Abstract
Free full text

An adolescent sensitive period for threat responding: impacts of stress and sex
Abstract
Anxiety and fear-related disorders peak in prevalence during adolescence, a window of rapid behavioral development and neural remodeling. However, understanding of the development of threat responding and the underlying neural circuits remains limited. Preclinical models of threat conditioning and extinction have provided an unparalleled glimpse into the developing brain. In this review we discuss mouse and rat studies on the development of threat response regulation with a focus on the adolescent period. Evidence of non-linear patterns of threat responding during adolescence and the continued development of the underlying circuitry is highly indicative of an adolescent sensitive period for threat response regulation. While we highlight literature in support of this unique developmental window, we also emphasize the need for causal studies to clarify the parameters defining such a sensitive period. In doing so, we explore how stress and biological sex impact the development and expression of threat response regulation during adolescence and beyond. Ultimately, a deeper understanding of how these factors interact with and impact developmental trajectories of learning and memory will inform treatment and prevention strategies for pediatric anxiety disorders.
Difficulty in assessing and appropriately responding to potential threats is a key feature of many psychiatric disorders, including anxiety disorders, which peak in prevalence during adolescence (1-3). Despite the emergence of anxiety in early adolescence, development of treatments for these disorders has been largely restricted to adulthood (4-7). Novel therapeutic and preventative strategies that are biologically-based and developmentally-informed are sorely needed (8). Part and parcel of developing clinically relevant and effective treatments for adolescent anxiety disorders is understanding the developmental neurobiology of threat response regulation, the process by which threat responding adapts depending on the environment (9). Increasingly over the past decade, research has begun to illuminate how threat responding is differentially expressed as a function of age (10-12) and how it is linked to concurrent development of reward and threat circuitry (13). In this review, we highlight developmental studies from mice and rats delineating patterns of threat responding, drawing focus to the adolescent period when threat response regulation is in flux and the neural circuits thought to mediate this regulation are undergoing rapid maturation and refinement (2). For more on developmental trajectories of anxiety in humans and non-human primates we direct the reader to other reviews in this Special Issue (Dylan Gee, Michelle Craske, Ned Kalin). Demarcation of the adolescent window is determined based on a variety of factors including physical, pubertal, neurobiological and social milestones (14, 15). Here, we consider adolescence in mice and rats to encompass the developmental time frame between postnatal day (P)29 and P55.
After presenting relevant behavioral and neural data on adolescent threat responding, we discuss known and potential impacts of stress and biological sex on the emergence of key behaviors and neural circuits used to delineate the adolescent sensitive period for threat response regulation. Recent studies have emphasized the importance of examining how threat responding is differentially expressed as a function of sex (16-18) and experience (19, 20). Moreover, a strong case has been made for the inclusion of sex as a biological variable for determining the developmental impact of stress (21). Understanding how these factors shape the timing of behavioral and neural milestones in the development of threat response regulation will provide insight and direction for experiments examining regulatory processes during adolescence and the potentially enduring impact of experiences across the lifespan.
Defining Sensitive Periods for Development
Experience drives behavioral and neural adaptations allowing an individual to effectively respond to our ever-evolving environment across the lifespan. However, the impact of experience is most marked during early life, when it can impact ongoing rapid maturation and reshaping of neural circuits and the behaviors they mediate. Critical periods of development are defined by the absolute necessity of experiencing specific external stimuli during a restricted period of time. During critical periods, high plasticity allows these experiences to shape brain development. As a result, their absence can have permanent consequences. In contrast, sensitive periods are also windows of heightened plasticity when the impact of experience can be maximal, but development can occur both in the absence of the experience or when the experience occurs outside of the sensitive window (22).
Although emotional experiences occur across the lifespan, the circuitry mediating affective behavior is undergoing rapid developmental changes during adolescence. Thus, emotional experiences have profound potential to shape the long-term functioning of affective circuitry (23-25). In this review, we focus on responding to threats in the environment as a robust form of affective behavior and consider whether adolescence is a sensitive period for threat response regulation, an outstanding question that has emerged from the developmental literature. As an important clarification, our intention is not to imply that learning about the presence (and more relevant for this review, the absence) of threats is restricted to a particular period of time, nor that the capacity for threat response regulation is dependent on exposure to threats. Instead, we examine evidence that certain experiences during the adolescent period may impact circuit maturation during adolescence, in turn modulating adult circuit function and the expression of threat response regulation. Although recent behavioral and neural analyses support the idea that adolescence is a sensitive period for threat response regulation, in our view, additional evidence in the form of causal analyses is still needed. The remainder of this review will highlight data from mice and rats consistent with the understanding of adolescence as a sensitive period for threat response regulation, while also identifying areas of future study needed to enhance our current understanding.
Threat Response Regulation in Adolescence
While responding to threats and the cues that predict them can be highly adaptive, the ability to alter the same responses in light of a changing environment is equally important. This flexible process is called threat response regulation. In this review, we cover literature on Pavlovian threat conditioning, with freezing as the primary measure of threat responding reported in these studies. Our review focuses on extinction, a common process of threat response regulation by which repeated exposure to a conditioned stimulus (CS) in the absence of an aversive unconditioned stimulus (US) leads to new inhibitory learning associated with reduced freezing to a CS. Principles of extinction have been incorporated into cognitive behavioral therapy, the predominant behavioral treatment for anxiety and fear-related disorders (26, 27). However, relatively little is known about the behavioral and neural features of extinction in developing populations, hindering treatment optimization.
The ability to learn a predictive association between a formerly neutral stimulus CS and an aversive US emerges early in development, as early as P10 in rats, and can be modulated by maternal presence (see Opendak & Sullivan in this Special Issue). Subsequently, the ability to retain long-term threat-associated memories emerges between the juvenile (P16-P17) and preadolescent (P23-P24) periods (28-30). The capacity to flexibly adjust responding to a threat-associated cue in a changing environment has also been shown to emerge by the juvenile period. Early studies examining within-session extinction learning have revealed comparable rates of reduced freezing to the CS by the end of extinction training between juvenile (P16-P17), preadolescent (P23-P24), adolescent (P35), and early adult (P70) male rats (28, 29, 31-33). One study using male mice found that adolescents (P29) showed limited extinction learning compared to juveniles (P23) and young adults (P70) when threat responding was measured across four consecutive days instead of one (34). The limited extinction learning observed in this study may be more reflective of a reduced ability to retain extinction learning between days, rather than the inability to reduce fear across extinction per se, which is discussed in more detail below. Thus, although threat learning emerges during infancy, long-term recall of threat-associated cues and the ability to reduce freezing to these cues during extinction learning does not emerge until the late juvenile and pre-adolescent periods, after which these processes largely remain stable.
In addition to the ability to reduce threat responding during extinction training, another key feature of threat response regulation is the ability to maintain extinction learning over time, also known as extinction retention (or recall). A common finding in the literature is that extinction retention is expressed non-linearly across development with limitations apparent specifically during adolescence. Indeed, adolescent male rats (P35) and mice (P29) show increased freezing to the CS 24 hours after extinction training (i.e. poorer extinction retention) when compared to pre-adolescents (P23-P24) or adults (P70) (32-35). However, disruptions in extinction retention are only observed when both threat conditioning and extinction learning take place during adolescence. For example, Baker and Richardson (2015) found that when threat conditioning occurred in pre-adolescence (P24) and extinction occurred in adolescence (P35), or when threat conditioning occurred in adolescence (P35) and extinction occurred in adulthood (P70), extinction retention was comparable to rats that received both threat conditioning and extinction during either pre-adolescence or adulthood (33). Thus, associative competition between memories of threat-associated cues and extinction memories specifically during adolescence may be responsible for extinction retention failures. Notably, although the longevity of extinction memories appears to be reduced during adolescence, this can be overcome by amending the extinction training procedures. Indeed, when the extinction training is doubled, adolescent male rats achieve levels of extinction retention (twenty-four hours later) comparable to juveniles and adults (32, 35). In addition, in adolescent male mice, if extinction training takes place in the threat conditioning context, extinction retention two-weeks later is greater than same age counterparts that underwent extinction training in a novel context (36). This study is one of the first to suggest the potential efficacy of context-based extinction during adolescence to significantly attenuate threat-associated memories. As discussed below, increasing the engagement of brain circuits involved in the inhibition of threat responding may be necessary for long-term extinction retention.
Neurobiology of Threat Response Regulation in Adolescence
Patterns of threat response regulation during adolescence parallel dynamic remodeling of long-range connectivity between components of threat circuitry (Figure 1a). Parts of the rodent brain homologous to the human and non-human primate prefrontal cortex (PFC) have been shown to be integral to threat response regulation in adults. Hereafter we will use the term PFC to describe these homologous regions in mice and rats (but see (37)). In mice and rats, development of the PFC is protracted, continuing throughout adolescence and even into young adulthood (38, 39), providing a possible explanation for diminished extinction retention during adolescence. By the same vein, markers of PFC development and the functional integration of connectivity between PFC and the amygdala correspond with the ability to effectively acquire, consolidate and retrieve extinction memories (40)(Figure 1a).
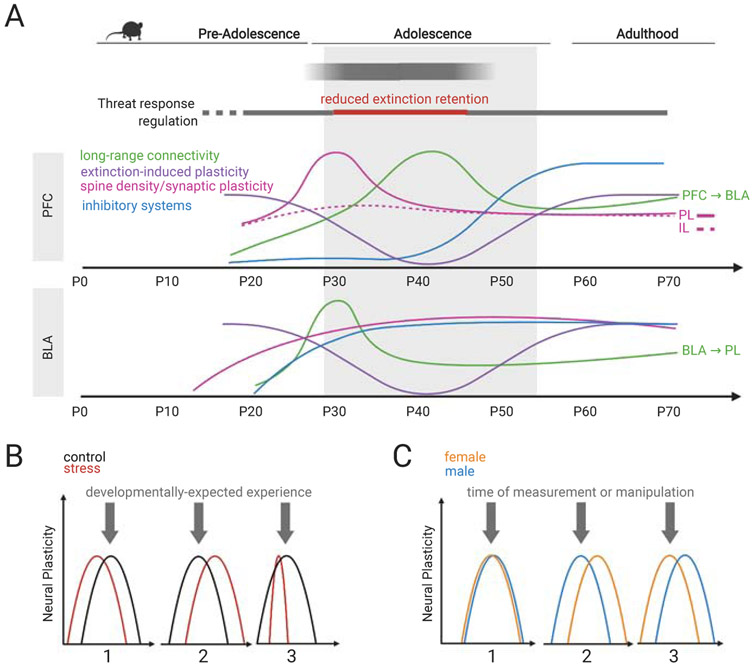
(a) Although memories of threat-associated cues emerge as early as postnatal day 10 (P10), long-term retention of these memories does not emerge until preadolescence. Similarly, the ability to extinguish a memory of a threat-associated cue and retain this extinction memory emerges in pre-adolescence. However, extinction retention is temporarily reduced during adolescence. Dynamic remodeling of the prefrontal cortex (PFC) (including the prelimbic (PL) and infralimbic (IL) subregions) and basolateral amygdala (BLA) parallels these behavioral changes. Key maturational changes discussed in the review are highlighted here. While some changes occur gradually across development (inhibitory system integration in the BLA), others are transiently increased (spine density in the PL) or decreased (extinction-induced plasticity) relative to pre-adolescence and adulthood. It is important to note that this schematic is based on studies done in male mice and rats and may differ for females. (b,c) Theoretical scenarios to consider when designing and interpreting developmental fear studies. (b) Little is known about the impact of adolescent stress on the maturation of circuitry related to threat responding. While accelerated development (i.e., heightened plasticity occurs earlier) may occur (scenario #1), it is important to consider how stress may delay the emergence of (scenario #2) or even truncate (scenario #3) the duration of neural plasticity, and thus the timing of sensitive windows. Misalignment in developmentally-expected experiences (such as increased exploration independent of caregivers and increased interaction with novel conspecifics (14, 25) (gray arrows) and periods of increased neural plasticity may disrupt canonical maturation of these threat response systems. (c) When studying potential sex differences, it is important to consider where a male or female rodent is in their development at the time measurements or manipulations are taking place. While a measurement or manipulation (gray arrows) may occur when both males and females are both in a state of heightened neural plasticity (scenario #1), others may occur when either only males (scenario #2) or only females (scenario #3) are experiencing maximum plasticity. For example, measuring extinction retention at the same chronological age in males and females may result in different behavioral phenotypes not because there is a sex difference but because males and females are at different stages of development.
Behavioral differences often used to delineate sensitive periods are reflective of changes in neural circuits, specifically alterations in plasticity (22). However, the timing of sensitive periods of heightened plasticity and structural reorganization can vary by brain region, leading to an imbalance in regional control over behavior. Within the PFC of adult mice and rats, the prelimbic (PL) and infralimbic (IL) sub-regions inversely regulate threat responding with PL activation increasing threat responding and impairing extinction and IL activation decreasing threat responding and facilitating extinction (27, 41-43). Notably, changes in neuroplasticity in both PL and IL occur during early adolescence along different timelines. In male mice, between P24 and P31 there is a surge in spine density in the PL, but not IL, that is followed by a subsequent pruning between P31 and P45 (36). Coinciding with increased synaptic plasticity in PL, a marked absence of learning-induced changes in plasticity has been observed in the IL of male mice (34). Indeed, while both preadolescent (P24) and adult (P70) male rats exhibit extinction retention 24 hours after training as well as post-extinction increases in phosphorylated mitogen-activated protein kinase (pMAPK; a marker of activity-dependent modulation of synaptic plasticity) in the IL, adolescents (P35) show neither (35). However, adolescents achieve comparable levels of extinction retention and pMAPK when extinction training is doubled (35). Thus, additional training may be necessary to mount an IL response sufficient to override concurrent elevations in PL activity. Together, these findings suggest that a temporary imbalance in relative PL and IL contributions to threat responding may bias memory recall such that threat associations are prioritized over safety associations acquired during extinction, although additional experiments are necessary to investigate this possibility. Coinciding with shifts in synaptic plasticity is a surge in neuronal projections to the PL, but not IL, from the basolateral amygdala (BLA), which plays a critical role in the acquisition, consolidation and maintenance of information about threats in mice and rats (7)(Figure 1a). Thus, increased BLA-PL connectivity may temporarily increase the strength of threat-associated memories, contributing to diminished extinction retention during adolescence.
While the PL in male mice also exhibits a transient surge in spine density in early adolescence followed by a period of pruning (36), spine density in the BLA gradually increases from P20 to P35, after which the number of spines remains constant (44). Although relatively little is known about amygdala engagement during extinction in adolescence, one study found that in juvenile, but not adolescent, male rats, extinction training increased pMAPK in both the IL and BLA when compared to no extinction controls (33). The lack of extinction-induced plasticity in the BLA in male mice and rats following extinction appears to be unique to adolescents as increased pMAPK is observed in juveniles (P17), pre-adolescents (P24), and adults (33, 45, 46).
In adult mice and rats, reciprocal connections between the PFC and amygdala enable the consolidation and retrieval of an extinction memory (41-43). A neuroanatomical tracing study in male mice found that the density of PFC (sub-region nonspecific) axons in the BLA peaks between P30 and P45 (39). Excitatory synaptic strengthening of PFC-BLA synapses gradually increases from P15 to P30, where it reaches adult levels. Another neuroanatomical tracing study in rats observed a similar peak in IL-BLA connectivity around P45 that was followed by a period of significant pruning (~50%) of projections (38). However, recent data indicates that although threat exposure leads to an increase in the capacity for IL to inhibit BLA activity in adult male rats, providing a means to modulate or inhibit inappropriate threat responding, the same capacity for inhibitory control is absent during adolescence (P28-P40)(47). This suggests that although the framework for BLA regulation via PFC is set early in adolescence, functional maturation may continue throughout the adolescent period.
A significant rise in inhibitory synapses and signaling across the PFC (subregion nonspecific) and BLA during adolescence in male mice and rats is presumed to facilitate fine-tuning of long-range connections established by early adolescence (48-51). Furthermore, new excitatory projections from the amygdala to the PFC (subregion non-specific) that sprout during adolescence in male rats gradually increase connections onto inhibitory interneurons (52). However, little is known about the role of inhibitory interneurons in threat response regulation during adolescence. A recent study in male mice found that while parvalbumin interneurons do not reach maturity until adulthood, somatostatin interneurons develop earlier in adolescence and exhibit an adolescent-specific surge in inhibition of pyramidal neurons in the IL (53). This surge in somatostatin-mediated inhibition of the IL may play a role in the diminished extinction-induced plasticity in the IL and reduced extinction retention observed in adolescent male mice (36). In conclusion, the timing of these changes suggests that the final stages of PFC-BLA maturation is ushered in by increased inhibitory transmission in adolescence (Figure 1a).
The Impact of Stress and Biological Sex on Adolescent Threat Response Regulation
As outlined above, threat response regulation undergoes tremendous change across early development (25). A more stable neural circuitry emerges in early adulthood as naturally-occurring periods of heightened plasticity come to a close. Transient ontogenetic adaptations allow a developing individual to maximally learn from their environment. However, periods of heightened plasticity can also leave the individual vulnerable to maladaptive circuit connectivity (22), possibly contributing to the emergence of various psychiatric disorders or conferring susceptibility to adverse experiences later in life. Concurrent adaptations driven by chronic stress (i.e. poverty, abuse) can have an indelible impact on the developing brain. A number of environmental and biological factors orchestrate trajectories of brain maturation (54, 55). Moreover, early life is not one continuous sensitive period but a collection of coordinated sensitive periods, defined by unique cellular and molecular changes across neural circuits. Accordingly, it is important to consider how environmental exposures, such as stress, as well as biological sex may impact not only the expression of threat responding during adolescence but also sensitive period onset and duration.
Evidence that threat responding is differentially regulated in adolescence often leads to the interpretation of adolescence as a sensitive period for threat response regulation. To be considered a sensitive period, it would need to be shown that experience (e.g. stress) during this window would exert an enduring influence not observed following the same experience in other periods (e.g., childhood or adulthood) (24). Additionally, correlative changes in brain and behavior do not make a sensitive period. For example, the lack of causal studies makes it difficult to determine whether reductions in extinction retention are a direct result of altered plasticity in the PL, IL, and BLA.
Stress
A growing literature has highlighted that exposure to stress during adolescence can have both short-and long-term effects on brain development (56). For example, three days of variable stress (P28-P30) in male rats impairs extinction in adulthood (P85-P87;(57)). In apparent contrast, 7 days of restraint stress in male rats (P42-P49) led to immediate reductions in extinction retention in stressed rats compared to controls, but these differences did not persist into adulthood (58). A lack of enduring deficits in extinction retention observed in this study suggests that stressor type, timing, and duration are important factors to consider when evaluating the developmental implications of stress exposure. In a similar vein, the extent to which stressful experiences can be anticipated markedly influences behavioral outcomes. Indeed, a study in male rats found that chronic unpredictable stress exposure from P28-P55 impaired late adolescent (P56) extinction and extinction retention and also decreased brain derived neurotrophic factor (BDNF; a mediator of synaptic plasticity that has been implicated in adult extinction) and plasticity marker pMAPK in the IL and PL compared to stress-naive controls (59). Interestingly, male rats that instead received predictable chronic mild stress had facilitated extinction, stronger extinction retention, and increased BDNF and pMAPK in the IL specifically. Although this study did not compare the effects of different stressors across ages, these findings suggest that homotypic, predictable stress can engender resilience to future stressful events in adolescent male rats. Studies comparing the short- or long-term effects of stress across ages are sorely needed to establish whether adolescence is a sensitive period for the effects of stress on threat response regulation. However, the majority of studies examining the impacts of adolescent stress exposure either focus solely on adolescence (lacking another age comparison) or include adolescents and adults, but lack a pre-adolescent comparison group.
Stress may lead to a precocious, delayed, or truncated emergence of the heightened neural plasticity that defines a sensitive period. Misalignment in heightened plasticity and developmentally-expected experiences may impact threat response patterns and alter the trajectory of future sensitive period development in such a way that truncates periods of adaptive and flexible learning (Figure 1b). Though yet to be fully examined in adolescence, there is growing evidence that negative experiences during infancy disrupt the timing of continued development (21, 60). Two separate studies found that maternal separation stress leads to both an early emergence of adult-like extinction retention in P35 rats (61) and strong retention into adolescence of threat-associated memories encoded at P17 (62). With respect to circuit development, Honeycutt and colleagues (2019) found that maternal separation stress leads to precocious development of BLA-PFC innervation during adolescence, with this shift occurring earlier in females (P28) than in males (P38)(63). It is plausible that stress anytime during early development can leave an indelible mark on maturing neural circuitry. Thus, additional studies are needed to determine which aspects of threat circuit development are most sensitive to adolescent (versus infant or pre-adolescent) stress.
Biological Sex
Anxiety disorder diagnoses are higher in females, a sex difference that emerges as early as six years of age (64, 65). Despite these known differences, threat response regulation is poorly understood in females, further hindering treatment development specific for a population at heightened risk. Studies in adult female mice and rats highlight unique behavioral responses, hormonal influences, and neural mechanisms underlying threat response regulation (16). However, very few developmental extinction studies have included females, making it difficult to draw conclusions about sex differences in the development of threat response regulation. Additionally, if temporal shifts in sensitive period development exist between sexes, experiences or experimental manipulations at one time point in development may in actuality impact different timeframes of development (Figure 1c). In other words, males and females of the same chronological age may respond to the same experience differently or an experience may have a greater impact depending on their developmental/pubertal stage.
Findings in juvenile and pre-adolescent rats suggest that long-term memory of a threat-associated cue may emerge earlier in females; however, it is difficult to determine without studies comparing extinction retention across additional ages or comparing between sexes (66, 67). Matsuda and colleagues (2018) included a range of developmental ages (P28, P42, P56, P70, and P105) and found that adult female mice (P70 and P105) show stronger threat-associated memory, reduced extinction learning, and decreased extinction-induced plasticity in the PFC (measured by pMAPK) compared to adolescent (P42) females (68). It is important to note that although adolescent females show increased extinction-induced plasticity compared to adult females (opposite to what is seen in male mice and rats), direct comparisons between sexes are necessary to establish sex differences in extinction-induced plasticity in adolescence. A recent study found that estrous stage during extinction affected extinction learning and retention in adolescent (P35) rats (69). Specifically, females in metestrus/diestrus or proestrus (when estrogen levels are rising) froze more during extinction compared to males, females in estrus (when estrogen levels drop), and females that had not yet undergone menarche. Furthermore, females in metestrus/diestrus during extinction exhibited reduced extinction retention 24 hours later compared to males. Interestingly, gonadectomy before pubertal onset (P21) facilitated extinction learning in females, while delaying it males, and enhanced extinction retention in females when compared to same-sex sham surgery controls. These findings suggest an inverse relationship between estrogen levels and extinction retention during adolescence, which contrasts with findings in adult females (70-72), possibly due to developmental differences in estrogen receptor expression in brain regions mediating threat responding (73).
Surprisingly little is known about the immediate and enduring impact of adolescent stress on threat response regulation in females. McCormick and colleagues (2013) found that female rats that underwent social instability stress from P30-P45, but not P70-P85, exhibited increased freezing during extinction immediately after stress in adolescence and later in adulthood (74). Interestingly, male rats that experienced social instability stress during adolescence showed comparable extinction learning to controls both immediately after stress and later in adulthood (75). It is important to note that the behavioral and structural changes discussed so far occur around pubertal onset (anywhere from P30-P37 in mice and P37-39 in rats – see (76)). Puberty is a time of sexually divergent physiological changes that influence behavior and brain development and puberty onset occurs earlier on average in female mice and rats (76). For example, in rats, while the number of PFC neurons decreases across adolescence in both sexes, neuronal loss is greater in females and coincides with pubertal onset (44, 77, 78). Developmental processes across adolescence, like synaptic overproduction, synaptic pruning, and myelination, have also been shown to occur earlier in female mice and rats (see (79)). However, it is unknown whether puberty plays a causal role in threat circuit maturation specifically, warranting further study.
It is prudent that we do not always assign one condition (e.g. adult or male) as the yardstick to design experiments or measure outcomes. For example, in many adult studies, male-focused experimental designs are simply extended to include females. Adopting a female-focused framework (80) in studies of development will enhance our understanding of the maturational milestones and neural mechanisms mediating threat response regulation in females and spur sex-specific and developmentally-informed interventions and treatments for anxiety disorders.
Concluding Remarks
The emergence of anxiety disorders during adolescence necessitates a deeper understanding of how neural substrates important for regulating adult behavior change across development. Mouse and rat studies discussed here show that extinction retention is impaired in adolescence but extended extinction training or context-based extinction training can improve extinction retention during adolescence. Furthermore, we consider how stress and biological sex may impact threat response regulation during adolescence as well as sensitive period onset and duration. Causal studies designed to uncover the circuits mediating unique patterns of adolescent threat responding are necessary to improve our understanding of adolescent development and the emergence of anxiety during adolescence. Rodent studies can aid ongoing human studies to deepen our understanding of early-emerging patterns of anxiety as well as resilience to trauma to help mitigate and prevent the manifestation of debilitating threat responding throughout life (8, 81).
Acknowledgements
The development and writing of this paper were supported by grants to D.M.G from the NIH National Center for Advancing Translational Science (TL1-TR-002386); to H.C.M. from the NIH Pathway to Independence Award (K99MH119320); and to F.S.L. from NIH (NS052819, MH123154) and the NewYork-Presbyterian Youth Anxiety Center. Figure created with BioRender.com.
Footnotes
Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.biopsych.2020.10.003
Read article for free, from open access legal sources, via Unpaywall:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7954972
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1016/j.biopsych.2020.10.003
Article citations
Violence victimisation and young adult cardiometabolic health: the role of timing and social identity.
Ann Hum Biol, 51(1):2390834, 01 Feb 2024
Cited by: 0 articles | PMID: 39301955
Developmental age and fatty acid amide hydrolase genetic variation converge to mediate fear regulation in female mice.
Dev Psychobiol, 65(6):e22409, 01 Sep 2023
Cited by: 0 articles | PMID: 37607892 | PMCID: PMC10454978
Through a Developmental Lens: Emerging Insights to Understand and Treat Pediatric PTSD.
Am J Psychiatry, 180(9):636-644, 01 Sep 2023
Cited by: 1 article | PMID: 37654114 | PMCID: PMC10636806
Review Free full text in Europe PMC
Towards a youth mental health paradigm: a perspective and roadmap.
Mol Psychiatry, 28(8):3171-3181, 14 Aug 2023
Cited by: 30 articles | PMID: 37580524 | PMCID: PMC10618105
Review Free full text in Europe PMC
Maturation of a cortical-amygdala circuit limits sociability in male rats.
Cereb Cortex, 33(13):8391-8404, 01 Jun 2023
Cited by: 1 article | PMID: 37032624 | PMCID: PMC10321102
Go to all (21) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Nonlinear developmental trajectory of fear learning and memory.
Ann N Y Acad Sci, 1304:62-69, 31 Oct 2013
Cited by: 11 articles | PMID: 24176014 | PMCID: PMC4155981
Review Free full text in Europe PMC
Sensitive periods in fear learning and memory.
Stress, 17(1):13-21, 28 May 2013
Cited by: 14 articles | PMID: 23611461 | PMCID: PMC4336785
Review Free full text in Europe PMC
Fear learning and memory across adolescent development: Hormones and Behavior Special Issue: Puberty and Adolescence.
Horm Behav, 64(2):380-389, 01 Jul 2013
Cited by: 34 articles | PMID: 23998679 | PMCID: PMC3761221
Review Free full text in Europe PMC
Anticipatory Threat Responding: Associations With Anxiety, Development, and Brain Structure.
Biol Psychiatry, 87(10):916-925, 15 Nov 2019
Cited by: 31 articles | PMID: 31955915 | PMCID: PMC7211142
Funding
Funders who supported this work.
NCATS NIH HHS (2)
Grant ID: UL1 TR002384
Grant ID: TL1 TR002386
NIMH NIH HHS (2)
Grant ID: R01 MH123154
Grant ID: K99 MH119320
NINDS NIH HHS (1)
Grant ID: R01 NS052819





