randomized evidence to support this treatment at this time. Particularly for those patients with mild thrombocytopenia, such treatment would carry a greater risk of bleeding [65].
Endothelium, von Willebrand Factor, and ABO Blood Group
COVID-19 pneumonia is associated with endothelial cell disruption, tissue factor expression, and activation of the coagulation cascade. These responses worsen oxygenation, and local hypoxia establishes a deleterious positive thromboinflammatory feedback loop [8]. Direct endothelial damage by the virus and/or endothelial activation by cytokines released during COVID-19 infection are possible mechanisms of thrombosis [66]. Activated or damaged endothelial cells release their Weibel-Palade bodies containing ultra-large molecular-weight multimers of von Willebrand factor (vWF). The ultra-large vWF multimers can spontaneously bind platelets and lead to microthrombosis if not trimmed by ADAMTS13.
vWF has previously been shown to play a role in the development of thrombocytopenia during viral infections. Intravenous administration of adenovirus (as used for gene therapy studies) is associated with platelet activation and acute thrombocytopenia. This cytopenia is not seen in adenovirus-treated vWF knock-out mice, however [67].
COVID-19 patients have significantly elevated vWF levels; mean vWF antigen has been reported to be 455–529% [10, 22]. Escher et al. [66, 68] reported elevated vWF antigen and function, together with increased FVIII clotting activity in COVID-19 patients, likely reflecting the combined effect of the greater release of Weibel-Palade bodies from endothelial cells and the acute-phase reaction that raises the FVIII level. ADAMTS13 activity is mild-to-moderately reduced in COVID-19 patients [68, 69, 70].
In a study from Yale New Haven hospital, markers of the activation of endothelial cells and platelets were significantly elevated in COVID-19 patients in the ICU compared to non-ICU patients (vWF antigen mean 565% [SD 199] vs. 278% [SD 133], p < 0.0001; soluble P-selectin mean 15.9 ng/mL [SD 4.8] vs. 11.2 ng/mL [3.1], p = 0.0014). Mortality was significantly correlated with vWF antigen (r = 0.38; p = 0.0022) and soluble thrombomodulin (r = 0.38; p = 0.0078) [71].
In a study on ABO blood group distribution in 2,173 COVID-19 patients by Zhao et al. [72], group A individuals were more likely to have symptomatic COVID-19 disease, while group O individuals were less likely to have it; this association is not universally identified, however. Latz et al. [73] did not show any correlation between ABO type and COVID-19-related intubation or death, but they did confirm that group O individuals were least likely to test positive for SARS-CoV-2. A genome-wide association study involving 1,980 patients in Europe showed the 9q34.2 locus (including the ABOglycosyltransferase gene) to be associated with severe COVID-19 with respiratory failure. This study also confirmed the higher risk in blood group A than in other blood groups (odds ratio [OR] 1.45; 95% CI 1.20–1.75) and a protective effect in blood group O versus the other blood groups (OR 0.65; 95% CI 0.53–0.79) [74]. These findings could be due to elevated vWF levels in non-group O individuals, which predispose to thromboembolic events, as has been well-described prior to the current pandemic [75].
An alternative explanation for this ABO-differentiated susceptibility could be the isohemagglutinin-mediated neutralization or clearance of the SARS-CoV-2 virus coated with A substance (as would be created by group A or AB individuals) [76]. Enveloped viruses (such as coronaviruses) are glycosylated, meaning that their envelope proteins are post-translationally modified by the addition of glycans. The interaction between SARS-CoV Spike protein and ACE2 can be specifically blocked by anti-A antibodies when the Spike protein is synthesized by cells that express group A substance [77]. Gallian et al. [78]showed a lower seroprevalence of antibodies neutralizing SARS-CoV-2 in group O donors, which they interpret as reflecting a lower rate of infection in these individuals, likely due to the natural protection from anti-A and anti-B isohemagglutinins.
Coagulation
Patients hospitalized for COVID-19 infection typically have elevated fibrinogen and D-dimer levels. On average, fibrinogen is increased to 5.0–7.0 g/dL in COVID-19 ICU patients [10, 24]. CRP is another acute-phase reactant that increases greatly during the acute-phase reaction [79] associated with elevated IL-6 [34, 80, 81].
D-dimers are significantly increased in COVID-19 [10, 24], likely reflecting pulmonary vascular bed thrombosis and fibrinolysis [8]. D-dimers reflect fibrin clot formation, clot crosslinking by FXIIIa, and fibrinolysis. The marked elevation of D-dimers in COVID-19 appears to reflect coagulation activation from viremia and cytokine storm, but superinfection and organ dysfunction are other possible causes. Unfortunately, often the mean D-dimer values cannot be directly compared across publications due to frequent omission of whether D-dimers are reported in D-dimer equivalent units (DDU) or fibrinogen equivalent units (FEU) [82, 83]. A D-dimer cut-off of >1 μg/mL (DDU/FEU units not reported) may stratify COVID-19 patients at a higher risk of poor outcomes [82]. Temporally increasing D-dimer levels indicate the progressive severity of COVID-19 infection and can be used as a predictor that more aggressive critical care will be needed. Of nearly 1,500 COVID-19 hospital admissions, Li et al. [25]found two admission covariates that correlated with an increased risk of death: age (OR 1.18; 95% CI 1.02–1.36) and baseline D-dimer level (OR 3.18; 95% CI 1.48–6.82).
Guan et al. [30]reported a series of 1,099 patients with COVID-19 from China; elevated D-dimer (>0.5 mg/L) (DDU/FEU units not reported) was found in 260/560 (46%) patients. Han et al. [24]reported elevated D-dimer and fibrinogen levels among patients with COVID-19 compared to healthy controls (mean D-dimer 10,400 vs. 260 ng/mL [DDU/FEU units not reported]; mean fibrinogen 500 vs. 290 mg/dL). Fibrinogen degradation products (FDP) were also significantly increased, i.e., 33.8 versus 1.6 mg/L [24]. D-dimers and FDP progressively increased with COVID-19 severity, but the fibrinogen level stayed elevated [24]. This is substantially different from typical DIC [62]. However, Tang et al. [2] did describe 15/21 of the non-survivors (71.4%, vs. 0.6% of the survivors) as meeting the criteria for DIC during hospitalization, with low fibrinogen levels present late in their course. In 183 consecutive COVID-19 patients from Wuhan, D-dimers and FDP levels as well as PT clotting times were significantly increased in non-survivors at admission [2]. At presentation, non-survivors had a mean D-dimer level of 2.12 (range 0.77–5.27) μg/mL, while survivors had a mean of 0.61 (range 0.35–1.29) μg/mL; FEU versus DDU was not specified [2]. Both survivors and non-survivors had elevated fibrinogen upon hospital admission and the levels in these two groups were not statistically different. According to Zhou et al. [84], factors associated with mortality included an elevated D-dimer >1.0 μg/mL (FEU or DDU units not specified) on admission and increased PT. In a multivariate regression, a D-dimer level >1.0 μg/mL at admission was associated with increased mortality with an OR 18.42 (95% CI 2.64–128.55). Huang et al. [85]showed that D-dimer levels on admission were higher in patients needing critical care support (median D-dimer level 2.4 mg/L [0.6–14.4]) than in those not requiring such support (median D-dimer level 0.5 mg/L [0.3–0.8], p = 0.0042) (DDU vs. FEU not specified). These data have prompted the ISTH interim guidelines for COVID-19 coagulopathy to suggest that patients who have a 3–4-fold increase in D-dimers should be considered for hospital admission, even in the absence of other severe symptoms, due to the evidence of increased thrombin generation [40].
In the study by Li et al. [25], fibrinogen was higher at baseline in the subjects who died (median 4.3 [IQR 3.2–5.2] vs. 3.6 [IQR 2.9–4.5] g/L) but also lower at nadir (2.6 [IQR 1.7–3.9] vs. 3.2 [IQR 2.6–3.9] g/L). This implies a greater degree of fibrinogen consumption in the COVID-19 mortalities.
Al-Samkari et al. [23] confirmed excess clotting in 400 COVID-19 inpatients in the USA, all treated with prophylactic anticoagulation. Overall, the thrombotic complication rate was 9.5% (6.8–12.8%) while the overall bleeding rate was 4.8% (2.9–7.3%). D-dimer >2.5 μg/mL at initial presentation was predictive of bleeding, thrombosis, critical illness, and death (DDU vs. FEU not specified). Additional markers at the initial presentation predictive of thrombosis during hospitalization included a platelet count >450 ×109/L (adjusted OR 3.6; 95% CI 1.3–10.0). Forty-one (10.3%) and 10 (2.5%) patients had a platelet count <100 ×109/L or <50 ×109/L during their hospital course, respectively. Patients with more severe thrombocytopenia (<50 ×109/L) had increased bleeding manifestations. They alsofound a very low rate of DIC (2% of critically ill patients) using ISTH criteria.
In a small-scale study by Panigada et al. [22], 55% of the COVID-19 patients had antithrombin levels below the reference range, while protein C was not decreased in any of the patients assessed. Antithrombin is known to be consumed during coagulation, and the mild antithrombin deficiency that they described is consistent with this. The lack of significant protein C deficiency is quite unusual for typical DIC [86], providing further support that the COVID-19-associated coagulopathy may be distinct from DIC.
aPTT waveform analysis was reported in three ICU patients with COVID-19 [87]. These patients had mostly mildly prolonged aPTT clotting times. A biphasic waveform (a marker for DIC) was not present in any of the patients. However, all three showed an increased maximum clot velocity, implying a prothrombotic state.
The finding of small-vessel endothelial damage in COVID-19 patients may help explain the increased incidence of arterial and microvascular thrombotic events [19, 88] in addition to VTE. Severely ill COVID-19 patients can experience arterial thrombosis-related acral ischemia [89]. Among 1,916 adults treated for COVID-19 in 2 New York hospitals, 1.6% experienced an ischemic stroke. In comparison, the stroke rate among influenza patients between 2016 and 2018 was 0.2%. The median time from COVID-19 symptom onset to stroke was 16 days [90].
COVID-19 patients commonly have lupus anticoagulants. Bowles et al. [91]described a 20% incidence of prolonged screening aPTT in 216 COVID-19 patients. Of the patients with prolonged screening aPTT, fully 91% had a detectable lupus anticoagulant. Helms et al. [10]showed 88% lupus anticoagulant positivity in tested COVID-19 ICU patients. Whether these frequent lupus anticoagulants are pathogenic for the increased thrombotic risk in COVID-19 patients, or are epiphenomena, is not known at this time. The markedly elevated CRP levels seen in COVID-19 patients may also be causing false positivity [92].
Viscoelastic Analysis
COVID-19 is associated with a hypercoagulable profile, mostly affecting clot formation kinetics and clot strength. Ranucci et al. [62] showed that, upon admission to the ICU, a group of intubated COVID-19 patients with normal platelet counts, mildly prolonged aPTT, and elevated plasma fibrinogen had a markedly increased fibrinogen contribution to clot strength on Quantra viscoelastic analysis. Similar findings are seen with the TEG 5000 instrument, with an increased α angle and maximum amplitude, using a kaolin activator with native blood in the presence of heparinase [22]. Pavoni et al. [81] showed similar hypercoagulable findings with ROTEM that persisted over the course of COVID-19 illness. In contrast to these findings with clot kinetics and clot strength, clot time (CT and R time) are less commonly hypercoagulable in COVID-19.
ICU patients with COVID-19 commonly show a complete absence of detectable fibrinolysis 30 min after achievement of maximum clot amplitude (LY30 0.0%) [93]. Patients with both 0.0% LY30 and a D-dimer level >2,600 ng/mL (FEU) had a VTE event rate of 50% compared with 0% in patients meeting neither criterion (p = 0.008), and a hemodialysis rate of 80% compared with 14% in patients meeting neither criterion (p = 0.004) [93]. In this way, COVID-19 coagulopathy is somewhat similar to sepsis-induced disseminated intravascular coagulation, which typically presents with suppressed fibrinolysis [94]. However, as compared to sepsis-associated DIC, D-dimer levels are typically more significantly elevated in COVID-19 [94]. While LY30 measurement during COVID-19 can seem to imply fibrinolytic shutdown, the markedly elevated D-dimer level clearly shows continuing fibrinolysis. The very low LY30 TEG values for these patients reveal an absence of elevated in vitrofibrinolysis. The details of in vivofibrinolysis in COVID-19 have not yet been investigated, but fibrinolysis is obviously continuing to some degree.
COVID-19 coagulopathy can often meet criteria for the relatively newly defined entity of sepsis-induced coagulopathy (SIC), which is defined and quantified according to a reduced platelet count, increased INR, and higher organ dysfunction score [95, 96].
Anticoagulation and Anticoagulation Monitoring
Critically ill patients in ICU settings experience VTE rates of 13–30% without chemoprophylaxis, and 5–15% with routine chemoprophylaxis [93]. However, Chinese COVID-19 inpatients were not routinely treated with prophylactic anticoagulation, as is common practice in the USA [12]. Routine thromboprophylaxis is not standard in Chinese hospitals, partly due to the lower rate of VTE detected in Asians [97]. This must be kept in mind when comparing D-dimer levels in COVID-19 studies.
In a retrospective study on 449 patients with severe COVID-19, no difference in 28-day mortality was found between heparin users and nonusers (30.3 vs. 29.7%; p = 0.910). However, the 28-day mortality of heparin users was lower than that of nonusers in patients with an ISTH SIC score ≥4 or a D-dimer level >3.0 μg/mL (FEU vs DDU not specified) [98]. Prophylactic-dose low-molecular-weight heparin is recommended for all hospitalized COVID-19 patients despite abnormal coagulation tests in the absence of active bleeding, and is withheld only if platelet counts are <25 ×109/L, or the fibrinogen level is <0.5 g/L [99].
Some have argued for the use of therapeutic dose anticoagulation in COVID-19 patients without identified thrombosis [100]. However, it must be kept in mind that therapeutic anticoagulation is associated with increased bleeding. In October 2020, the American Society of Hematology published guidelines suggesting using prophylactic-intensity over intermediate-intensity or therapeutic-intensity anticoagulation in patients with COVID-19 related critical illness who do not have suspected or confirmed VTE [105]. VTE rates remain quite high in COVID-19 patients despite therapeutic anticoagulation [13]. The mild prolongation of aPTT in COVID-19 patients may necessitate the monitoring of unfractionated heparin with anti-Xa assays.
Bleeding is rare in the setting of COVID-19. Transfusion therapy should not be instituted on the basis of laboratory results alone, but rather be reserved for those with active bleeding, requiring an invasive procedure, or who are at an otherwise high risk for bleeding complications. If bleeding does develop, patients can be managed with principles similar to those in ISTH guidelines for DIC [101].
Conclusion
The currently available evidence suggests that the COVID-19 coagulopathy represents a combination of localized pulmonary platelet consumption, low-grade DIC (only rarely meeting the ISTH DIC criteria), and variably a thrombotic microangiopathy. Elevated vWF levels and soluble thrombomodulin imply activated or damaged endothelium, as has been seen histologically in autopsy studies. It would be anticipated that damaged endothelium would result in the release of ultra-large vWF multimers capable of interacting with platelets, leading to platelet activation, microthrombi, and platelet consumption. Elevated soluble P-selectin levels and platelet flow cytometric studies suggest increased activation of endothelium and/or circulating platelets in COVID-19 patients [102]. Evidence of a direct stimulatory role of SARS-CoV-2 Spike protein upon platelets has also recently been presented [64]. Severe COVID-19 illness is associated with increased platelet activation as well as platelet-monocyte aggregation [104]. Platelets from severely ill COVID-19 patients can induce monocyte TF expression (in a P-selectin and αIIb/β3 dependent manner) [104], which may amplify inflammation and hypercoagulability in these patients.
The homeostatic response to platelet consumption is increased platelet production with an increase in the IPF. This compensatory response is robust in COVID-19 and can be out of proportion to the degree of thrombocytopenia, with elevated MPV and IPF occurring even in COVID-19 patients with normal platelet counts.
Despite the large number of publications focusing on the hemostatic changes associated with COVID-19, it should be kept in mind that all severe infectious disorders are associated with changes in hemostasis laboratory values as well as thrombotic and bleeding events. Al-Samkari et al. [23] have shown that the VTE rate in critically ill COVID-19 patients receiving thromboprophylaxis is similar to the previously published rates in patients with non-COVID-19 critical illness. They showed similar findings for bleeding events. This raises the question as to whether COVID-19 has a unique effect on the hemostatic system or simply causes the expected activation of the hemostatic system in the setting of severe inflammation [23].
Continued controlled studies are necessary to guide best treatment for COVID-19 patients and better elucidate the role that platelets play in COVID-19 pathophysiology.
Conflict of Interest Statement
G.D.W. is on an advisory committee at Diagnostica Stago and receives honoraria. J.L.M. declares no conflicts of interest.
Acknowledgements
The authors would like to thank Kara Newton and Drs. Megan Parilla and Sandeep Gurbuxani for assistance with patients' identification and data extraction.
References
Full text links
Read article at publisher's site: https://doi.org/10.1159/000512007
Read article for free, from open access legal sources, via Unpaywall:
https://karger.com/pat/article-pdf/88/1/15/3404370/000512007.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1159/000512007
Article citations
Evaluating Personalized Add-On Ayurveda Therapy in Oxygen-Dependent Diabetic COVID-19 Patients: A 60-Day Study of Symptoms, Inflammation, and Radiological Changes.
Cureus, 16(9):e68392, 01 Sep 2024
Cited by: 0 articles | PMID: 39355453 | PMCID: PMC11444340
Addressing Post-Acute COVID-19 Syndrome in Cancer Patients, from Visceral Obesity and Myosteatosis to Systemic Inflammation: Implications in Cardio-Onco-Metabolism.
Biomedicines, 12(8):1650, 24 Jul 2024
Cited by: 1 article | PMID: 39200115 | PMCID: PMC11351439
Review Free full text in Europe PMC
Impaired instructive and protective barrier functions of the endothelial cell glycocalyx pericellular matrix is impacted in COVID-19 disease.
J Cell Mol Med, 28(16):e70033, 01 Aug 2024
Cited by: 0 articles | PMID: 39180511 | PMCID: PMC11344469
Review Free full text in Europe PMC
Development and validation of a scoring system to predict the mortality of hospitalized patients with SARS-CoV-2 Omicron: a nationwide, multicentre study.
BMC Pulm Med, 24(1):312, 03 Jul 2024
Cited by: 0 articles | PMID: 38961438 | PMCID: PMC11223413
Effects of Pycnogenol® in people with post-COVID-19 condition (PYCNOVID): study protocol for a single-center, placebo controlled, quadruple-blind, randomized trial.
Trials, 25(1):385, 15 Jun 2024
Cited by: 0 articles | PMID: 38879571 | PMCID: PMC11179231
Go to all (238) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
COVID-19 coagulopathy: is it disseminated intravascular coagulation?
Intern Emerg Med, 16(2):309-312, 24 Dec 2020
Cited by: 50 articles | PMID: 33368021 | PMCID: PMC7758412
Review Free full text in Europe PMC
Coagulopathy in COVID-19.
J Thromb Haemost, 18(9):2103-2109, 21 Jul 2020
Cited by: 311 articles | PMID: 32558075 | PMCID: PMC7323352
Review Free full text in Europe PMC
Covid-19: The Rollercoaster of Fibrin(Ogen), D-Dimer, Von Willebrand Factor, P-Selectin and Their Interactions with Endothelial Cells, Platelets and Erythrocytes.
Int J Mol Sci, 21(14):E5168, 21 Jul 2020
Cited by: 104 articles | PMID: 32708334 | PMCID: PMC7403995
Review Free full text in Europe PMC
Prothrombotic Phenotype in COVID-19: Focus on Platelets.
Int J Mol Sci, 22(24):13638, 20 Dec 2021
Cited by: 16 articles | PMID: 34948438 | PMCID: PMC8705811
Review Free full text in Europe PMC





