Abstract
Free full text

Maintenance therapy for FLT3-ITD-mutated acute myeloid leukemia
Associated Data
Abstract
FLT3-ITD is a constitutively activated variant of the FLT3 tyrosine kinase receptor. Its expression in acute myeloid leukemia (AML) is associated with a poor prognosis. Due to this, the development of tyrosine kinase inhibitors (TKI) blocking FLT3-ITD became a rational therapeutic concept. This review describes key milestones in the clinical development of different FLT3-specific TKI with a particular focus on FLT3-TKI maintenance therapy in remission after allogeneic hematopoietic stem cell transplantation (HCT). Recent evidence from randomized trials using sorafenib in FLT3-ITD mutated AML provided a proof of concept that targeted post-HCT maintenance therapy could become a new treatment paradigm in AML.
Why FLT3 as a therapeutic target in acute myeloid leukemia?
AML is a clonal stem cell malignancy. Although AML prognosis is governed by genomic features,1-3 therapeutic targeting of recurrently mutated genes is a complex task.4 For example, while mutations in epigenetic regulator genes such as DNMT3A or ASXL1 are recurrently detected in AML5 and are linked to a dismal prognosis,3,6 the same mutations are also very frequently found in hematopoietic stem cells of healthy elderly individuals with 'clonal' (so-called 'age-related hematopoiesis' or CHIP/ARCH-associated mutations).7,8 Thus, although ARCH mutations seem to be instrumental drivers of clonal progression towards AML,5,8 their detection in AML per se does not qualify them as therapeutic targets in AML.9 This is because the expression of mutant oncoproteins is therapeutically exploitable only if their inhibition induces differentiation, restricts growth, or reduces viability of the AML bulk. A perfect target oncoprotein in AML is the constitutively activated FLT3 receptor tyrosine kinase which results from mutations in the FMS-like tyrosine kinase 3 (FLT3) gene. FLT3 mutations emerge very late during AML pathogenesis.3,10 They are found in approximately 30% of all AML patients and can be either FLT3-tyrosine kinase point mutations (FLT3-TKD) or FLT3 internal tandem duplication mutations (FLT3-ITD).11 Only FLT3-ITD mutations are associated with a poor outcome in AML.12,13 FLT3-TKD and FLT3-ITD cause uncontrolled signaling through the ERK-signaling, PI3-kinase signaling, and, in the case of FLT3-ITD, also STAT5-signaling,14 and drive stem cell transformation. 15,16 By 'hijacking' the signal transduction machinery of AML cells, FLT3 oncoproteins generate a strong dependence on FLT3-signaling pathways to sustain survival. As a result, AML cells undergo apoptosis in vitro and in vivo when FLT3 signaling output is blocked by a TKI.17,18 Dependence on FLT3 oncoproteins provided the biological rationale for the clinical development of FLT3 inhibitors in FLT3- mutated AML (reviewed by Kindler et al.18 and Daver et al.19).
In this review, I will discuss evidence that illustrates the value of FLT3-TKI when used as maintenance therapy in remission after allogeneic hematopoietic stem cell transplantation (HCT) compared with its use outside the context of a HCT.
The main characteristics and approval status of currently developed FLT3 inhibitors are shown in Table 1.
A long road for FLT3-TKI in acute myeloid leukemia
In spite of the preclinical data showing a promising activity of FLT3 inhibitors in FLT3-mutated AML, it has proven difficult to translate these preclinical results into a clinical benefit in vivo, especially in the monotherapy setting. For example, the first-generation FLT3 and multikinase inhibitor midostaurin failed to induce complete remissions (CR) or even partial remissions (PR) in relapsed or refractory (r/r)-FLT3-mutated AML.23,24 Likewise, lestaurtinib, another multitargeted FLT3 TKI, kills FLT3-ITD+ AML cells in vitro and reduces bone marrow blasts in 5 out of 17 r/r-FLT3-ITD+ AML patients. However, PR or CR are not achieved with lestaurtinib as monotherapy.25,26 In elderly relapsed FLT3-mutated AML patients, lestaurtinib given after chemotherapy did not improve response rates or survival.27 In newly diagnosed younger AML with FLT3-activating mutations, lestaurtinib given after first-line induction and consolidation chemotherapy failed to improve either relapse-free survival (RFS) or overall survival (OS).28
Table 1.
Important characteristics of FLT3 kinase inhibitor therapy in acute myeloid leukemia.
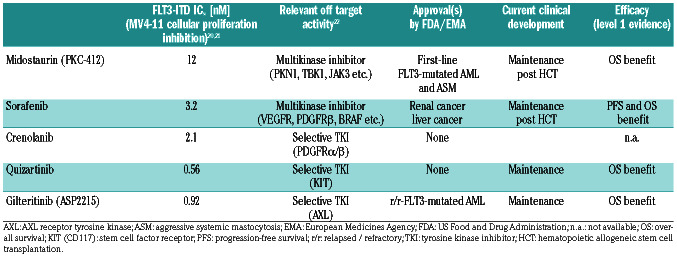
Sorafenib is a multitargeted TKI that was originally developed as a B-RAF and multi-kinase inhibitor in renal/hepatocellular cancer. However, the compound also shows potent FLT3-ITD inhibitory activity. Intriguingly, in a phase I trial recruiting FLT3-ITD mutated r/r-AML patients,29 sorafenib monotherapy induced PR and CR. A case series later confirmed activity of sorafenib in r/r- FLT3-ITD and also in FLT3-wild-type AML.30-32 However, no randomized sorafenib monotherapy trial was ever launched in AML. Intriguingly, when sorafenib monotherapy was given to FLT3-ITD+ AML patients relapsing after HCT, some cases achieved durable remissions, suggesting a remarkable synergism between proapoptotic FLT3-kinase inhibition and anti-AML immunity through the allogeneic immune system.32,33
The Quantum-R and ADMIRAL trials were the first randomized, placebo-controlled trials to provide evidence that second-generation, FLT3-selective inhibitors (quizartinib and gilteritinib) improved OS (by 2 to 4 months) in r/r-FLT3-mutated AML.34,35
Although the potential for clinical efficacy of FLT3-TKI in AML has been clearly demonstrated, in the r/r-AML setting, responses to FLT3-inhibitor monotherapy are usually only temporary. Treatment-emergent FLT3-TKI resistance restricts efficacy regardless of the type of inhibitor used.17,25,32,35-41 To address this problem, and find synergistic therapeutic modalities, TKI were integrated into available first-line AML treatments. For example, in the SORMAL trial42 and the RATIFY study,43 FLT3-TKI were combined with chemotherapy followed by a TKI maintenance phase with either sorafenib or midostaurin. In SORAML, sorafenib led to reduced rates of relapse, progression or death.42 In the double blind, randomized RATIFY study, the addition of midostaurin to conventional induction/consolidation chemotherapy followed by 12 months of midostaurin maintenance improved OS in FLT3-mutated AML.43
FLT3-TKI maintenance outside the context of allogeneic hematopoietic stem cell transplantation
Although BCR-ABL and FLT3-ITD drive oncogene dependence, they fail to induce leukemic self-renewal,44,45 which explains why FLT3-ITD inhibition alone fails to eliminate leukemic stem cells. In several studies, FLT3- TKI were combined with intensive chemotherapy or hypomethylating agents (HMA).28,42,43,46-54 Three of these combination therapy trials (which were randomized and placebo-controlled: RATIFY,43 SORAML,42 and clinicaltrials. gov identifier NCT0037337346) included a TKI maintenance therapy after first-line chemotherapy/TKI induction and consolidation.42,43,46 In all three trials, TKI maintenance was discontinued once patients underwent HCT. A post hoc efficacy analysis of the midostaurin-maintenance phase in the RATIFY trial suggested that midostaurin maintenance might not further reduce the probability of relapse,43 even though RATIFY was not designed to test this.55 In the SORAML study, RFS curves further separated over time, including during the maintenance phase of the trial.42 However, once again, the trial could not determine to what extent the sorafenib maintenance phase in particular contributed to the improved RFS.
FLT3-TKI maintenance after allogeneic stem cell transplantation
With a probability of disease recurrence of 50% or over, AML relapse remains the most frequent type of treatment failure after HCT, especially in high-risk patients with FLT3-ITD+ AML.56,57 Prognosis of relapsed AML after HCT is generally poor due to a lack of effective treatments. Chemotherapy, donor lymphocyte infusion or second HCT only achieve long-term outcomes in around 5% of cases.58,59
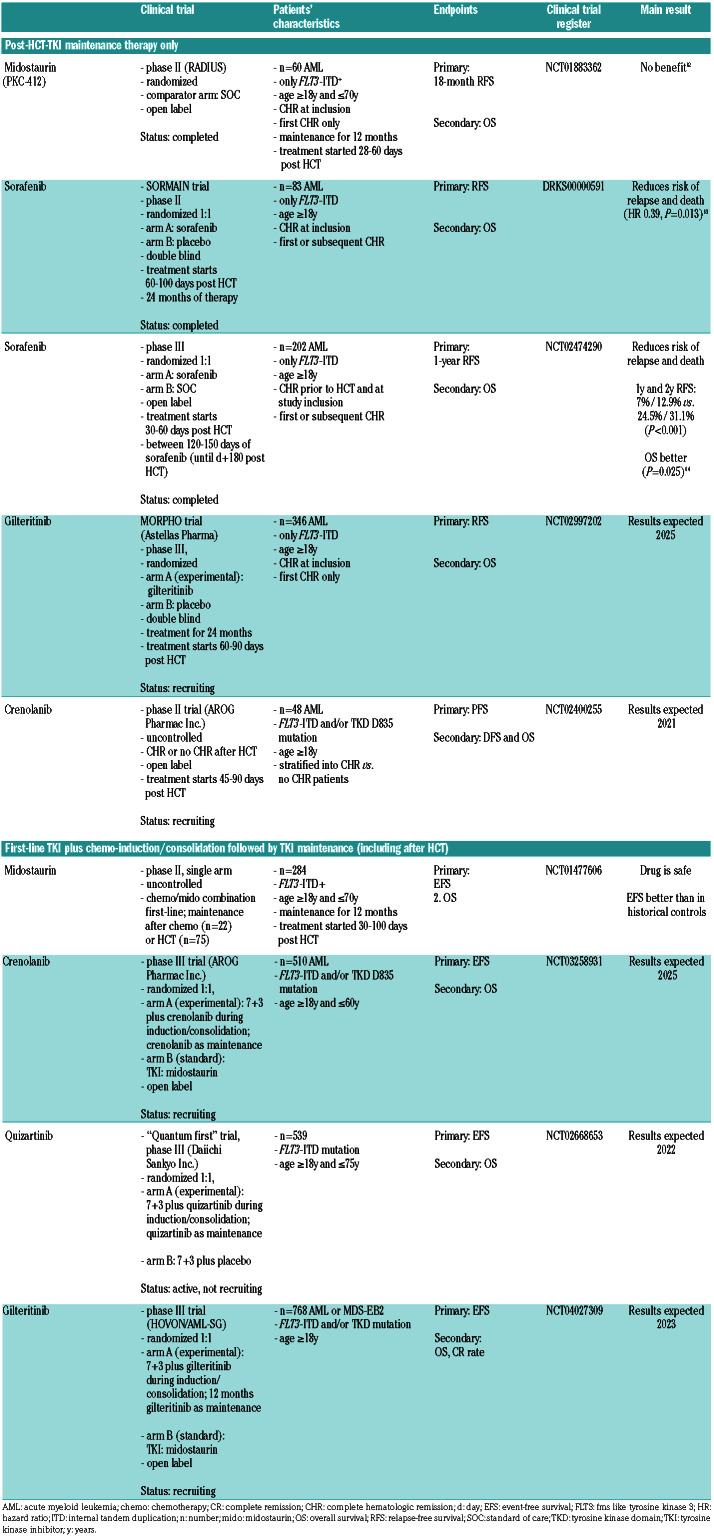
Table 2.
Trials testing post-hematopoietic stem cell transplantation (HCT) maintenance therapy.
Intriguingly, sorafenib monotherapy resulted in durable remissions in a small, but nonetheless important proportion of FLT3-ITD+ AML patients relapsing after HCT.32,33 This clinical observation implied that sorafenib might prevent AML relapse after HCT by inhibiting FLT3-ITDdriven AML outgrowth, thereby giving the new immune system more time to elicit immune responses against residual AML blasts. In a murine FLT3-ITD AML model, FLT3-ITD inhibition by sorafenib promoted anti-leukemic T-cell immunity by triggering IL-15 secretion.60 On the other hand, sorafenib-induced autocrine IL-15 secretion cannot explain the positive results in the SORAML trial since this study mainly included FLT3-ITD- AML patients. Indeed, in mice, sorafenib aggravates T-cell mediated allo-immunity independently from FLT3-ITD inhibition.61 Taken together, it seems that different mechanisms might contribute to the beneficial effects of sorafenib observed post HCT. A first placebo-controlled post-HCT maintenance therapy trial (the SORMAIN trial) started in 2010 using the multi-kinase and FLT3-TKI sorafenib (see below).
As of today, five randomized controlled clinical trials have investigated whether FLT3 TKI maintenance therapy post HCT improves outcome (Table 2). Three recent randomized trials addressed the value of FLT3-TKI (crenolanib, quizartinib or gilteritinib) in combination with chemotherapy followed by post-HCT TKI maintenance (Table 2), and some of these are ongoing with results expected between 2021 and 2025.
Midostaurin
In the RADIUS trial, Maziarz et al. randomized 60 FLT3-ITD+ AML midostaurin versus standard of care (SOC).62 This was an open label trial and midostaurin treatment was given for 12 months. The primary outcome, RFS, was comparable for midostaurin- and SOCtreated patients. Thus, the RADIUS-trial does not support a role for midostaurin as maintenance drug post HCT in FLT3-ITD-mutated AML.
An uncontrolled phase II study suggested that midostaurin maintenance post HCT was more efficacious than midostaurin maintenance after conventional chemotherapy/midostaurin combination therapy (Table 2). Of interest, only 75 of 134 patients (56%) ultimately proceeded to post-HCT maintenance, and most of these patients (59%) stopped maintenance earlier (after a median of 9 months).49
Gilteritinib and crenolanib
Maintenance trials with gilteritinib and crenolanib are ongoing (Table 2). The placebo-controlled, Astellas-sponsored trial (MORPHO) plans to randomize 346 FLT3- mutated AML patients who were transplanted in first complete hematologic remission. Recruited patients will be stratified according to minimal residual disease (MRD) levels post HCT. The MORPHO trial is expected to report results in 2025.
Sorafenib
Evidence is available from two recently published randomized trials: 1) the placebo controlled SORMAIN trial (recruitment period 2010-2015);63 and 2) an open label phase III trial from China (recruitment period 2015-2018)64 evaluating whether sorafenib maintenance post HCT improves progression-free survival (PFS) and OS in FLT3-ITD+ AML.
In SORMAIN, 83 patients were recruited. The primary endpoint, RFS, was significantly better with sorafenib. After a median follow-up of 41.8 months in SORMAIN, median RFS was not reached with sorafenib versus 30.9 months with placebo (HR 0.39, 95%CI: 0.18-0.85; P=0.013).63 During the first two years after randomization, the risk of relapse or death was reduced by 75% (HR 0.25, P=0.002).63 In the Chinese phase III trial, 202 patients were randomized to receive sorafenib versus placebo. The median follow-up duration is 21.3 months.64 The 2-year leukemia-free survival was 78.9% versus 56.6% (HR 0.37, 95%CI: 0.22-0.63; P<0.0001), which is comparable to that in the SORMAIN trial. At 24 months, OS was higher with sorafenib versus placebo in both the SORMAIN trial (90.5% vs. 66.2%; HR 0.24, 95%CI: 0.08-0.74; P=0.007) and the phase III trial (82.1% vs. 68.0%, HR 0.48, 95%CI: 0.27-0.86; P=0.012).
In both trials, sorafenib was well tolerated because toxicities could be managed with dose reductions without losing efficacy.
An important aspect of the SORMAIN trial were prospectively acquired MRD data. Although based on a relatively small number of patients, data suggest that sorafenib maintenance is especially beneficial for patients who are already in very good remission at the time of transplantation; among MRD- patients prior to allo-HCT, there were 0 of 9 relapses with sorafenib versus 5 of 12 relapses with placebo (P=0.028).63
Because a large number of retrospective studies had previously established that MRD levels prior and post HCT inversely correlate with probabilities of RFS and OS,65,66 SORMAIN data lend further support to the notion that achieving MRD-negativity prior to HCT could become an important treatment goal in this high-risk AML subtype. Thus, achievement of low MRD level prior to HCT, such as, for example, through a chemotherapy/ FLT3-TKI combination treatment67 (see trials in Table 2), would support the use of sorafenib maintenance post HCT.
Open questions and future directions
SORMAIN data and the phase III results from Xuan et al. establish TKI maintenance treatment post HCT as a novel and efficacious therapy.63,64 Data from these two trials reveal an unprecedented therapeutic potency of an FLT3-kinase inhibitor if applied in the context of CR after HCT. In such a context, FLT3-inhibition could maintain CR in the vast majority of patients, who would otherwise relapse. In particular, especially patients with no MRD prior to HCT (but also patients with MRD-positivity after HCT) seem to gain great benefit from FLT3-TKI (sorafenib) maintenance. Thus FLT3-TKI treatment in remission might be a clue as to how to significantly improve the detrimental natural course of FLT3-ITD mutated AML.63
Many important questions remain. For example, what is the mechanism underlying these potentially curative effects of sorafenib? It should be emphasized once again that sorafenib is a multi-targeted TKI and that its efficacy in AML can be also FLT3-ITD independent, as evidenced by the SORAML trial, which treated mainly FLT3-ITDAML patients.42 Results from ongoing randomized TKI maintenance therapy trials will help clarify whether the benefit of FLT3-TKI maintenance is compound (sorafenib)-specific or whether less promiscuous, highly FLT3-specific inhibitors such as gilteritinib or quizartinib offer comparable or even better benefits (Table 2).
Secondly, the optimal duration of maintenance therapy is unclear: 12 months (as in the phase III study) versus 24 months (as in SORMAIN), or potentially even longer. Finally, it will be important to explore whether the concept of targeted maintenance therapy in remission after HCT is generally applicable also to novel, AML-approved signaling inhibitors such as the BCL-2 inhibitor venetoclax68 or the IDH1/2-inhibitors enasidenib/ivosidenib.69,70 Recent positive results from maintenance trials using the oral hypomethylating compound CC-486,71 azacytidine72 or decitabine73 demonstrate that maintenance chemotherapy can meaningfully improve OS.
Funding Statement
Funding: AB was supported by the DFG (GRK 2573) and the German Carreras Leukemia Foundation (16R/2019).
References
Articles from Haematologica are provided here courtesy of Ferrata Storti Foundation
Full text links
Read article at publisher's site: https://doi.org/10.3324/haematol.2019.240747
Read article for free, from open access legal sources, via Unpaywall:
https://haematologica.org/article/download/haematol.2019.240747/72787
Citations & impact
Impact metrics
Article citations
Long-term remissions with Gilteritinib in early relapse after allogeneic stem cell transplantation of FLT3/NPM1 mutated acute myeloid leukemia.
Blood Cell Ther, 7(3):75-78, 14 Jun 2024
Cited by: 0 articles | PMID: 39263622 | PMCID: PMC11384128
Lipid metabolism dynamics in cancer stem cells: potential targets for cancers.
Front Pharmacol, 15:1367981, 27 Jun 2024
Cited by: 1 article | PMID: 38994204 | PMCID: PMC11236562
Review Free full text in Europe PMC
Targeting BMAL1 reverses drug resistance of acute myeloid leukemia cells and promotes ferroptosis through HMGB1-GPX4 signaling pathway.
J Cancer Res Clin Oncol, 150(5):231, 04 May 2024
Cited by: 0 articles | PMID: 38703241 | PMCID: PMC11069489
ABT199/venetoclax synergism with thiotepa enhances the cytotoxicity of fludarabine, cladribine and busulfan in AML cells.
Oncotarget, 15:220-231, 14 Mar 2024
Cited by: 0 articles | PMID: 38484153
BRCC36 associates with FLT3-ITD to regulate its protein stability and intracellular signaling in acute myeloid leukemia.
Cancer Sci, 115(4):1196-1208, 30 Jan 2024
Cited by: 3 articles | PMID: 38288901 | PMCID: PMC11007003
Go to all (20) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials
- (1 citation) ClinicalTrials.gov - NCT00373373
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Sorafenib Maintenance After Allogeneic Hematopoietic Stem Cell Transplantation for Acute Myeloid Leukemia With FLT3-Internal Tandem Duplication Mutation (SORMAIN).
J Clin Oncol, 38(26):2993-3002, 16 Jul 2020
Cited by: 219 articles | PMID: 32673171
Sorafenib Maintenance Appears Safe and Improves Clinical Outcomes in FLT3-ITD Acute Myeloid Leukemia After Allogeneic Hematopoietic Cell Transplantation.
Clin Lymphoma Myeloma Leuk, 15(5):298-302, 12 Dec 2014
Cited by: 52 articles | PMID: 25550214
Haematopoietic cell transplantation with and without sorafenib maintenance for patients with FLT3-ITD acute myeloid leukaemia in first complete remission.
Br J Haematol, 175(3):496-504, 19 Jul 2016
Cited by: 101 articles | PMID: 27434660 | PMCID: PMC5083189
FLT3 inhibitors in the treatment of acute myeloid leukemia: current status and future perspectives.
Minerva Med, 111(5):427-442, 21 Sep 2020
Cited by: 6 articles | PMID: 32955823
Review





