Abstract
Free full text

Frequent neurocognitive deficits after recovery from mild COVID-19
Abstract
Neuropsychiatric complications associated with coronavirus disease 2019 caused by the Coronavirus SARS-CoV-2 (COVID-19) are increasingly appreciated. While most studies have focussed on severely affected individuals during acute infection, it remains unclear whether mild COVID-19 results in neurocognitive deficits in young patients. Here, we established a screening approach to detect cognitive deficiencies in post-COVID-19 patients. In this cross-sectional study, we recruited 18 mostly young patients 20–105 days (median, 85
days (median, 85 days) after recovery from mild to moderate disease who visited our outpatient clinic for post-COVID-19 care. Notably, 14 (78%) patients reported sustained mild cognitive deficits and performed worse in the Modified Telephone Interview for Cognitive Status screening test for mild cognitive impairment compared to 10 age-matched healthy controls. While short-term memory, attention and concentration were particularly affected by COVID-19, screening results did not correlate with hospitalization, treatment, viremia or acute inflammation. Additionally, Modified Telephone Interview for Cognitive Status scores did not correlate with depressed mood or fatigue. In two severely affected patients, we excluded structural or other inflammatory causes by magnetic resonance imaging, serum and cerebrospinal fluid analyses. Together, our results demonstrate that sustained sub-clinical cognitive impairments might be a common complication after recovery from COVID-19 in young adults, regardless of clinical course that were unmasked by our diagnostic approach.
days) after recovery from mild to moderate disease who visited our outpatient clinic for post-COVID-19 care. Notably, 14 (78%) patients reported sustained mild cognitive deficits and performed worse in the Modified Telephone Interview for Cognitive Status screening test for mild cognitive impairment compared to 10 age-matched healthy controls. While short-term memory, attention and concentration were particularly affected by COVID-19, screening results did not correlate with hospitalization, treatment, viremia or acute inflammation. Additionally, Modified Telephone Interview for Cognitive Status scores did not correlate with depressed mood or fatigue. In two severely affected patients, we excluded structural or other inflammatory causes by magnetic resonance imaging, serum and cerebrospinal fluid analyses. Together, our results demonstrate that sustained sub-clinical cognitive impairments might be a common complication after recovery from COVID-19 in young adults, regardless of clinical course that were unmasked by our diagnostic approach.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first identified in December 2019 as the cause of a respiratory illness designated coronavirus disease 2019 (COVID-19) (Huang et al., 2020). While patients with COVID-19 frequently suffer from respiratory symptoms, neurologic and neuropsychiatric complications have been increasingly reported (Ellul et al., 2020; Varatharaj et al., 2020). Moreover, histopathologic examination of brains from deceased COVID-19 patients indicate the potential of SARS-CoV-2 to infiltrate the central nervous system (CNS) (Solomon et al., 2020).
Reported neuropsychiatric manifestations include milder symptoms like dizziness and anosmia (Hornuss et al., 2020) but also in rare cases severe manifestations such as acute demyelinating encephalopathy (Reichard et al., 2020), meningitis (Moriguchi et al., 2020) and strokes (Helms et al., 2020; Oxley et al., 2020). Recently, the symptoms of 153 patients with COVID-19 from the UK who reported neurologic and psychiatric complications during the acute phase of the disease were reported and intra-cerebral haemorrhages and altered mental status were the most common complications (Varatharaj et al., 2020). Similarly, in a study from Wuhan with 214 patients, 78 patients reported unspecific neurological symptoms and 13 patients had a new cerebrovascular diagnosis during acute infection (Mao et al., 2020).
These studies have focussed on severe neurologic and neuropsychiatric complications during the acute infection but did not include sustained neuropsychological deficits after full recovery from COVID-19. Moreover, severe neurologic complications have been mostly investigated in patients with multiple risk factors who developed severe COVID-19 with complications but not in young adults after recovery. For the outbreaks of the closely related SARS-CoV-2 and middle-east-respiratory-syndrome-coronavirus (MERS-CoV), acute delirium and encephalitis have been reported during acute disease but also sustained neuropsychologic syndromes (Rogers et al., 2020). Thus, deeper analysis and epidemiologic (Ritchie et al., 2020) studies as well as development of screening tools for mild cognitive deficits in young adults are an important unmet clinical need to detect sub-clinical neuropsychologic symptoms and help to differentiate unspecific post-illness-manifestations.
Here, we established a facile screening approach for cognitive deficits in 18 young patients without diagnosed cognitive pre-conditions after recovery from COVID-19 and discovered widespread sub-clinical deficits.
Materials and methods
Patient cohorts
For this cross-sectional study, we randomly interviewed patients from the outpatient clinic of the University Medical Centre Hamburg-Eppendorf (UKE) and included only the patients who did not stay at intensive care unit. In total, 21 patients were approached and 18 agreed to participate in our study. The severity of COVID-19 into mild, moderate, severe, critical and lethal disease courses was classified using the WHO criteria (WHO reference number: 451 WHO/2019-nCoV/clinical/2020.5). We included only the patients who suffered from mild and moderate COVID-19 and were not admitted to our intensive care unit. Since our patient cohort was considerably younger (mean age, 42.2 years; SD 14.3
years; SD 14.3 years), we additionally tested healthy individuals with similar age (mean age, 38.4
years), we additionally tested healthy individuals with similar age (mean age, 38.4 years; SD 14.4
years; SD 14.4 years). Randomly selected healthy individuals were the employees of the University Medical Center Hamburg-Eppendorf, who did not have prior knowledge of the Modified Telephone Interview for Cognitive Status (TICS-M) or similar neuropsychological screenings and were matched for age within a range of 5
years). Randomly selected healthy individuals were the employees of the University Medical Center Hamburg-Eppendorf, who did not have prior knowledge of the Modified Telephone Interview for Cognitive Status (TICS-M) or similar neuropsychological screenings and were matched for age within a range of 5 years but not for sex. Except for one patient who still visited high school, all patients and healthy participants received in total more than 12
years but not for sex. Except for one patient who still visited high school, all patients and healthy participants received in total more than 12 years of education. The patient-reported symptoms were collected by individual reports and documented symptoms during the inpatient and subsequent outpatient stays at the University Medical Center Hamburg-Eppendorf. During the interviews, we questioned the patients in a structured manner that included all organ systems and specifically asked for neuropsychological deficits.
years of education. The patient-reported symptoms were collected by individual reports and documented symptoms during the inpatient and subsequent outpatient stays at the University Medical Center Hamburg-Eppendorf. During the interviews, we questioned the patients in a structured manner that included all organ systems and specifically asked for neuropsychological deficits.
Assessment tools
The interviews were either conducted by phone or directly with the patient. Individuals were recruited until 14 July 2020. To sensitively screen for mild cognitive deficiencies, we utilized the TICS-M that was originally developed to broadly screen for mild cognitive impairment in elderly adults (MCI) by telephone. This questionnaire has been validated for amnestic mild cognitive impairment (reference for mean age, 74.9 years;
years;  amnestic mild cognitive impairment, <34) (Cook et al., 2009) and Alzheimer’s dementia (Manly et al., 2011). We performed TICS-M according to the previously published protocols (Cook et al., 2009). The total interview lasted 15–20
amnestic mild cognitive impairment, <34) (Cook et al., 2009) and Alzheimer’s dementia (Manly et al., 2011). We performed TICS-M according to the previously published protocols (Cook et al., 2009). The total interview lasted 15–20 min. Four domains are tested by the TICS-M: (i) orientation, (ii) recent memory and delayed memory, (iii) attention and (iv) semantic memory, comprehension and repetition (language/concentration) (De Jager et al., 2003). The TICS-M included the following items: (i) name, (ii) age, (iii) date, (iv) weekday, (v) season, (vi) phone number (each 1 point), (vii) counting backward (2 points), (viii) first, a 10-word list learning exercise and then a delayed (21) recall of that word list (10 points each), (ix) subtractions (5 points); (x–xiii) responsive naming (4 points), (xiv–xv) repetition (2 points), (xvi) current chancellor and (xvii) president of Germany (each 2 points), (xviii) finger tapping (2 points) and (xix, xx) word opposites (2 points). The total score was 50 points. The TICS-M score has been validated to test episodic memory for words, episodic memory for non-verbal information and attention (Crooks et al., 2006). We controlled for possible biases by the Patient Health Questionnaire-9 Depression Scale (PHQ-9) and Fatigue Assessment Scale. Two patients with peculiarly low TICS-M scores (Vignette A, 39 points; Vignette B, 31 points) who reported severe restrictions in their everyday life due to their reported COVID-19-associated neuropsychological symptoms underwent further neurologic and neuropsychologic evaluation.
min. Four domains are tested by the TICS-M: (i) orientation, (ii) recent memory and delayed memory, (iii) attention and (iv) semantic memory, comprehension and repetition (language/concentration) (De Jager et al., 2003). The TICS-M included the following items: (i) name, (ii) age, (iii) date, (iv) weekday, (v) season, (vi) phone number (each 1 point), (vii) counting backward (2 points), (viii) first, a 10-word list learning exercise and then a delayed (21) recall of that word list (10 points each), (ix) subtractions (5 points); (x–xiii) responsive naming (4 points), (xiv–xv) repetition (2 points), (xvi) current chancellor and (xvii) president of Germany (each 2 points), (xviii) finger tapping (2 points) and (xix, xx) word opposites (2 points). The total score was 50 points. The TICS-M score has been validated to test episodic memory for words, episodic memory for non-verbal information and attention (Crooks et al., 2006). We controlled for possible biases by the Patient Health Questionnaire-9 Depression Scale (PHQ-9) and Fatigue Assessment Scale. Two patients with peculiarly low TICS-M scores (Vignette A, 39 points; Vignette B, 31 points) who reported severe restrictions in their everyday life due to their reported COVID-19-associated neuropsychological symptoms underwent further neurologic and neuropsychologic evaluation.
Neuropsychological and neurological assessment
Two patients were invited for further neuropsychological and neurological assessment at the outpatient clinic for neuroimmunological diseases at the Department of Neurology of the University Medical Center Hamburg-Eppendorf, Germany. Neurological examination was performed by board-certified neurologists. Further diagnostic measures included analyses of serological parameters, cerebrospinal fluid (CSF) and cranial imaging.
SARS-CoV-2 diagnostic procedures
We used Cobas6800 system (Roche, Mannheim, Germany) for the detection of SARS-CoV-2 RNA from nasopharyngeal smears by polymerase chain reaction as previously described in detail (Pfefferle et al., 2020). For serology, we used Liaison XL system for quantitative SARS-CoV-2 IgG detection according to the manufacturer’s recommendation.
Statistical analysis
Data were analysed within the R environment (Version 1.2.5.002) on a Mac OS X. Unless stated otherwise, comparisons between two experimental groups are presented as violin plot with median or 95% confidence intervals and differences were determined using two-tailed, unpaired Wilcoxon–Mann–Whitney test and were false-discovery rate (FDR)-corrected for multiple comparisons. To find the predictors of screening results, we used multiple linear regression models. The results were FDR-corrected for multiple comparisons. Exact P-values are reported in respective sections of the article and figure legends. We analysed the scores of Fatigue Assessment Scale and Patient Health Questionnaire-9 Depression Scale as well as age, length of hospitalization, sickness duration, time from recovery to neurocognitive assessment, maximal PCR cycle threshold values, maximal antibody titres, maximal CRP, IL-6, ferritin and d-dimers as predictors. The outcome variable was the TICS-M score. For effect size of non-parametric comparisons, we calculated Rosenthals r. Significant results are indicated by *P <
<  0.05, **P
0.05, **P <
<  0.01 and ***P
0.01 and ***P <
<  0.001.
0.001.
Ethics statement
The study was approved by the Ärztekammer Hamburg. All patients and healthy participants gave consent for participation, data analysis and publication.
Data availability
Data are available from the corresponding author, upon reasonable request. Data are not publicly available due to ethical restrictions because the information contained on those data could compromise the privacy of the reported patients.
Results
The aim of this study was to establish a screening that sensitively and specifically detects subtle neurocognitive deficits. Therefore, we screened our post-COVID-19 outpatient clinic for mostly young patients with mild to moderate disease courses according to the WHO criteria (WHO reference number: 451 WHO/2019-nCoV/clinical/2020.5) without known cognitive pre-conditions who recovered without complications. We included 10 females and 8 males at ages ranging from 17 to 71 years (mean, 42.2
years (mean, 42.2 years; SD, 14.3
years; SD, 14.3 years). Our cohort consisted of 11 inpatients (61%), 6 outpatients (33%) and 1 patient did not seek medical care (6%). During the acute infection, four patients received supplementary oxygen, two patients were treated with remdesivir and one patient with tocilizumab due to cytokine storm. None of the patients received intensive care and no vascular or structural neurological event was recorded. All patients recovered without severe complications 20–105
years). Our cohort consisted of 11 inpatients (61%), 6 outpatients (33%) and 1 patient did not seek medical care (6%). During the acute infection, four patients received supplementary oxygen, two patients were treated with remdesivir and one patient with tocilizumab due to cytokine storm. None of the patients received intensive care and no vascular or structural neurological event was recorded. All patients recovered without severe complications 20–105 days (median, 85
days (median, 85 days from COVID-19 recovery to assessment time) prior to the timepoint of our screening (a summary of patients’ characteristics is provided in Table 1). In addition, we tested 10 healthy individuals with similar age (n
days from COVID-19 recovery to assessment time) prior to the timepoint of our screening (a summary of patients’ characteristics is provided in Table 1). In addition, we tested 10 healthy individuals with similar age (n =
= 10; mean age, 38.4
10; mean age, 38.4 years; SD, 14.4
years; SD, 14.4 years; a summary of healthy controls’ characteristics is provided in Table 1) as control group.
years; a summary of healthy controls’ characteristics is provided in Table 1) as control group.
Table 1
Summary of characteristics and manifestations of post-COVID-19 patients and healthy control subjects
| Characteristics | Post-COVID-19 patients | Healthy controls |
|---|---|---|
| Mean age (range) | 42.11 (17–71) | 38.4 (22–59) |
| Age distribution (%) | ||
 <20 <20 | 1 (5.6) | 0 (0) |
 20–40 20–40 | 8 (42.2) | 6 (60) |
 40–60 40–60 | 8 (47.4) | 4 (40) |
 >60 >60 | 1 (5.2) | 0 (0) |
| Sex (%) | ||
 Female Female | 10 (57.9) | 4 (40) |
 Male Male | 8 (42.1) | 6 (60) |
| Pre-conditions (%) | ||
 Asthma bronchiale Asthma bronchiale | 3 (16.7) | Not assessed |
 Hypothyreosis Hypothyreosis | 3 (16.7) | Not assessed |
 Hypertonus Hypertonus | 2 (11.1) | Not assessed |
 Coagulation disorder Coagulation disorder | 2 (11.1) | Not assessed |
 Diabetes mellitus type 2 Diabetes mellitus type 2 | 1 (5.6) | Not assessed |
 Multiple sclerosis Multiple sclerosis | 1 (5.6) | Not assessed |
 Autoimmune hepatitis Autoimmune hepatitis | 1 (5.6) | Not assessed |
 Follicular lymphoma Follicular lymphoma | 1 (5.6) | Not assessed |
| Clinical stay (%) | ||
 Outpatient clinic Outpatient clinic | 6 (68.5) | Not assessed |
 Inpatient clinic Inpatient clinic | 11 (31.5) | Not assessed |
| Treatment (%) | ||
 Oxygen supplementation Oxygen supplementation | 6 (33.3) | Not assessed |
 Remdesivir Remdesivir | 3 (16.7) | Not assessed |
 Antibiotics Antibiotics | 2 (11.1) | Not assessed |
 Tocilizumab Tocilizumab | 1 (5.6) | |
| Neuropsychiatric symptoms (%) | ||
 Attention deficits Attention deficits | 9 (50.0) | 0 (0) |
 Concentration deficits Concentration deficits | 8 (44.4) | 0 (0) |
 Short-term memory deficits Short-term memory deficits | 8 (44.4) | 0 (0) |
 Troubles in finding words Troubles in finding words | 5 (27.8) | 0 (0) |
 Fatigue Fatigue | 3 (16.7) | 0 (0) |
 Severe mood swings Severe mood swings | 2 (11.1) | 0 (0) |
 Lack of energy Lack of energy | 1 (5.6) | 0 (0) |
 Phonophobia Phonophobia | 1 (5.6) | 0 (0) |
 Incoherent thoughts Incoherent thoughts | 1 (5.6) | 0 (0) |
| Test screening results (range) | ||
 TICS-M TICS-M | 38.83 (31–46) | 45.8 (43–50) |
 Fatigue Assessment Scale Fatigue Assessment Scale | 24.17 (13–40) | 18.1 (18–19) |
 Patient Health Questionnaire-9 Depression Scale Patient Health Questionnaire-9 Depression Scale | 2.83 (0–9) | 0.7 (0–2) |
We chose the TICS-M as primary tool (Cook et al., 2009) as it has been extensively validated for screening of mild cognitive deficiencies by telephone. This we considered important when conducting larger studies that include affected individuals who might not seek professional health care and stay in home quarantine. Strikingly, post-COVID-19 patients scored significantly lower results in the TICS-M (mean, 38.83; range, 31–46) compared to healthy controls (mean, 45.8; range, 43–50) (Fig. 1A), especially regarding short-term memory, attention and concentration/language tasks (Fig. 1B). Notably, results from screening for depression (Personal Health Questionnaire 9) and for fatigue (Fatigue Assessment Scale) did not show significant correlation with TICS-M scores (Fig. 1C and D). In terms of patients’ self-reported symptoms, out of 18 included individuals 9 (50%) reported attention deficits, 8 (44.4%) concentration deficits, 8 (44.4%) short-term memory deficits, 5 (27.8%) troubles in finding words, 3 (16.7%) fatigue, 2 (11.1%) severe mood swings and 1 (5.6%) sustained lack of energy, phonophobia and incoherent thoughts (Fig. 1E).
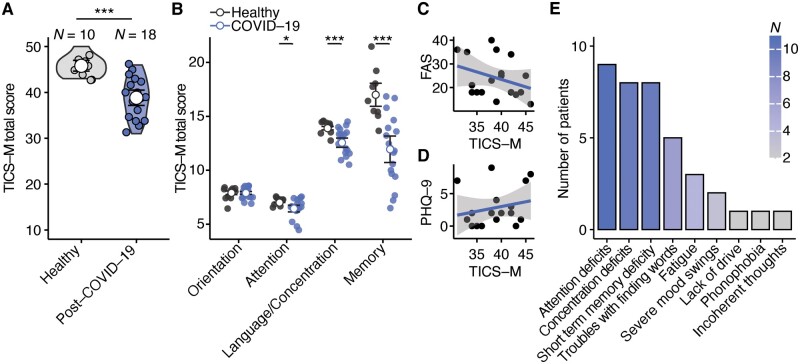
Cognitive deficiencies in post-COVID-19 patients. (A) Comparison of TICS-M total scores (P =
= 0.0002) between healthy individuals (n
0.0002) between healthy individuals (n =
= 10) and post-COVID-19 patients (n
10) and post-COVID-19 patients (n =
= 18). Two-tailed Wilcoxon-test was used and mean with 95% confidence interval is shown. (B) Comparison of the different cognitive domains orientation (P
18). Two-tailed Wilcoxon-test was used and mean with 95% confidence interval is shown. (B) Comparison of the different cognitive domains orientation (P =
= 0.9643), attention (P
0.9643), attention (P =
= 0.029), language and concentration (P
0.029), language and concentration (P =
= 0.009) and memory (P
0.009) and memory (P =
= 0.004) that were tested with the TICS-M. Two-tailed Wilcoxon-test was used and mean with 95% confidence interval is shown. (C, D) Linear regression analysis of TICS-M scores and Fatigue Assessment Scale (C; t = −1.3653, FDR-adjusted P
0.004) that were tested with the TICS-M. Two-tailed Wilcoxon-test was used and mean with 95% confidence interval is shown. (C, D) Linear regression analysis of TICS-M scores and Fatigue Assessment Scale (C; t = −1.3653, FDR-adjusted P =
= 0.3820, Estimate = –0.165) and Patient Health Questionnaire-9 Depression Scale (D; t
0.3820, Estimate = –0.165) and Patient Health Questionnaire-9 Depression Scale (D; t =
= 0.8957, FDR-adjusted P
0.8957, FDR-adjusted P =
= 0.3836, Estimate = 0.324) scores of post-COVID-patients. (E) Reported neuropsychiatric symptoms that sustained after recovery.
0.3836, Estimate = 0.324) scores of post-COVID-patients. (E) Reported neuropsychiatric symptoms that sustained after recovery.
Next, we aimed to find the predictors of our observed cognitive deficits. First, we analysed patients’ characteristics and found that neither sex (Fig. 2A) nor age (Fig. 2B) could explain the observed differences. Additionally, we investigated whether severity of the acute COVID-19 disease could be an explanation. Therefore, we next analysed whether hospitalization had an impact on post-COVID-19 manifestations. However, we found that TICS-M scores in post-COVID-19 patients did not correlate with the time interval from our interview to recovery (Fig. 2C), length of sickness (Fig. 2D) and length of inpatient stay (Fig. 2E). To further evaluate the impact of disease severity, we correlated the neurocognitive deficits with acute and sustained somatic symptoms (Fig. 2F) and treatments during the acute infection. Our analysis revealed that the number of somatic symptoms did not correlate with the number of sustained self-reported neurocognitive deficits (Fig. 2G) and the results in our screening (Fig. 2H). Moreover, treatments such as oxygen supplementation (Fig. 3A) and drugs such as remdesivir, tocilizumab or antibiotics (Fig. 3B) in acute infection could not predict the observed cognitive impairments. Thus, our data imply that post-COVID-19 neuropsychologic deficits are independent from hospitalization and disease severity.
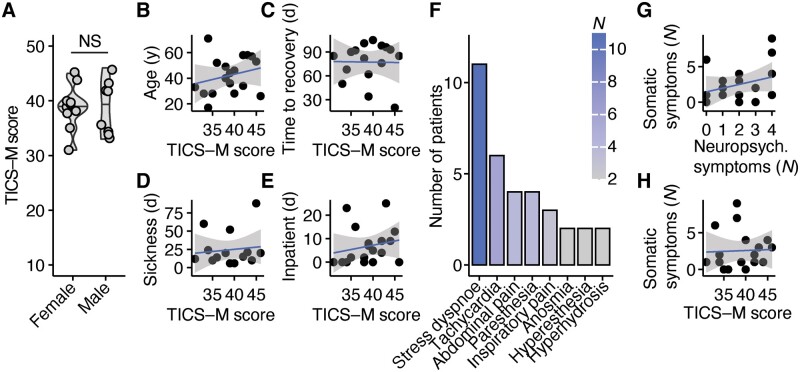
Cognitive deficits are independent from hospitalization and sickness duration. (A) Comparison of TICS-M total scores (P =
= 0.9644) between female (n
0.9644) between female (n =
= 10) and male (n
10) and male (n =
= 8) post-COVID-19 patients. Two-tailed Wilcoxon-test was used. (B–E) Linear regression analysis of TICS-M scores and age in years (B; t
8) post-COVID-19 patients. Two-tailed Wilcoxon-test was used. (B–E) Linear regression analysis of TICS-M scores and age in years (B; t =
= 1.0241, FDR-adjusted P
1.0241, FDR-adjusted P =
= 0.6420, Estimate = 0.057), time to recovery from acute COVID-19 in days (C; t = −0.0576, FDR-adjusted P
0.6420, Estimate = 0.057), time to recovery from acute COVID-19 in days (C; t = −0.0576, FDR-adjusted P =
= 0.9548, Estimate = −0.003), duration of sickness in days (D; t = −0.0576, FDR-adjusted P
0.9548, Estimate = −0.003), duration of sickness in days (D; t = −0.0576, FDR-adjusted P =
= 0.9548, Estimate = 0.023) and duration of inpatient treatment in days (E; t
0.9548, Estimate = 0.023) and duration of inpatient treatment in days (E; t =
= 0.8254, FDR-adjusted P
0.8254, FDR-adjusted P =
= 0.7021, Estimate = 0.112) of post-COVID-patients. (F) Self-reported somatic symptoms that appeared at least once after recovery from COVID-19 and were reported from at least two patients. (G,H) Linear regression analysis of number of somatic and neurocognitive symptoms (G; t
0.7021, Estimate = 0.112) of post-COVID-patients. (F) Self-reported somatic symptoms that appeared at least once after recovery from COVID-19 and were reported from at least two patients. (G,H) Linear regression analysis of number of somatic and neurocognitive symptoms (G; t =
= 1.282, FDR-adjusted P
1.282, FDR-adjusted P =
= 0.2181, Estimate = 0.177) and number of somatic symptoms and TICS-M scores (H; t
0.2181, Estimate = 0.177) and number of somatic symptoms and TICS-M scores (H; t =
= 0.161, FDR-adjusted P
0.161, FDR-adjusted P =
= 0.874, Estimate = 0.068).
0.874, Estimate = 0.068).
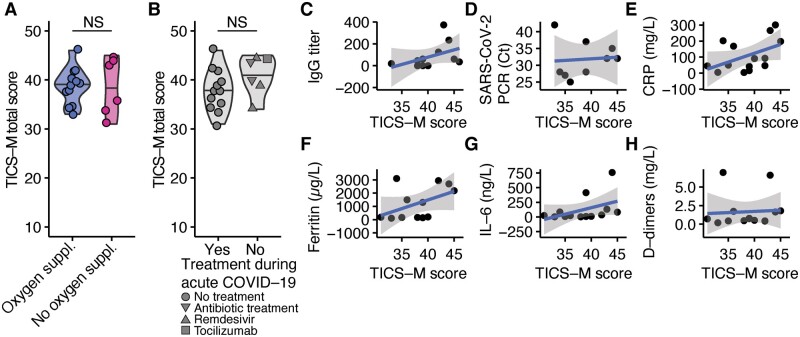
Cognitive deficits are independent from acute disease severity and viremia. (A) Comparison of TICS-M total scores (P =
= 0.9251) between post-COVID-19 patients who received supplementary oxygen during acute disease (n
0.9251) between post-COVID-19 patients who received supplementary oxygen during acute disease (n =
= 6) and patients who recovered without supplementary oxygen (n
6) and patients who recovered without supplementary oxygen (n =
= 12). Two-tailed Wilcoxon-test was used. (B) Comparison of TICS-M total scores (P
12). Two-tailed Wilcoxon-test was used. (B) Comparison of TICS-M total scores (P =
= 0.1589) between post-COVID-19 patients who received no treatment (n
0.1589) between post-COVID-19 patients who received no treatment (n =
= 12), antibiotics (n
12), antibiotics (n =
= 2), remdesivir (n
2), remdesivir (n =
= 2) or tocilizumab (n
2) or tocilizumab (n =
= 1) during acute COVID-19. Two-tailed Wilcoxon-test was used. (C–H) Linear regression analysis of TICS-M scores and maximal anti-SARS-CoV2-IgG titre (C; t
1) during acute COVID-19. Two-tailed Wilcoxon-test was used. (C–H) Linear regression analysis of TICS-M scores and maximal anti-SARS-CoV2-IgG titre (C; t =
= 1.4352, FDR-adjusted P
1.4352, FDR-adjusted P =
= 0.4626, Estimate = 0.014), cycle threshold values in SARS-CoV-2 PCR (D; t
0.4626, Estimate = 0.014), cycle threshold values in SARS-CoV-2 PCR (D; t =
= 0.8422, FDR-adjusted P
0.8422, FDR-adjusted P =
= 0.9358, Estimate = 0.064), CRP (E; t = −0.0811, FDR-adjusted P
0.9358, Estimate = 0.064), CRP (E; t = −0.0811, FDR-adjusted P =
= 0.4363, Estimate = 0.021), ferritin (F; t
0.4363, Estimate = 0.021), ferritin (F; t =
= 0.1266, FDR-adjusted P
0.1266, FDR-adjusted P =
= 0.4363, Estimate = 0.002), IL-6 (G; t = −0.1309, FDR-adjusted P
0.4363, Estimate = 0.002), IL-6 (G; t = −0.1309, FDR-adjusted P =
= 0.4363, Estimate = 0.009), d-dimers (H; t
0.4363, Estimate = 0.009), d-dimers (H; t =
= 0.8330, FDR-adjusted P
0.8330, FDR-adjusted P =
= 0.4363, Estimate = 0.121) during acute COVID-19 infection.
0.4363, Estimate = 0.121) during acute COVID-19 infection.
Subsequently, we analysed the impact of acute inflammation and maximal viremia in acute COVID-19 on neurocognitive deficits. Therefore, we accounted the cycle threshold from SARS-CoV-2 PCR, antibodies against SARS-CoV-2 and inflammatory serum markers. Our analysis revealed that maximal SARS-CoV-2 IgG-titres (Fig. 3C), SARS-CoV-2-PCR cycle threshold values (Fig. 3D), CRP (Fig. 3E), ferritin (Fig. 3F), IL-6 (Fig. 3G) and d-dimer (Fig. 3H) serum concentration during acute COVID-19 were not significant predictors of our observed neurocognitive deficits.
For further diagnostic measures, we investigated the two most severely affected patients by cranial MRI and lumbar puncture that excluded structural pathologies and acute inflammation. Detailed neuropsychologic evaluation confirmed deficits of attention, executive functions and memory (detailed case vignettes are reported in the supplementary material).
Discussion
SARS-CoV-2 affects multiple organ systems by infiltrating endothelial cells of blood vessels throughout the whole body (Varga et al., 2020). Thus, a multitude of symptoms and clinical disease courses have been described (Gupta et al., 2020). Here, we focussed on the evaluation of neurocognitive post-COVID-19 manifestations in mostly young adults who recovered from acute uncomplicated COVID-19. This study demonstrates substantial neurocognitive deficits that sustain after recovery and advocate screening routines for cognitive deficits during medical care of post-COVID-19 patients.
Cerebrovascular events and altered mental status have been described to be common neuropsychiatric manifestations in acute COVID-19 in a nationwide surveillance study in the UK (Varatharaj et al., 2020). In total, 59% of patients with altered mental status could be assigned to a neuropsychiatric disorder, underlining the diversity of COVID-19-associated manifestations. We screened patients after recovery from COVID-19 and found substantial neurocognitive deficits that sustained after acute infection. Moreover, we detected subtle cognitive deficits that did not restrain most patients in daily life and were only unmasked by our specific screening, including deficits in short-term memory, attention and concentration. Retrospective meta-analysis of SARS and MERS outbreaks has revealed acute and long-term neuropsychological deficits. Similar to our findings, most-common post-illness manifestations included impaired concentration and attention in 19.9% and impaired memory in 18.9% of patients after recovery (Rogers et al., 2020). Therefore, our study represents first indications that warrant broad screenings in post-COVID-19 patients to clarify the diversity of neuropsychological deficits and prevent potential further harm.
Screening methods for mild cognitive deficits are mostly used in the diagnostics of dementia that were validated in elderly adults (Castanho et al., 2014). Here, we chose TICS-M as it has been validated as a telephone screening method which is important for prospective studies that include patients who did not seek professional medical care during or after COVID-19. However, TICS-M has been validated for the diagnosis of mild amnestic dementia in a patient cohort with an average age of 74.9 years (Cook et al., 2009). Since the average age of our patient cohort (42.1
years (Cook et al., 2009). Since the average age of our patient cohort (42.1 years) was considerably lower, we additionally tested a healthy control group with similar age as control. Thus, further optimization of a standardized screening tool is needed. Our findings suggest to especially focus the screening on short-term memory, attention and concentration. In contrast to currently available screening tools, validation should include all age groups since we and others (Dinakaran et al., 2020; Nalleballe et al., 2020) observed neurocognitive deficits in young adults.
years) was considerably lower, we additionally tested a healthy control group with similar age as control. Thus, further optimization of a standardized screening tool is needed. Our findings suggest to especially focus the screening on short-term memory, attention and concentration. In contrast to currently available screening tools, validation should include all age groups since we and others (Dinakaran et al., 2020; Nalleballe et al., 2020) observed neurocognitive deficits in young adults.
Post-viral-syndromes have been described for multiple viral infections, such as Epstein–Barr–Virus or influenza (Hotchin et al., 1989) and are characterized by severe fatigue (Thomas, 1987). Smartphone-App-based patients’ reports assessing fatigue in COVID-19 (Menni et al., 2020) and histopathological findings of viral infiltrates and diffuse immune cell activation in brains from deceased COVID-19 patients (Polak et al., 2020) may indicate similar clinical presentations. However, systematic analysis with large patient cohorts in different cultural settings and different countries is needed for clarification. To exclude potential biases and classical post-viral-syndromes, we additionally screened for fatigue and depression. Our data indicate that neurocognitive deficits after recovery from COVID-19 are independent from fatigue and mood alterations and therefore might be different from the classical post-viral syndrome (Perrin et al., 2020) but a specific post-COVID-19 manifestation. SARS-CoV-2 might infiltrate the CNS through the nose (Riel et al., 2015) and trigger a reactive immune response in the brain that could alter neuronal signalling. In addition, exposure of human brain organoids to SARS-CoV-2 revealed direct infection of neurons with subsequent alterations of intracellular signalling and cell death (Ackermann et al., 2020) that could disturb neuronal connectivity. Notably, TICS-M scores of post-COVID-19 patients did not significantly correlate with the maximal inflammatory response during the acute infection. Studies that investigated chronic fatigue syndrome in rheumatic disorders (Korte and Straub, 2019) and multiple sclerosis (Giovannoni, 2006) found that chronic but not acute dysregulation of the immune metabolism and especially cytokine composition correlated with fatigue and individual suffering. Although serum profiling during acute COVID-19 revealed distinct cytokine profiles that correlated with disease outcome (Lucas et al., 2020), detailed immunological profiling of post-COVID-19 patients is sparse. Our study is limited by the sample size. Therefore, we cannot report representative frequencies of post-COVID-19 manifestations and longitudinal monitoring and analysis of large cohorts of post-COVID-19 patients is warranted to find clear correlates with long-lasting symptoms after recovery.
Furthermore, we used the TICS-M that was originally developed to screen for MCI in elderly adults because we aimed to establish a tool that was validated for telephone interviews. However, our test results should be validated by neuropsychological tests that were established in young adults such as the Cambridge Automated Test Battery (Crooks et al., 2006). Furthermore, confounders for cognitive testing such as years of education and substance abuse were not assessed. However, we documented the patients’ profession and except for one participant who still visited high school all patients received at least 12 years of education. Moreover, only two patients received detailed neuropsychological and neurological assessment that confirmed the results of our screening as well as cranial MRI and lumbar puncture to exclude other potential pathologies. However, we provide a clear description of the observed deficits and provide distinct symptoms that will instruct prospective screenings in larger cohorts to sensitively identify post-COVID-19 patients with cognitive deficits.
years of education. Moreover, only two patients received detailed neuropsychological and neurological assessment that confirmed the results of our screening as well as cranial MRI and lumbar puncture to exclude other potential pathologies. However, we provide a clear description of the observed deficits and provide distinct symptoms that will instruct prospective screenings in larger cohorts to sensitively identify post-COVID-19 patients with cognitive deficits.
Together, the results of our study demonstrate that young patients who recovered from uncomplicated COVID-19 can have sustained neuropsychologic deficits that can be unmasked by targeted screening.
Supplementary Material
fcaa205_Supplementary_Data
Acknowledgements
The authors thank the UKE ID COVID-19 study team for their support. They thank members of the Friese and Schulze zur Wiesch laboratories for discussions. They also thank Marc Lütgehetmann for providing PCR and antibody titres. The authors thank Stephanie Lau for helping with neuropsychological assessments. The graphical abstract was created using biorender.com. M.A.F. received honoraria for consultation and travel expenses from Biogen, Merck KGaA, Novartis and Roche unrelated to this Correspondence.
Funding
A.H., M.M.A., J.S.Z.W. are funded by the Deutsche Zentrum für Infektionsforschung (DZIF).
Competing interests
The authors declare no competing interests and no conflict of interest.
Glossary
| CNS = | central nervous system |
| COVID-19 = | Coronavirus disease 2019 caused by the Coronavirus SARS-CoV-2 |
| CSF = | cerebrospinal fluid |
| FDR = | false-discovery rate; |
| MCI = | mild cognitive impairment in elderly adults |
| MERS-CoV = | Middle-east-respiratory-syndrome-coronavirus |
| SARS-CoV-2 = | severe acute respiratory syndrome coronavirus 2 |
| TICS-M = | Modified Telephone Interview for Cognitive Status |
Contributor Information
Marcel S Woo, Institute of Neuroimmunology and Multiple Sclerosis, University Medical Center Hamburg-Eppendorf, 20251 Hamburg, Germany.
Jakob Malsy, Division of Infectious Diseases, University Medical Center Hamburg-Eppendorf, 20246 Hamburg, Germany. Department of Medicine, University Medical Center Hamburg-Eppendorf, 20246 Hamburg, Germany.
Jana Pöttgen, Institute of Neuroimmunology and Multiple Sclerosis, University Medical Center Hamburg-Eppendorf, 20251 Hamburg, Germany.
Susan Seddiq Zai, Institute of Neuroimmunology and Multiple Sclerosis, University Medical Center Hamburg-Eppendorf, 20251 Hamburg, Germany.
Friederike Ufer, Institute of Neuroimmunology and Multiple Sclerosis, University Medical Center Hamburg-Eppendorf, 20251 Hamburg, Germany. Department of Neurology, University Medical Center Hamburg-Eppendorf, 20246 Hamburg, Germany.
Alexandros Hadjilaou, Institute of Neuroimmunology and Multiple Sclerosis, University Medical Center Hamburg-Eppendorf, 20251 Hamburg, Germany. Department of Neurology, University Medical Center Hamburg-Eppendorf, 20246 Hamburg, Germany.
Stefan Schmiedel, Division of Infectious Diseases, University Medical Center Hamburg-Eppendorf, 20246 Hamburg, Germany. Department of Medicine, University Medical Center Hamburg-Eppendorf, 20246 Hamburg, Germany.
Marylyn M Addo, Division of Infectious Diseases, University Medical Center Hamburg-Eppendorf, 20246 Hamburg, Germany. Department of Medicine, University Medical Center Hamburg-Eppendorf, 20246 Hamburg, Germany. German Center for Infection Disease (DZIF), University Medical Center Hamburg-Eppendorf, 20246 Hamburg, Germany.
Christian Gerloff, Department of Neurology, University Medical Center Hamburg-Eppendorf, 20246 Hamburg, Germany.
Christoph Heesen, Institute of Neuroimmunology and Multiple Sclerosis, University Medical Center Hamburg-Eppendorf, 20251 Hamburg, Germany. Department of Neurology, University Medical Center Hamburg-Eppendorf, 20246 Hamburg, Germany.
Julian Schulze Zur Wiesch, Division of Infectious Diseases, University Medical Center Hamburg-Eppendorf, 20246 Hamburg, Germany. Department of Medicine, University Medical Center Hamburg-Eppendorf, 20246 Hamburg, Germany. German Center for Infection Disease (DZIF), University Medical Center Hamburg-Eppendorf, 20246 Hamburg, Germany.
Manuel A Friese, Institute of Neuroimmunology and Multiple Sclerosis, University Medical Center Hamburg-Eppendorf, 20251 Hamburg, Germany.
References
- Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F., et al.Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N Engl J Med 2020; 383: 120–8. [Europe PMC free article] [Abstract] [Google Scholar]
- Castanho TC, Amorim L, Zihl J, Palha JA, Sousa N, Santos NC. Telephone-based screening tools for mild cognitive impairment and dementia in aging studies: a review of validated instruments. Front Aging Neurosci 2014; 6: 16. 10.3389/fnagi.2014.00016. [Europe PMC free article] [Abstract] [Google Scholar]
- Cook SE, Marsiske M, McCoy KJM. The use of the Modified Telephone Interview For Cognitive Status (TICS-M) in the detection of amnestic mild cognitive impairment. J Geriatr Psychiatry Neurol 2009; 22: 103–9. [Europe PMC free article] [Abstract] [Google Scholar]
- Crooks VC, Petitti DB, Robins SB, Buckwalter JG. Cognitive domains associated with performance on the telephone interview for cognitive status-modified. Am J Alzheimers Dis Other Dement 2006; 21: 45–53. [Abstract] [Google Scholar]
- De Jager CA, Budge MM, Clarke R. Utility of TICS-M for the assessment of cognitive function in older adults. Int J Geriatr Psychiatry 2003; 18: 318–24. [Abstract] [Google Scholar]
- Dinakaran D, Manjunatha N, Naveen Kumar C, Suresh BM. Neuropsychiatric aspects of COVID-19 pandemic: a selective review. Asian J Psychiatr 2020; 53: 102188. [Europe PMC free article] [Abstract] [Google Scholar]
- Ellul MA, Benjamin L, Singh B, Lant S, Michael BD, Easton A., et al.Neurological associations of COVID-19 [Internet]. Lancet Neurol 2020; 19: 767–783. 10.1016/S1474-4422(20)30221-0. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Giovannoni G. Multiple sclerosis related fatigue. J Neurol Neurosurg Psychiatry 2006; 77: 2–3. [Europe PMC free article] [Abstract] [Google Scholar]
- Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS., et al.Extrapulmonary manifestations of COVID-19. Nat Med 2020; 26: 1017–32. [Abstract] [Google Scholar]
- Helms J, Kremer S, Merdji H, Clere-Jehl R, Schenck M, Kummerlen C., et al.Neurologic features in severe SARS-CoV-2 infection. N Engl J Med 2020; 382: 2268–70. [Europe PMC free article] [Abstract] [Google Scholar]
- Hornuss D Lange B Schröter N Rieg S Kern WV Wagner D. Anosmia in COVID-19 patients. Clin Microbiol Infect 2020; 26: 1426–7. 10.1016/j.cmi.2020.05.017. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Hotchin NA, Read R, Smith DG, Crawford DH. Active Epstein-Barr virus infection in post-viral fatigue syndrome. J Infect 1989; 18: 143–50. [Abstract] [Google Scholar]
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y., et al.Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. [Europe PMC free article] [Abstract] [Google Scholar]
- Korte SM, Straub RH. Fatigue in inflammatory rheumatic disorders: pathophysiological mechanisms. Rheumatology 2019; 58: V35–50. [Europe PMC free article] [Abstract] [Google Scholar]
- Lucas C, Wong P, Klein J, Castro TBR, Silva J, Sundaram M., et al.; Yale IMPACT Team. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature 2020; 584: 463–9. [Europe PMC free article] [Abstract] [Google Scholar]
- Manly JJ, Schupf N, Stern Y, Brickman AM, Tang MX, Mayeux R. Telephone-based identification of mild cognitive impairment and dementia in a multicultural cohort. Arch Neurol 2011; 68: 607–14. [Europe PMC free article] [Abstract] [Google Scholar]
- Mao L, Jin H, Wang M, Hu Y, Chen S, He Q., et al.Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 2020; 77: 683–90. [Europe PMC free article] [Abstract] [Google Scholar]
- Menni C, Valdes AM, Freidin MB, Sudre CH, Nguyen LH, Drew DA., et al.Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat Med 2020; 26: 1037–40. [Europe PMC free article] [Abstract] [Google Scholar]
- Moriguchi T, Harii N, Goto J, Harada D, Sugawara H, Takamino J., et al.A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis 2020; 94: 55–8. [Europe PMC free article] [Abstract] [Google Scholar]
- Nalleballe K, Reddy Onteddu S, Sharma R, Dandu V, Brown A, Jasti M., et al.Spectrum of neuropsychiatric manifestations in COVID-19 [Internet]. Brain Behav Immun 2020; 88: 71–74. 10.1016/j.bbi.2020.06.020. [Europe PMC free article] [Abstract] [Google Scholar]
- Oxley TJ, Mocco J, Majidi S, Kellner CP, Shoirah H, Singh IP., et al.Large-vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med 2020; 382: e60. [Europe PMC free article] [Abstract] [Google Scholar]
- Perrin R, Riste L, Hann M, Walther A, Mukherjee A, Heald A. Into the looking glass: post-viral syndrome post COVID-19. Med Hypotheses 2020; 144: 110055. [Europe PMC free article] [Abstract] [Google Scholar]
- Pfefferle S, Reucher S, Nörz D, Lütgehetmann M. Evaluation of a quantitative RT-PCR assay for the detection of the emerging coronavirus SARS-CoV-2 using a high throughput system. Eurosurveill 2020; 25: 2000152. [Europe PMC free article] [Abstract] [Google Scholar]
- Polak SB, Van Gool IC, Cohen D, von der Thüsen JH, van Paassen J. A systematic review of pathological findings in COVID-19: a pathophysiological timeline and possible mechanisms of disease progression. Mod Pathol 2020; 33: 2128–11. [Europe PMC free article] [Abstract] [Google Scholar]
- Reichard RR, Kashani KB, Boire NA, Constantopoulos E, Guo Y, Lucchinetti CF. Neuropathology of COVID-19: a spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. Acta Neuropathol 2020; 140: 1–6. [Europe PMC free article] [Abstract] [Google Scholar]
- Riel DV, Verdijk R, Kuiken T. The olfactory nerve: a shortcut for influenza and other viral diseases into the central nervous system. J Pathol 2015; 235: 277–87. [Abstract] [Google Scholar]
- Ritchie K, Chan D, Watermeyer T. The cognitive consequences of the COVID-19 epidemic: collateral damage? [Internet]. Brain Commun 2020; 2. 10.1093/braincomms/fcaa069. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Rogers JP, Chesney E, Oliver D, Pollak TA, McGuire P, Fusar-Poli P., et al.Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry 2020; 7: 611–27. [Europe PMC free article] [Abstract] [Google Scholar]
- Solomon IH, Normandin E, Bhattacharyya S, Mukerji SS, Keller K, Ali AS., et al.Neuropathological features of Covid-19 [Internet]. N Engl J Med 2020; 383: 989–92. 10.1056/NEJMc2019373. [Europe PMC free article] [Abstract] [Google Scholar]
- Thomas PK. Postviral fatigue syndrome. Lancet 1987; 329: 218–9. [Abstract] [Google Scholar]
- Varatharaj A, Thomas N, Ellul MA, Davies NWS, Pollak TA, Tenorio EL., et al.Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study [Internet]. Lancet Psychiatry 2020; 7: 875–882. 10.1016/S2215-0366(20)30287-X. [Europe PMC free article] [Abstract] [Google Scholar]
- Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS., et al.Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020; 395: 1417–8. [Europe PMC free article] [Abstract] [Google Scholar]
Articles from Brain Communications are provided here courtesy of Oxford University Press
Full text links
Read article at publisher's site: https://doi.org/10.1093/braincomms/fcaa205
Read article for free, from open access legal sources, via Unpaywall:
https://academic.oup.com/braincomms/article-pdf/2/2/fcaa205/38696611/fcaa205.pdf
Citations & impact
Impact metrics
Article citations
Structural and functional brain markers of cognitive impairment in healthcare workers following mild SARS-CoV-2 infection during the original stream.
Brain Commun, 6(5):fcae340, 30 Sep 2024
Cited by: 0 articles | PMID: 39416878 | PMCID: PMC11481020
Cognitive outcomes and psychological symptoms in an Italian cohort with post-acute COVID-19 condition (PACC).
Heliyon, 10(20):e39431, 16 Oct 2024
Cited by: 0 articles | PMID: 39469684 | PMCID: PMC11513557
Deep into Cognition: The Neuropsychological Identikit of Younger and Older Individuals after COVID-19 Infection.
Biology (Basel), 13(10):754, 24 Sep 2024
Cited by: 0 articles | PMID: 39452064 | PMCID: PMC11504078
Altered functional brain connectivity, efficiency, and information flow associated with brain fog after mild to moderate COVID-19 infection.
Sci Rep, 14(1):22094, 27 Sep 2024
Cited by: 0 articles | PMID: 39333726 | PMCID: PMC11437042
Dissociable effects of mild COVID-19 on short- and long-term memories.
Brain Commun, 6(4):fcae270, 14 Aug 2024
Cited by: 0 articles | PMID: 39210912 | PMCID: PMC11358641
Go to all (181) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Multidomain cognitive impairment in non-hospitalized patients with the post-COVID-19 syndrome: results from a prospective monocentric cohort.
J Neurol, 270(3):1215-1223, 23 Nov 2022
Cited by: 16 articles | PMID: 36422669 | PMCID: PMC9686246
Cognitive Complaints Assessment and Neuropsychiatric Disorders After Mild COVID-19 Infection.
Arch Clin Neuropsychol, 38(2):196-204, 01 Feb 2023
Cited by: 4 articles | PMID: 36464245
Neurocognitive screening in patients following SARS-CoV-2 infection: tools for triage.
BMC Neurol, 22(1):285, 30 Jul 2022
Cited by: 12 articles | PMID: 35907815 | PMCID: PMC9338515
Cognitive and Neuropsychiatric Manifestations of COVID-19 and Effects on Elderly Individuals With Dementia.
Front Aging Neurosci, 12:588872, 26 Oct 2020
Cited by: 90 articles | PMID: 33192483 | PMCID: PMC7649130
Review Free full text in Europe PMC
Funding
Funders who supported this work.