Abstract
Free full text

Altered functional brain connectivity, efficiency, and information flow associated with brain fog after mild to moderate COVID-19 infection
Abstract
COVID-19 is associated with increased risk for cognitive decline but very little is known regarding the neural mechanisms of this risk. We enrolled 49 adults (55% female, mean age =
= 30.7
30.7 ± 8.7), 25 with and 24 without a history of COVID-19 infection. We administered standardized tests of cognitive function and acquired brain connectivity data using MRI. The COVID-19 group demonstrated significantly lower cognitive function (W
± 8.7), 25 with and 24 without a history of COVID-19 infection. We administered standardized tests of cognitive function and acquired brain connectivity data using MRI. The COVID-19 group demonstrated significantly lower cognitive function (W =
= 475, p
475, p <
< 0.001, effect size r
0.001, effect size r =
= 0.58) and lower functional connectivity in multiple brain regions (mean t
0.58) and lower functional connectivity in multiple brain regions (mean t =
= 3.47
3.47 ±0.36, p
±0.36, p =
= 0.03, corrected, effect size d
0.03, corrected, effect size d =
= 0.92 to 1.5). Hypo-connectivity of these regions was inversely correlated with subjective cognitive function and directly correlated with fatigue (p
0.92 to 1.5). Hypo-connectivity of these regions was inversely correlated with subjective cognitive function and directly correlated with fatigue (p <
< 0.05, corrected). These regions demonstrated significantly reduced local efficiency (p
0.05, corrected). These regions demonstrated significantly reduced local efficiency (p <
< 0.026, corrected) and altered effective connectivity (p
0.026, corrected) and altered effective connectivity (p <
< 0.001, corrected). COVID-19 may have a widespread effect on the functional connectome characterized by lower functional connectivity and altered patterns of information processing efficiency and effective information flow. This may serve as an adaptation to the pathology of SARS-CoV-2 wherein the brain can continue functioning at near expected objective levels, but patients experience lowered efficiency as brain fog.
0.001, corrected). COVID-19 may have a widespread effect on the functional connectome characterized by lower functional connectivity and altered patterns of information processing efficiency and effective information flow. This may serve as an adaptation to the pathology of SARS-CoV-2 wherein the brain can continue functioning at near expected objective levels, but patients experience lowered efficiency as brain fog.
Introduction
COVID-19 is a highly infectious disease caused by the novel coronavirus SARS-CoV-2. As of this publication, current estimates by the World Health Organization indicate over 103 million total cases in the U.S1. Although COVID-19 is an acute respiratory syndrome, it is now recognized as a multi-organ disease with a broad range of symptoms2. The brain is among the organs directly and indirectly affected, resulting in a variety of neurological complications including cognitive decline3. While most patients recover from the acute disease phase, many COVID-19 survivors will experience significant ongoing symptoms4,5. These symptoms commonly include persistent cognitive impairment6–10, which negatively impacts quality of life and ability to return to work11,12.
Neuroimaging provides valuable insights regarding the underlying mechanisms of cognitive impairment. It has been used to predict cognitive outcomes related to injury and disease13, to phenotype, or biotype distinct subgroups within a diagnostic category14, and predict response to cognitive therapies15. Importantly, neuroimaging is frequently more sensitive to mild or subtle changes in brain function compared to behavioral testing13. In fact, traditional neuropsychological tests may lack sensitivity to detect subtle cognitive deficits in conditions that share similarities to COVID-19 such as cancer-related cognitive impairment and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)16,17. Neuroimaging has also been shown to detect intervention-related effects when cognitive testing does not18,19.
However, there have been few neuroimaging studies of post-COVID-19 related cognitive deficits. Most studies have focused on patients who had been hospitalized for COVID-19, have not separated those with mild/moderate symptoms from those with severe symptoms in analyses, or have used mild/moderate cases as controls for severe ones20–29. Therefore, these results may reflect an increased disease burden and do not provide insight regarding the brain changes that may occur in mild to moderate cases. It is also known that ICU survivors are at high risk for significant cognitive deficits, regardless of diagnosis30.
Cognitive impairment is not limited to severe cases but also seems to be prevalent among those with mild to moderate disease not requiring hospitalization. Studies, including our own, have shown that 22–78% of patients with mild to moderate disease may be affected, depending on the sample characteristics and assessments utilized31–35. Given that most COVID-19 cases are mild to moderate, with more recent SARS-CoV-2 variants also leading to Long COVID, further cognitive outcomes research is needed that focuses on these survivors.
Of the few available neuroimaging studies that focused on non-critical/non-hospitalized cases, widespread abnormalities in gray matter volumes, white matter integrity or cerebral perfusion have been documented36–39. These studies have examined COVID-19 survivors 30–179 days post-infection. Most studies observed relationships between neuroimaging metrics and cognitive performance, although not all studies measured these relationships. There have been few functional connectivity studies focused on cognitive effects of COVID-19. The brain is a functional network, or connectome, and the analysis of functional connectivity can provide unique and valuable insights regarding the organization of this network with respect to information processing.
One study found both increased and decreased functional connectivity affecting left cingulate, anterior cingulate, and insula40. However, these were patients being treated for neurological complications related to COVID-19 and were compared with patients being treated for other neurological conditions. The study also did not specify whether COVID-19 infection was mild, moderate or severe. Another study showed increased functional connectivity in the default mode network, but also did not specify the severity of COVID-19 infection41. Jin et al. noted decreased connectivity in the bilateral postcentral gyrus following mild infection42. Bungenberg et al. demonstrated regions of increased and decreased connectivity in patients being treated for long COVID after mild infection43. Another study demonstrated hyperconnectivity in subcortical, somatosensory motor, and cerebellar regions in moderate compared to mild cases but did not evaluate mild cases26. Churchill et al. observed widespread alterations in functional connectivity 4–5 months post mild to moderate COVID-19 infection44. Regions of hyper- and hypo-connectivity were observed although overall mean connectivity was reduced in COVID-19 survivors.
Using a standardized neuropsychological testing battery, we previously demonstrated significant cognitive deficit in non-hospitalized survivors of mild to moderate COVID-19 who were assessed approximately 4 months post-diagnosis34. However, we did not have a control group or imaging data. In the present study, we examined a new cohort of mild to moderate COVID-19 survivors who were longer term post-infection (around 10 months) and compared them to a frequency matched group of non-infected, fully vaccinated controls. We obtained cognitive testing (objective and subjective), neuropsychiatric symptom assessment (e.g., fatigue, anxiety, depression), anosmia assessment, and functional magnetic resonance imaging (fMRI) data to examine the neural mechanisms of post-COVID-19 related cognitive impairment. We hypothesized that COVID-19 survivors would demonstrate lower cognitive function and altered functional connectome (brain network) organization compared to controls. Given that prior imaging studies of mild to moderate COVID-19 have indicated widespread abnormalities in gray matter, white matter integrity, and cerebral perfusion, we expected widespread alterations in functional connectivity potentially with areas of hypo- and hyper-connectivity.
Results
COVID-19 group characteristics
As shown in Table Table1,1, participants with a history of COVID-19 infection were nearly evenly split between mild and moderate disease severity with relatively low symptom burden during the acute infection. There was a wide range in the interval between COVID-19 diagnosis and study enrollment (12–719 days, mean of 10 ± 7 months).
± 7 months).
Table 1
Demographic and COVID-19 characteristics.
COVID N = = 25 25 | Control N = = 24 24 | Stat | p value | |
|---|---|---|---|---|
| Age (years) | 30.3 (8.0) | 30.3 (9.0) | t = = -0.003 -0.003 | 0.997 |
| Education (years) | 16.4 (1.8) | 16.9 (1.6) | t = = 0.995 0.995 | 0.325 |
| Face mask during fMRI | N = = 2 (8%) 2 (8%) | N = = 3 (12.5%) 3 (12.5%) | X2 = = 0.223 0.223 | 0.637 |
| Female gender | N = = 14 (56.0%) 14 (56.0%) | N = = 13 (54.2%) 13 (54.2%) | X2 = = 0.017 0.017 | 0.897 |
Income < < $100K $100K | N = = 12 (48%) 12 (48%) | N = = 8 (33.3%) 8 (33.3%) | X2 = = 1.113 1.113 | 0.292 |
| Racial/ethnic minority | N = = 3 (12.0%) 3 (12.0%) | N = = 11 (45.8%) 11 (45.8%) | X2 = = 6.868 6.868 | 0.009 |
| Time since diagnosis (days) | 307 (223) range: 12–719 | |||
| COVID severity moderate | N = = 11 (44.0%) 11 (44.0%) | |||
| No. of symptoms during active COVID infection | Median Range |
Data are shown as mean (standard deviation) unless otherwise indicated.
Neuropsychiatric symptoms
The COVID-19 group demonstrated lower performance on all objective cognitive tests (effect size r =
= 0.15 to 0.30) as well as the PROMIS Cognitive score, a measure of subjective cognitive function. However, only PROMIS Cognitive score was significantly different between groups (p
0.15 to 0.30) as well as the PROMIS Cognitive score, a measure of subjective cognitive function. However, only PROMIS Cognitive score was significantly different between groups (p <
< 0.001, corrected, effect size r
0.001, corrected, effect size r =
= 0.58). The COVID-19 group also endorsed greater symptoms of anxiety and depression on the PROMIS-57 measure; however, these were not significantly different between groups after correction for multiple comparisons (Table (Table22).
0.58). The COVID-19 group also endorsed greater symptoms of anxiety and depression on the PROMIS-57 measure; however, these were not significantly different between groups after correction for multiple comparisons (Table (Table22).
Table 2
Neuropsychiatric testing performance.
COVID N = = 25 25 | Control N = = 24 24 | Stat (W) | Uncorrected p value | FDR corrected p value | Rank biserial correlation | |
|---|---|---|---|---|---|---|
| Trails A | 97.2 (11.9) | 104.4 (13.7) | 372.5 | 0.081 | 0.211 | 0.296 |
| Trails B | 98.6 (11.9) | 100.8 (15.5) | 333.0 | 0.353 | 0.386 | 0.158 |
| Digit symbol substitution | 95.0 (14.8) | 99.2 (15.1) | 353.5 | 0.289 | 0.386 | 0.178 |
| Stroop | 94.4 (19.4) | 101.1 (15.8) | 341.5 | 0.269 | 0.386 | 0.188 |
| Immediate recall | 100.2 (18.9) | 106.5 (9.8) | 344.0 | 0.356 | 0.386 | 0.147 |
| Delayed recall | 102.4 (13.8) | 106.5 (10.5) | 359.5 | 0.219 | 0.386 | 0.198 |
| PROMIS cognitive | 49.4 (11.4) | 60.1 (6.2) | 475.0 | 0.000 |  < < 0.001 0.001 | 0.583 |
| PROMIS social role performance | 57.2 (9.7) | 59.1 (8.4) | 332.0 | 0.494 | 0.494 | 0.107 |
| PROMIS anxiety | 55.0 (11.6) | 48.0 (9.4) | 195.0 | 0.035 | 0.199 | − 0.350 |
| PROMIS depression | 48.9 (8.9) | 43.7 (5.4) | 202.5 | 0.046 | 0.199 | − 0.325 |
| PROMIS fatigue | 49.9 (9.9) | 44.4 (9.2) | 212.0 | 0.078 | 0.211 | − 0.293 |
| PROMIS sleep disturbance | 50.9 (9.8) | 46.5 (7.2) | 236.0 | 0.202 | 0.386 | − 0.213 |
| PROMIS pain | 45.5 (7.1) | 43.8 (6.0) | 260.0 | 0.333 | 0.386 | − 0.133 |
Data are shown as mean (standard deviation). Rank biserial correlation is the effect size.
FDR false discovery rate correction for multiple comparisons.
false discovery rate correction for multiple comparisons.
Demographic and clinical predictors of cognitive performance
A multiple linear regression model for predicting PROMIS Cognitive score from demographic and clinical variables was significant (adjusted R2 =
= 0.545, F
0.545, F =
= 4.20, p
4.20, p =
= 0.007, Table Table3).3). However, fatigue was the only significant predictor (beta
0.007, Table Table3).3). However, fatigue was the only significant predictor (beta =
= −0 .896, p
−0 .896, p

 ≤ 0.001).
≤ 0.001).
Table 3
Demographic and clinical predictors of subjective cognitive function.
| Predictor | Unstandardized coefficient | Standardized coefficient | p value |
|---|---|---|---|
| Female | 1.31 | 0.736 | |
| Age | − 0.017 | − 0.012 | 0.948 |
| Time since COVID-19 diagnosis | − 0.006 | − 0.115 | 0.479 |
| Education | 0.987 | 0.162 | 0.329 |
| Fatigue | − 0.896 | − 0.784 |  < < 0.001 0.001 |
| Loss of smell (during infection) | 1.83 | 0.742 | |
| Loss of taste (during infection) | − 4.78 | 0.435 | |
| Moderate COVID-19 severity | − 0.621 | 0.881 | |
| Racial/ethnic minority | − 1.48 | 0.782 |
Functional connectome group differences
As shown in Fig. 1 and Supplementary Table 1, Network-based Statistic (NBS) analysis of functional brain connectivity (functional connectome organization) identified one large subnetwork of edges (functional connections) that was significantly different between the groups. Specifically, the COVID-19 group demonstrated significantly lower functional connectivity in multiple brain regions representing all cerebral lobes, subcortical structures, and cerebellum, involving 7 out of 8 functional networks [t =
= 3.47
3.47 ± 0.36, range
± 0.36, range =
= 3.1–5.14, p
3.1–5.14, p =
= 0.033, family-wise error rate (FWE) corrected, effect size d
0.033, family-wise error rate (FWE) corrected, effect size d =
= 0.92 to 1.5]. These edges are hereafter referred to as “hypo-connected connectome edges”. The COVID-19 group did not show any areas of higher functional connectivity compared to controls. Most (82%) of the hypo-connected connectome edges in the COVID-19 group involved the default mode network, primarily (66%) dorsolateral prefrontal/orbitofrontal regions.
0.92 to 1.5]. These edges are hereafter referred to as “hypo-connected connectome edges”. The COVID-19 group did not show any areas of higher functional connectivity compared to controls. Most (82%) of the hypo-connected connectome edges in the COVID-19 group involved the default mode network, primarily (66%) dorsolateral prefrontal/orbitofrontal regions.
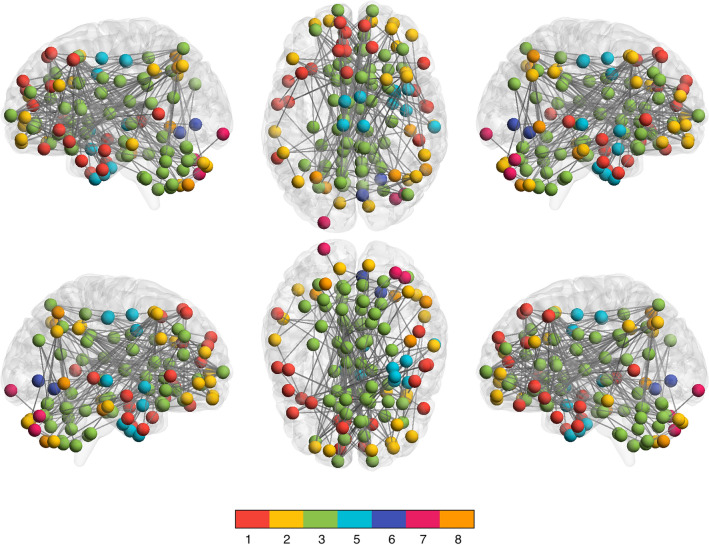
Hypo-connected functional connectome edges. Network-based statistic (NBS) analysis indicated that the COVID-19 group demonstrated significantly lower functional connectivity compared to controls in multiple cortical and subcortical regions across the entire brain, especially the default mode network (mean t =
= 3.47
3.47 ± 0.36, range
± 0.36, range =
= 3.1–5.14, p
3.1–5.14, p =
= 0.033, FWE corrected, d
0.033, FWE corrected, d =
= 0.92 to 1.5). The functional connectome is shown here as a graph with spheres representing nodes (regions) and lines representing their functional connections (edges). Nodes are colored by their membership in 1 of 8 functional networks evaluated (see color bar): 1: medial frontal, 2: frontoparietal, 3: default mode, 4: subcortical/cerebellar, 5: motor, 6: visual 1, 7: visual 2, 8: visual association (there were no significant differences in network 4). There were no regions of significant hyper-connectivity.
0.92 to 1.5). The functional connectome is shown here as a graph with spheres representing nodes (regions) and lines representing their functional connections (edges). Nodes are colored by their membership in 1 of 8 functional networks evaluated (see color bar): 1: medial frontal, 2: frontoparietal, 3: default mode, 4: subcortical/cerebellar, 5: motor, 6: visual 1, 7: visual 2, 8: visual association (there were no significant differences in network 4). There were no regions of significant hyper-connectivity.
Functional connectome correlates of neuropsychiatric function, demographic and COVID-19 characteristics
One subnetwork of hypo-connected connectome edges was negatively correlated with PROMIS Cognitive score in the COVID-19 group [mean r =
= −0.411
−0.411 ±0.048, range
±0.048, range =
= −0.366 to −0.476, p
−0.366 to −0.476, p <
< 0.043, false discovery rate (FDR) corrected]. Edges included those in left cingulate, bilateral inferior orbitofrontal gyri, left caudate, bilateral fusiform gyri, right superior temporal gyrus and right cerebellum crus 1, connecting default mode network with medial frontal and frontoparietal networks and motor network with medial frontal network (Fig. 2a, Supplementary Table 2). There were no significant correlations with demographics or COVID-19 characteristics (p
0.043, false discovery rate (FDR) corrected]. Edges included those in left cingulate, bilateral inferior orbitofrontal gyri, left caudate, bilateral fusiform gyri, right superior temporal gyrus and right cerebellum crus 1, connecting default mode network with medial frontal and frontoparietal networks and motor network with medial frontal network (Fig. 2a, Supplementary Table 2). There were no significant correlations with demographics or COVID-19 characteristics (p >
> 0.25, FDR corrected).
0.25, FDR corrected).
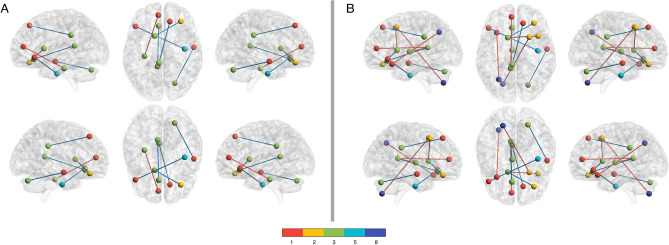
Hypo-connected Functional Connectome Edges Correlated with PROMIS Cognitive and Fatigue Scores. In the COVID group, several hypo-connected edges were significantly correlated with (A) PROMIS Cognitive score (p <
< 0.043, FDR corrected) and (B) PROMIS Fatigue score (p
0.043, FDR corrected) and (B) PROMIS Fatigue score (p <
< 0.034, FDR corrected). Most (83%) of the edges correlated with Cognition were also correlated with Fatigue and these edges are colored in blue. Nodes are colored by their membership in 1 of 8 functional networks evaluated (see color bar): 1: medial frontal, 2: frontoparietal, 3: default mode, 4: subcortical/cerebellar, 5: motor, 6: visual 1, 7: visual 2, 8: visual association (not all networks were significant and therefore are not represented).
0.034, FDR corrected). Most (83%) of the edges correlated with Cognition were also correlated with Fatigue and these edges are colored in blue. Nodes are colored by their membership in 1 of 8 functional networks evaluated (see color bar): 1: medial frontal, 2: frontoparietal, 3: default mode, 4: subcortical/cerebellar, 5: motor, 6: visual 1, 7: visual 2, 8: visual association (not all networks were significant and therefore are not represented).
Fatigue is the most reported and fundamental symptom of Long COVID5 and was the only significant predictor of PROMIS Cognitive score in our COVID-19 cohort. Therefore, we also conducted NBS correlation analysis between hypo-connected connectome edges and PROMIS Fatigue score in the COVID-19 group. As shown in Fig. 2b and Supplementary Table 2, one subnetwork of edges significantly correlated with fatigue. Most were positively correlated and there was notable overlap with the edges that correlated with PROMIS Cognitive score. Specifically, 5/6 edges (83%) correlating with PROMIS Cognitive score also correlated with PROMIS Fatigue score and 5/10 edges (50%) correlating with PROMIS Fatigue also correlated with PROMIS Cognitive score.
Efficiency of information processing in fatigue-related regions
Given the overlap between cognition and fatigue in terms of functional connectome correlates, the evidence that lower brain network information processing efficiency is directly related to lower metabolic cost45, and the association between chronic fatigue and hypometabolic states46, we hypothesized that fatigue-related hypo-connected functional connectome edges would be the most likely to demonstrate lower information processing efficiency. Therefore, we measured local efficiency only for the nodes encompassing fatigue-related hypo-connected edges. This strategy also served to reduce the number of comparisons for this analysis. As shown in Table Table4,4, local efficiency was significantly lower in the COVID-19 group for 5/17 (29%) fatigue-related nodes, after correction for multiple comparisons (p <
< 0.026, FDR corrected). These nodes included left cingulate and right cerebellum crus 1 and were primarily located in the default mode network.
0.026, FDR corrected). These nodes included left cingulate and right cerebellum crus 1 and were primarily located in the default mode network.
Table 4
Efficiency of information processing for nodes encompassing fatigue-related hypo-connected connectome edges.
| Node | COVID | Control | W | Uncorrected p value | FDR corrected p value |
|---|---|---|---|---|---|
| Net_1_node_012_RSuperiorFrontalGyrus | 0.826 (0.026) | 0.839 (0.032) | 393 | 0.032 | 0.075 |
| Net_1_node_064_RSuperiorTemporalGyrus | 0.829 (0.026) | 0.831 (0.028) | 310 | 0.425 | 0.499 |
| Net_1_node_140_LAnteriorCingulate | 0.820 (0.021) | 0.845 (0.032) | 429 | 0.005 | 0.024 |
| Net_1_node_151_LInferiorFrontalGyrusOrbitalis | 0.816 (0.026) | 0.831 (0.029) | 396 | 0.028 | 0.075 |
| Net_1_node_153_LInferiorFrontalGyrusOrbitalis | 0.813 (0.026) | 0.827 (0.021) | 391 | 0.035 | 0.075 |
| Net_2_node_014_RMiddleFrontalGyrus | 0.817 (0.023) | 0.821 (0.026) | 321 | 0.341 | 0.499 |
| Net_2_node_017_RInferiorFrontalGyrusOrbitalis | 0.818 (0.024) | 0.818 (0.031) | 298 | 0.520 | 0.552 |
| Net_2_node_030_RMiddleFrontalGyrus | 0.820 (0.029) | 0.828 (0.029) | 346 | 0.181 | 0.343 |
| Net_3_node_134_LAnteriorCingulate | 0.830 (0.023) | 0.848 (0.025) | 427 | 0.006 | 0.024 |
| Net_3_node_223_LPosteriorCingulate | 0.821 (0.025) | 0.847 (0.030) | 448 | 0.002 | 0.024 |
| Net_3_node_227_LPosteriorCingulate | 0.817 (0.029) | 0.836 (0.027) | 422 | 0.008 | 0.026 |
| Net_3_node_114_RCerebelumCrus1 | 0.809 (0.024) | 0.829 (0.021) | 437 | 0.003 | 0.024 |
| Net_3_node_258_LCaudateNucleus | 0.816 (0.028) | 0.822 (0.023) | 338 | 0.227 | 0.385 |
| Net_3_node_263_LThalamus | 0.823 (0.029) | 0.823 (0.019) | 317 | 0.371 | 0.499 |
| Net_5_node_058_RFusiformGyrus | 0.830 (0.031) | 0.815 (0.025) | 205 | 0.972 | 0.972 |
| Net_8_node_177_LInferiorParietalLobule | 0.814 (0.029) | 0.814 (0.025) | 309 | 0.433 | 0.499 |
| Net_8_node_240_LCerebelumVIII | 0.815 (0.031) | 0.818 (0.022) | 308 | 0.440 | 0.499 |
Data are shown as mean (standard deviation).
FDR false discovery rate.
Nodes that were significantly different between groups after correction for multiple comparisons are highlighted in bold text. Net: functional network; net 1: medial frontal, net 2: frontoparietal, net 3: default mode, net 4: subcortical/cerebellar, net 5: motor, net 6: visual 1, net 7: visual 2, net 8: visual association. Node: node number used to differentiate regions of interest within the parcellation atlas that have similar labels but different anatomical boundaries.
Effective (causal) connectivity among fatigue-related regions
We measured effective connectivity only for fatigue-related edges as well. Visually, the two directed acyclic graph (DAGs) representing the information flow among brain regions are very different considering the low number of true positive edges compared to the large number of false positive and false negative edges in the COVID-19 network compared to controls (Fig. 3). In fact, the number of false negatives (mean difference =
= 60, permutation distribution 95%CI 22 to 41) and false positives (mean difference
60, permutation distribution 95%CI 22 to 41) and false positives (mean difference =
= 43, permutation distribution 95%CI 15 to 32) in the COVID-19 group were significant (p
43, permutation distribution 95%CI 15 to 32) in the COVID-19 group were significant (p <
< 0.001, FDR corrected).
0.001, FDR corrected).
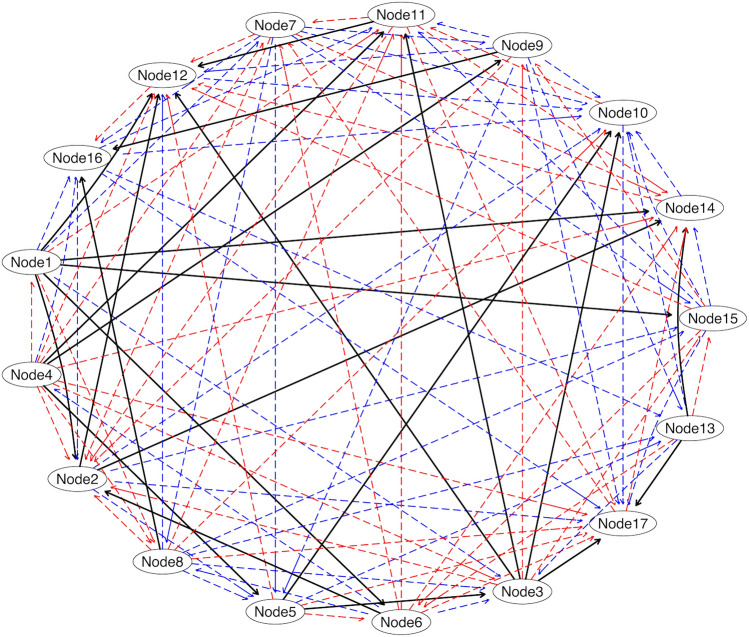
Effective (Causal) Connectivity Differences in COVID-19 Survivors Compared to Control. A true positive edge (shown in black) was defined as a connection between two regions in the COVID-19 group that existed in the control group with the same pathway (i.e., same child, same parent regions). A false positive (shown in blue) was defined as a connection between two regions in the COVID-19 group that did not exist in the control group or did not reflect the same pathway (e.g., different set of parent regions, different child region). A false negative (shown in red) was defined as the absence of a connection between regions for which there was an existing connection in the control group. The number of false negatives (mean difference =
= 60, permutation distribution 95%CI 22 to 41) and false positives (mean difference
60, permutation distribution 95%CI 22 to 41) and false positives (mean difference =
= 43, permutation distribution 95%CI 15 to 32) in the COVID-19 group were significant (p
43, permutation distribution 95%CI 15 to 32) in the COVID-19 group were significant (p <
< 0.001). See Supplementary Table 3 for node anatomic labels.
0.001). See Supplementary Table 3 for node anatomic labels.
Discussion
Our current findings are consistent with our previous study involving a different cohort34. However, given the inclusion here of a comparison group, we observed less cognitive impairment than in our prior study. This might also relate to the difference in assessment timing as our prior cohort were approximately 4 months post-infection, on average, and the current cohort was approximately 10 months post-infection. However, consistent with our prior results as well as those from other groups47, subjective cognitive impairment in the present COVID-19 cohort was markedly greater than objective impairment.
Network-based statistic (NBS) analysis indicated widespread hypo-connectivity of the functional connectome in the COVID-19 group compared to controls. Our findings are consistent with prior studies showing widespread alterations in functional connectivity post-COVID-19 infection42–44. Regions from 7 out of 8 functional networks demonstrated significantly lower connectivity, especially the default mode network. This network is believed to support implicit learning, prospection, monitoring, and self-reflection, among others48. Consistent with prior neuroimaging studies of non-critical COVID-19, orbitofrontal regions were among the most affected25,36,37. These regions are important for emotion processing, especially decision making related to reward systems49.
We observed a significant relationship between brain fog (subjective cognitive dysfunction) and fatigue in terms of both behavioral and neurofunctional measures. It is therefore likely that these represent a similar construct or overlapping neural mechanisms. The brain is highly plastic throughout the lifespan and can adapt remarkably well even to very significant injuries through reorganization of neural networks. However, the brain is an economical, critical system that must balance many competing demands50. A large-scale reduction in connectivity could theoretically reduce the energy demands in the brain but this would also reduce information processing efficiency45. Accordingly, we demonstrated that several fatigue-related hypo-connected connectome edges had significantly reduced local efficiency in the COVID-19 group compared to controls. This finding indicates that these brain regions had fewer direct connections, which can reduce parallel information processing, but also results in lower brain network cost.
Additionally, the effective (causal) functional connectivity of these edges was significantly altered in the COVID-19 group, meaning that the typical paths of information flow were significantly reorganized, which would again result in reduced information processing efficiency. These adaptations may allow the brain to continue functioning at near expected objective levels, but a patient would likely be very subjectively aware of the changes in efficiency and would thus endorse brain fog. Future studies should examine the effects of exertion on the relationships between cognitive function, fatigue and connectome efficiency. One study demonstrated reduced functional connectivity after cognitive exertion in patients with long COVID51, but the effects of physical exertion remain unclear. It is likely that there is individual variance in the post-COVID brain’s ability to adapt to additional energy demands, which may be important for understanding cognitive effects.
There were no effects of demographic or clinical characteristics on cognitive, imaging or COVID-19 outcomes. We previously found that age and gender may contribute to certain cognitive outcomes in non-critical COVID-19 survivors34 though few if any other studies have examined these factors. The demographic contributors to cognitive and connectome dysfunction following non-critical COVID-19 require further investigation.
Post COVID neurologic deficits share similarities with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). ME/CFS has been consistently associated with increased spatial extent of functional brain activation during tasks and cerebral hyporesponsiveness52,53. Prior studies have also demonstrated significant default mode network functional hypo-connectivity in patients with ME/CFS54. Default mode network has the highest glucose metabolic rate of all functional brain networks55, making it particularly vulnerable to hypometabolic states and ME/CSF is associated with hypometabolism46. Our local efficiency findings in COVID-19 are consistent with a prior study that demonstrated reduced brain network efficiency in ME/CSF utilizing alternative efficiency metrics56. We were unable to find a prior study of effective connectivity in ME/CSF, however, reduced cognitive efficiency has been proposed as the neural mechanism of subjective cognitive dysfunction in these patients, based on a systematic review of evidence across different neuroimaging analyses52.
Cognitive effects of COVID-19 have also been compared to cancer-related cognitive impairment (CRCI) in terms of molecular and neurobiologic mechanisms57. Studies demonstrate that CRCI is consistently characterized by significant subjective cognitive impairment and widespread brain abnormalities in the context of objectively normal cognitive performance58. CRCI is associated with chronic fatigue58 as well as altered effective connectivity and reduced brain network local efficiency, especially in frontal regions and default mode network59. The CRCI literature suggests that subjective and objective cognitive dysfunction represent different phenotypes of this syndrome and have distinct neural mechanisms60.
Therefore, as an exploratory post-hoc analysis, we examined the relationship between fatigue and Trails A score in our COVID-19 cohort, given that this was the most significantly different objective test between the COVID-19 and control groups. There was no correlation between PROMIS Fatigue and Trails A scores (r = −0.047, p
= −0.047, p =
= 0.823) or between PROMIS Cognitive and Trails A scores (r
0.823) or between PROMIS Cognitive and Trails A scores (r =
= 0.087, p
0.087, p =
= 0.678). Trails A score correlated with several hypo-connected connectome edges though none of these overlapped with those correlated with PROMIS Fatigue or PROMIS Cognitive scores (Supplementary Table 2). Furthermore, correlations between hypo-connected connectome edges and Trails A score were all positive indicating that lower connectivity among these brain regions negatively impacted Trails A performance.
0.678). Trails A score correlated with several hypo-connected connectome edges though none of these overlapped with those correlated with PROMIS Fatigue or PROMIS Cognitive scores (Supplementary Table 2). Furthermore, correlations between hypo-connected connectome edges and Trails A score were all positive indicating that lower connectivity among these brain regions negatively impacted Trails A performance.
Comparatively, correlations between hypo-connected connectome edges and PROMIS Cognitive score were all negative indicating that lower connectivity among these specific brain regions was beneficial for subjective cognition. Thus, it seems reasonable that the lower metabolic cost of reduced brain connectivity would result in lower cognitive fatigue and therefore lower subjective cognitive complaint, whereas the lower efficiency of reduced connectivity would result in lower objective cognitive ability. Not all the differences identified by NBS may be entirely pathological; some may reflect the brain’s adaptive response to the SARS-CoV-2 infection.
There are several limitations to this study including the small sample size and the cross-sectional design. It is also impossible to ensure that control participants did not have a prior asymptomatic, undetected COVID-19 infection, which is a limitation of all post-COVID studies. There is some evidence of altered cerebral perfusion among non-critical COVID-19 survivors37, which may impact the neurovascular coupling that fMRI signal relies upon. However, there is evidence supporting the reliability and reproducibility of functional connectomes in other syndromes where neurovascular coupling is an even larger issue61. Additionally, face masks worn during acquisition may affect the baseline fMRI signal, although there seem to be negligible effects on resting state functional connectivity62. Few participants in our study wore a face mask during the MRI (N =
= 5, 10%) and there was no difference between the groups in terms of this number. A more comprehensive battery of tests may be required to detect objective cognitive deficits. There are several alternative methods of functional and effective connectivity analyses which may yield different findings.
5, 10%) and there was no difference between the groups in terms of this number. A more comprehensive battery of tests may be required to detect objective cognitive deficits. There are several alternative methods of functional and effective connectivity analyses which may yield different findings.
In conclusion, this comprehensive functional and effective connectome study demonstrates novel insights regarding the neural mechanisms of COVID-19 related cognitive dysfunction. Our results suggest that COVID-19 may have a diffuse impact on functional brain connectivity, resulting in hypo-connected cortical and subcortical regions. We found that local efficiency as well as effective or directional connectivity were also affected, disrupting the pattern of information flow. These perturbations in functional connectivity across brain regions likely make brain function less efficient, which could result in the patients’ observed cognitive complaints and decline. The use of neuroimaging findings as potential intervention targets will require further study to differentiate between pathological and compensatory changes as well as how this technique may be used to track patient recovery. Further research is needed to determine the biomarkers of subjective versus objective cognitive effects of COVID, but our findings suggest that default mode network hypo-connectivity and inefficiency play an important role. Our findings also suggest that future studies may benefit from including more specific, sensitive, objective behavioral measures of processing speed or multitasking that involve parametric difficulty levels, stress testing or exertion to test the limits of neuroplastic adaptation.
Methods
Participants
Between October 2021 and January 2023, we recruited adults with and without history of COVID-19 in central Texas. Potential participants were recruited via social media, community boards, and hospital referrals. In total, we enrolled 50 adults (54% female) aged 21 to 50 years (mean =
= 30.7
30.7 ±8.7). Twenty-six participants had a self-reported history of positive COVID-19 test and the remaining 24 participants self-reported no history of infection by test or associated symptoms. Participants were not screened based on the presence or absence of cognitive or other neurologic symptoms. We excluded one participant in the COVID-19 group for receiving treatment in a Post-COVID Clinic for Long COVID given that this study focused on post-COVID-19 related cognitive deficits and not Long COVID. Participants in the COVID-19 group were excluded for self-reported signs or symptoms of severe infection including self-rating of severity or hospitalization. Any potential participant in either group was excluded for pre-existing history of developmental, medical, or psychiatric disorder known to affect cognition function, significant sensory impairment (e.g., blindness), or MRI contraindications (e.g., magnetic biomedical implants, certain orthodontia, claustrophobia). There were no significant differences between the two groups in any demographic characteristics except for racial/ethnic minority status which was significantly higher in the control group (Table (Table1).1). This study was approved by the University of Texas at Austin Institutional Review Board (protocol# 00001337), was conducted in accordance with the Declaration of Helsinki, and all participants provided written, informed consent.
±8.7). Twenty-six participants had a self-reported history of positive COVID-19 test and the remaining 24 participants self-reported no history of infection by test or associated symptoms. Participants were not screened based on the presence or absence of cognitive or other neurologic symptoms. We excluded one participant in the COVID-19 group for receiving treatment in a Post-COVID Clinic for Long COVID given that this study focused on post-COVID-19 related cognitive deficits and not Long COVID. Participants in the COVID-19 group were excluded for self-reported signs or symptoms of severe infection including self-rating of severity or hospitalization. Any potential participant in either group was excluded for pre-existing history of developmental, medical, or psychiatric disorder known to affect cognition function, significant sensory impairment (e.g., blindness), or MRI contraindications (e.g., magnetic biomedical implants, certain orthodontia, claustrophobia). There were no significant differences between the two groups in any demographic characteristics except for racial/ethnic minority status which was significantly higher in the control group (Table (Table1).1). This study was approved by the University of Texas at Austin Institutional Review Board (protocol# 00001337), was conducted in accordance with the Declaration of Helsinki, and all participants provided written, informed consent.
Demographic and COVID-19 symptom measures
We used instruments recommended by the National Institutes of Health to facilitate COVID-19-related research. Specifically, from the NIH Repository of COVID-19 Research Tools, we administered the RADxUP Sociodemographic Questionnaire and the COVID-19 Experiences (COVEX) questionnaire to measure COVID-severity, and symptoms that occurred during active COVID-19 infection, such as shortness of breath, loss of taste and smell, etc. The COVEX questionnaire defines illness severity as mild (“dry cough, headache, nausea/diarrhea, aches and pains, low-grade fever, no need to see a doctor or hospitalization”), moderate [“coughing, high fever (above 100.00 Fahrenheit or 37.80 Celsius), chills, feeling that you cannot get out of bed, shortness of breath],” severe (“breathlessness, complications leading to pneumonia”), and critical (“respiratory failure, septic shock, and/or organ dysfunction or failure”). We also assessed current anosmia using the Pocket Smell Test63.
Neuropsychiatric symptoms
We administered BrainCheck, a computerized battery of neuropsychological tests including Trails A (attention, processing speed, executive function), Trails B (attention, processing speed, executive function), Immediate List Recall (verbal memory learning), Delayed List Recall (verbal memory recall), Stroop (response inhibition), and Digit Symbol (graphomotor processing speed)64. BrainCheck provides age normalized test scores (mean =
= 100
100 ± 15). We also administered the Patient Reported Outcome Measures Information System (PROMIS) Cognitive Function Short Form 8a to measure subjective cognitive function65. This is an 8-item, self-rating questionnaire regarding the frequency of cognitive symptoms. We also administered PROMIS-57 Profile v2.1 to measure depressive symptoms, fatigue, anxiety, sleep disturbance, pain, and social role functioning66. PROMIS provides standardized T-scores with a mean of 50 and standard deviation of 10.
± 15). We also administered the Patient Reported Outcome Measures Information System (PROMIS) Cognitive Function Short Form 8a to measure subjective cognitive function65. This is an 8-item, self-rating questionnaire regarding the frequency of cognitive symptoms. We also administered PROMIS-57 Profile v2.1 to measure depressive symptoms, fatigue, anxiety, sleep disturbance, pain, and social role functioning66. PROMIS provides standardized T-scores with a mean of 50 and standard deviation of 10.
Functional connectome construction
All MRI sequences were collected using a Siemens Vida 3T (Siemens Medical Solutions, Malvern, PA) whole body scanner. Functional connectomes (brain networks) were defined as a matrix of nodes (brain regions) and edges (correlation between brain regions) using the preprocessed fMRI time series as previously described67. This resulted in a 268 ×
× 268 functional connectivity matrix for each participant (see Supplementary methods for further details). The 268 connectome nodes were ordered by their membership in 1 of 8 previously defined functional networks: medial frontal, frontal-parietal, default mode, subcortical/cerebellar, motor, visual 1, visual 2 and visual association68.
268 functional connectivity matrix for each participant (see Supplementary methods for further details). The 268 connectome nodes were ordered by their membership in 1 of 8 previously defined functional networks: medial frontal, frontal-parietal, default mode, subcortical/cerebellar, motor, visual 1, visual 2 and visual association68.
Statistical analysis
Sample characteristics and neuropsychiatric symptoms
Demographic and COVID-19 related variables were examined using descriptive statistics and compared between groups when appropriate using t-tests for continuous variables and Chi Squared tests for categorical variables (p <
< 0.05). We compared scores between groups using Mann Whitney tests (p
0.05). We compared scores between groups using Mann Whitney tests (p <
< 0.05) with FDR correction for multiple comparisons. The rank biserial correlation effect size was computed for Mann Whitney tests.
0.05) with FDR correction for multiple comparisons. The rank biserial correlation effect size was computed for Mann Whitney tests.
Predictors of cognitive function
To examine predictors of cognitive function in the COVID-19 group, we performed a multiple linear regression. Independent variables were selected based on our prior research34 as well as the literature regarding post-COVID cognitive function as presented in the Introduction. Specifically, we included racial/ethnic minority status (0 =
= yes, 1
yes, 1 =
= no), age (years), education (years), COVID-19 severity (1
no), age (years), education (years), COVID-19 severity (1 =
= moderate, 0
moderate, 0 =
= mild), gender (1
mild), gender (1 =
= female, 0
female, 0 =
= male), time since COVID-19 positive test (days), PROMIS Fatigue score, loss of smell during active COVID-19 infection (1
male), time since COVID-19 positive test (days), PROMIS Fatigue score, loss of smell during active COVID-19 infection (1 =
= yes, 0
yes, 0 =
= no), and loss of taste during active COVID-19 infection (1
no), and loss of taste during active COVID-19 infection (1 =
= yes, 0
yes, 0 =
= no). Pocket Smell Test score was not included as no participants demonstrated anosmia on this test and scores did not have adequate variance (92% of participants obtained a score of 3/3).
no). Pocket Smell Test score was not included as no participants demonstrated anosmia on this test and scores did not have adequate variance (92% of participants obtained a score of 3/3).
Brain network functional connectivity
Functional connectomes were compared between groups using the Network-Based Statistic (NBS) Toolbox, which identifies connected subnetworks within the whole brain network that differ in connectivity between groups69. Specifically, the network-based statistic was conducted using nonparametric permutation testing with 5000 permutations [t threshold =
= 3.1 (default), p
3.1 (default), p <
< 0.05, NBS extent], covarying for age and gender, to determine edge weight differences in subnetworks (sets of edges) between the groups, controlling for multiple comparisons using family-wise error (FWE). Cohen’s d effect sizes with 95% confidence intervals were calculated for NBS results by converting the significant edge t statistic as a function of the degrees of freedom70. NBS results were visualized using BrainNet Viewer71.
0.05, NBS extent], covarying for age and gender, to determine edge weight differences in subnetworks (sets of edges) between the groups, controlling for multiple comparisons using family-wise error (FWE). Cohen’s d effect sizes with 95% confidence intervals were calculated for NBS results by converting the significant edge t statistic as a function of the degrees of freedom70. NBS results were visualized using BrainNet Viewer71.
Functional connectome correlates
We conducted NBS correlation (p <
< 0.05, FDR corrected, 10,000 permutations) between edges that differed significantly between groups and cognitive test performance, demographics, and COVID-19 characteristics to examine the relationships between altered functional brain subnetworks and these factors in the COVID-19 group. In this case, NBS identifies subnetworks whose edge weights are correlated with a variable of interest. Both positive and negative correlations were examined. NBS utilizes the t statistic for correlation analysis, so these were converted to correlation coefficients:
0.05, FDR corrected, 10,000 permutations) between edges that differed significantly between groups and cognitive test performance, demographics, and COVID-19 characteristics to examine the relationships between altered functional brain subnetworks and these factors in the COVID-19 group. In this case, NBS identifies subnetworks whose edge weights are correlated with a variable of interest. Both positive and negative correlations were examined. NBS utilizes the t statistic for correlation analysis, so these were converted to correlation coefficients:  =
= degrees of freedom.
degrees of freedom.
Efficiency of information processing
In addition to the strength of functional connectivity between brain regions, we also examined the topological organization of the connectivity patterns. Specifically, we were interested in the efficiency of information processing within the functional connectome given that brain fog is frequently associated with perception of mental slowing72. We measured local efficiency by applying graph theory to the functional connectome matrices. In this context, efficient information processing is assumed to follow the shortest, or most direct paths between brain regions. Therefore, local efficiency is defined as the inverse shortest average path length (number of edges) of a node’s neighboring nodes73. We compared local efficiencies between groups using Mann Whitney tests (p <
< 0.05) with FDR correction for multiple comparisons.
0.05) with FDR correction for multiple comparisons.
Brain network effective connectivity
We also measured the effective connectivity of the functional connectome to examine the direction of information flow. Unlike functional connectivity, which is bidirectional or undirected, effective connectivity measures the causal, hierarchical relationships between brain regions. Specifically, we performed a Bayesian network analysis to identify the conditional dependencies between brain regions74. The conditional independence/dependence relationships among regions were encoded with a directed acyclic graph (DAG). Specifically, if node A (child) directly depended on node B (parent) irrespective of all other nodes (p <
< 0.001, after FDR correction), then this dependence was encoded as a directed edge to node A from node B75. We implemented a hill-climbing approach with Bayesian Information Criterion to determine the best fit network. We calculated the effective connectivity only of the nodes that were determined to have significantly different functional connectivity via NBS.
0.001, after FDR correction), then this dependence was encoded as a directed edge to node A from node B75. We implemented a hill-climbing approach with Bayesian Information Criterion to determine the best fit network. We calculated the effective connectivity only of the nodes that were determined to have significantly different functional connectivity via NBS.
Effective connectivity was compared graphically between groups to indicate false positive, false negative and true positive edges. A true positive edge was defined as a connection between two regions in the COVID-19 group that existed in the control group with the same pathway (i.e., same child, same parent regions). A false positive was defined as a connection between two regions in the COVID-19 group that did not exist in the control group or did not reflect the same pathway (e.g., different set of parent regions, different child region). A false negative was defined as the absence of a connection between regions for which there was an existing connection in the control group76. We used permutation testing to determine if the number of false negative/positive edges in the COVID-19 group was significantly greater than that of random groups59. Specifically, we generated random DAGs with the same edge probability as the original groups 5000 times. The number of false positives/negatives in the randomized experimental groups comprised the permutation distributions. The significance of the permutation test (p value) was determined by calculating the mean number of instances that the permutation networks had greater number of false negatives/positives than the original COVID-19 group. Bayesian network analyses, including graphical comparisons and permutation testing, were conducted in the R Statistical Package v4.3 (R Foundation, Vienna, Austria).
Acknowledgements
The authors would like to thank the faculty and staff of the Biomedical Imaging Center at The University of Texas at Austin.
Author contributions
This study was developed by the principal investigator (SRK). Data acquisition, preparation and analysis was conducted by OFR, ADS and SRK. The manuscript was prepared and edited by OFR and SRK and reviewed and edited by all other authors. All authors approved the manuscript for submission. OFR, ADS and SRK had access to the raw data.
Data availability
The datasets generated and analyzed during the current study are not publicly available due the fact that they constitute an excerpt of research in progress but will be available on the EBRAINS platform (https://www.ebrains.eu) under the corresponding author’s name at study completion. Interested parties may contact the corresponding author for further information.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-73311-0.
References
Articles from Scientific Reports are provided here courtesy of Nature Publishing Group
Citations & impact
This article has not been cited yet.
Impact metrics
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/168599086
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Neural basis of fatigue in post-COVID syndrome and relationships with cognitive complaints and cognition.
Psychiatry Res, 340:116113, 03 Aug 2024
Cited by: 0 articles | PMID: 39146616
Impact of Total Knee Arthroplasty with General Anesthesia on Brain Networks: Cognitive Efficiency and Ventricular Volume Predict Functional Connectivity Decline in Older Adults.
J Alzheimers Dis, 62(1):319-333, 01 Jan 2018
Cited by: 26 articles | PMID: 29439328 | PMCID: PMC5827939
Long-range connections are more severely damaged and relevant for cognition in multiple sclerosis.
Brain, 143(1):150-160, 01 Jan 2020
Cited by: 38 articles | PMID: 31730165 | PMCID: PMC6938033
Connectome gradient dysfunction contributes to white matter hyperintensity-related cognitive decline.
CNS Neurosci Ther, 30(7):e14843, 01 Jul 2024
Cited by: 1 article | PMID: 38997814 | PMCID: PMC11245402

 1,2
1,2 

