Abstract
Free full text

Through a Developmental Lens: Emerging Insights to Understand and Treat Pediatric PTSD
In July 1976, 26 children (and one adult) courageously escaped from a partially buried box trailer after being abducted and held for ransom by three armed men who had hijacked their school bus outside Chowchilla, Calif. (1–3). Upon reunion with their families, the children underwent a cursory medical examination and were declared no worse for wear by medical doctors and local officials, who assured worried parents that the children were highly unlikely to experience psychological problems stemming from the abduction. A psychiatrist who spoke with parents warranted that no more than one of the 26 would show any lingering effects of the experience. This assertion was deemed reasonable in 1976, when children were widely assumed to be invariably resilient to trauma (4) (despite evidence to the contrary [e.g., 5, 6]), and few psychiatrists claimed expertise in regard to the rare exceptions. Unsurprisingly to clinicians now, many of the kidnapping victims presented a debilitating constellation of emotional, behavioral, and cognitive symptoms (1–3). The children went untreated for months, however, in part “because no parent was willing to admit that his or her child was the 1 in 26” (1).
The timing of these events coincided with the formalization of criteria for a refined diagnostic “post-traumatic stress disorder” (PTSD), capturing symptoms observed following what was then described, in DSM-III, as a “psychologically traumatic event … outside the range of usual human experience.” The addition of PTSD to DSM-III sparked a decades-long odyssey of research and debate driving ongoing refinement of the core criteria for PTSD, as clinical science continues its attempts to better carve the disorder “at its joints” (8). The DSM workgroup members who formulated the original diagnostic criteria drew exclusively from research conducted with trauma-exposed adults (9, 10). This decision may have been motivated by the paucity of knowledge related to psychiatric symptoms among trauma-exposed children (1, 3), an area that has received expansive study in the four decades since (11–16). However, modest revisions of PTSD have continued to derive primarily from research findings in adults (8, 17, 18).
The reliance on adult-derived research for understanding and treating pediatric PTSD may come at a cost for youths (here defined as children and adolescents) in terms of detection, diagnosis, and treatment (19–21). As one example, field trials for DSM-5, which did evaluate the test-retest reliability of PTSD criteria in separate samples of adults and youths, found test-retest reliability to be good for the former, but poor for the latter (22). In this overview, we summarize the current phenomenological and neurobehavioral research in pediatric PTSD, which suggests that it is a neurodevelopmental trauma disorder with important differences from adult PTSD. Such an understanding will be critical for the estimated 5% of youths who will develop the disorder (23) and for whom there are few treatment options (12).
Symptomics and Syndromics
Although Freud (24) first suggested the possibility that psychic trauma might have unique effects on children, Terr’s (1, 3) reporting on the “Children of Chowchilla” in the pages of the Journal constitutes one of the earliest efforts to delineate differences in post-trauma symptoms across youths and adults. The expectation of absolute resilience in these youths may have stemmed in part from a failure to recognize the remarkably different symptoms presented by trauma-exposed children relative to trauma-exposed adults. Since PTSD’s inception in DSM-III, the criteria for the diagnosis have acknowledged the vulnerability of both adults and children in the aftermath of traumatic experiences. Subsequent revisions continue to incrementally recognize developmental differences in PTSD by adding or revising text describing special considerations for youths (25). Informed by accumulating work investigating PTSD in preschool-age children (28), separate diagnostic criteria for PTSD in early childhood were added in DSM-5. However, diagnosis of school-age children and adolescents continues to be guided by an algorithm largely designed for an adult population, despite increasing evidence indicating the poor sensitivity of these criteria in trauma-exposed youths (20, 21, 29, 30). We begin by detailing concerns with criterion A—the “gateway” to PTSD—which circumscribes the experiences that may engender traumatic stress symptoms.
PTSD is unique among psychiatric disorders in that diagnosis is predicated on exposure to a “traumatic” event, specifically defined as actual or threatened death, serious injury, or sexual violence experienced directly or vicariously (31, 32) (as of DSM-5) through exposure to details of a loved one’s trauma experience. Notably, this definition has carried for nearly 30 years with only modest revision (e.g., addition of vicarious exposure). A review of the research works informing its design in DSM-III and major revision in DSM-III-R suggests that criterion A was largely informed by the characteristics of inciting events in adults, particularly combat veterans (8, 10, 35). For youths (in contrast to adults), cognitive and emotional systems guiding subjective interpretation are in a state of developmental flux, and therefore the evaluation and lasting effects of impactful life events can vary considerably from childhood to adolescence to adulthood. Moreover, many studies have identified PTSD symptoms among children and adolescents exposed to a variety of experiences that may be more common or impactful among youths, such as bullying, unplanned pregnancy, forcible separation from caregivers, anticipated death, romantic rejection, racial discrimination, natural disaster, and media-based exposure to trauma (36–41).
The last is explicitly excluded as a criterion A event (as of DSM-5), a decision partly attributed (8) to a 2012 study that failed to detect an association between hours spent viewing disaster-related television coverage in the first week following the event and long-term risk of PTSD diagnosis (42). Yet, this work is an exception to two decades of research revealing posttraumatic stress symptoms among youths exposed to media coverage of horrific events such as the September 11 terrorist attacks (43), Hurricane Katrina (44), and the Boston Marathon bombing (45). Indeed, the authors of the originally cited study have since published a meta-analysis affirming the link between media consumption and diagnostic risk and symptom severity of PTSD in both youths and adults (46). Although the mechanism of transmission remains poorly understood (46), it may stem from an impoverished ability to engage in psychological distancing when confronted with depictions of traumatic events. Given that such emotion regulation skills are still underdeveloped in youths, they are at particularly high risk for being overwhelmed and traumatized from media-based exposure (47).
Societal changes since DSM-5 make the exclusion of this means of trauma exposure particularly problematic. The ubiquity of smartphone, surveillance, and body cameras allows rapid dissemination of video footage captured during extreme scenes of violence and catastrophe ranging from war to mass shootings to natural disasters to police brutality. Such videos are regularly popularized through social media platforms (e.g., TikTok), making them ubiquitous to adolescent audiences. Although the harmful effects of such media may be intuitive, there is a critical need for expanded empirical investigation into the magnitude of its effects in youths (as called for in the recent U.S. Surgeon General’s Advisory [48]), particularly in relation to traumatic stress.
Starting in DSM-5, the diagnosis of PTSD requires meeting or exceeding thresholds in four symptom domains, versus three in DSM-IV—intrusion (criterion B), avoidance (criterion C), negative thoughts or mood (criterion D), and hyperarousal (criterion E)—a change justified by psychometric work indicating that the new structure more closely captures PTSD in adults. Yet, evidence suggests that the diagnostic fit of this model is moderated by age, with PTSD becoming more “adult-like” only later in adolescence (49). As affirmed in ongoing research, these new criteria show limited sensitivity in youths, particularly at younger ages (20, 21). Psychiatric diagnosis relies heavily on the patient’s ability to provide detailed insight about their own thoughts, feelings, and behaviors. Immaturity in the advanced cognitive processes necessary to provide such insight (e.g., metacognition) might effectively restrict endorsement of symptoms in youths who are still developing these abilities. Although it could be argued that this emphasizes the need to derive diagnostic information from caregivers, parents’ assessments of their children’s PTSD symptoms demonstrate poor validity in capturing aspects of diagnostic criteria (50).
The developmental differences in pediatric PTSD may be most clear from work evaluating the disorder through a network lens, in which disorders are modeled as interactive webs of symptoms organized into a stable and self-reinforcing structure (51). As an example, physiological arousal in response to event-related cues may condition hypervigilance for those stimuli, with the associated psychological stress leading individuals to avoid people or situations featuring those cues, ultimately effecting anhedonia in relation to previously enjoyed activities. Indeed, a wealth of research now considers psychopathology networks (52), particularly among symptoms of adult PTSD (53). Of the handful of investigations applying network modeling to pediatric PTSD, Russell et al. (41) identified developmental differences in the substrates of PTSD by contrasting symptom networks across children (under 13 years of age) and adolescents (13 or older) who completed the UCLA PTSD Reaction Index in the aftermath of Hurricane Katrina with findings from comparable adult research (54). Whereas a tightly integrated subnetwork linked anger/irritability to sleep difficulties and concentration problems in prior work with adults, no comparable process was observed in youths. Furthermore, while a sense of foreshortened future and hypervigilance emerged as “hub” symptoms (i.e., symptoms with an outsized influence over all others) for adults (54), neither demonstrated such centrality in youths. Rather, activity avoidance and physiological reactivity emerged as “hub” symptoms in children, compared to hypervigilance and sense of foreshortened future in adolescents. Similar differences were observed across major network connections. For example, children showed a strong link between numbing of negative emotions and trauma-related amnesia, while teens did not, suggesting that these symptoms differentiate with development (and originally derive from a common substrate). Where present, commonalities across children, teens, and adults provided support for contemporary theories about the etiology (and enduring nature) of PTSD. Notably, all groups demonstrated strong links between physiological arousal and avoidance behaviors, providing further affirmation for aberrant threat learning as a model of PTSD pathophysiology across the lifespan. However, studies have only recently begun to examine developmental changes in threat learning and how these may contribute to differential expression of pediatric PTSD over development.
Threat Learning
Altered threat learning is an established translational model of pathophysiology in adult PTSD. Evidence to date suggests alterations in multiple aspects of threat learning in adult PTSD: increased threat acquisition (conditioning), threat generalization, decreased threat inhibition, and decreased threat extinction (55). Across animal models and humans, threat learning is typically evaluated in Pavlovian paradigms that condition a threat response by repeatedly pairing an innocuous stimulus (conditioned stimulus; CS) with an aversive event, and then extinguish that conditioning by presenting the CS alone (56). In an expanded, differential threat learning paradigm, trials pairing the benign stimulus to the aversive event (CS+) are compared with trials pairing a separate stimulus to a neutral event (CS−) (55). The contrast between responses to threat versus safety cues indexes subjects’ ability to discriminate between the two. Investigations using these paradigms find that adults with PTSD demonstrate faster acquisition and slower extinction of conditioning, as well as an exaggerated fear response relative to healthy individuals (55). However, as we review below, far less data exist on threat learning in pediatric PTSD, which must be considered in the context of normative maturation of this system.
Whereas the neural substrates of threat learning show relative stability in healthy adults, these circuits undergo considerable developmental change in youths (57–59). Although longitudinal studies of threat learning in normative pediatric samples remain few, cautious inference may be drawn from several cross-sectional works. Specifically, development may moderate the ability to discriminate between threat and safety cues in differential threat learning paradigms, as summative findings suggest that adults outperform adolescents, who in turn outperform children (60). These gains may stem in part from maturation in functional connectivity between regulatory prefrontal regions and the amygdala, including the ventromedial prefrontal cortex (vmPFC), which can exert inhibitory influence on amygdala-based threat responses, and the dorsolateral prefrontal cortex (dlPFC), a region shown to categorize stimuli signaling threat versus safety (61).
Additionally, replicated findings from animal and human studies reveal attenuated extinction learning in typically developing adolescents compared to children or adults (62). Interestingly, the developmental timing of maximal fear response in adolescence matches a crest in the growth of amygdala volumes (63). The comparative immaturity of downregulatory prefrontal regions may manifest in an adolescent threat response system wherein a maximally engaged amygdala impedes the attempts of higher-order brain regions to signal a change in meaning of threat cues. Indeed, fMRI research in adolescents reveals greater recruitment of the amygdala and hippocampus during threat learning in comparison to adults, although prefrontal responses are less discriminative (61, 64, 65). However, in children it may be that the immaturity of the amygdala actually promotes extinction learning, as does a fully developed prefrontal cortex in adults.
Knowledge about altered threat learning in pediatric PTSD may be derived from a parent body of work considering effects of trauma exposure generally. With regard to threat acquisition, trauma-exposed youths show reduced threat-safety discrimination (66–68) (although see null findings in references 69, 70), but with mixed findings in relation to PTSD symptoms. Relatedly, childhood trauma has been associated with reduced contextual encoding of threat stimuli in youths (8–19 years), an effect that becomes more pronounced with age and may contribute to impaired threat-safety discrimination (71, 72). With regard to extinction, early-adolescent refugees from Syria (average age, 12.7 years) with probable PTSD were found to show impaired extinction learning (but intact acquisition) compared to those with subthreshold PTSD symptoms (70). Mirroring these findings, children (ages 6–11 years) with trauma exposure were found to show poorer extinction recall (but intact acquisition) compared to non-traumatized children, as indicated by greater avoidance of both threat and safety stimuli (69). Our recent pilot study in pediatric PTSD (average age, 13 years) found that reexperiencing symptoms are associated with increased skin conductance responses during extinction recall, driven primarily by response to threat cues (Heyn et al., in revision).
Taken together, these studies suggest that trauma exposure, and potentially PTSD, in youths may be associated with reduced threat-safety discrimination during threat acquisition as well as impaired extinction recall. These findings bear similarity to those in adults, with the notable potential difference of heightened threat acquisition in adult PTSD (55), which has not yet been demonstrated in youths with trauma or PTSD. However, only two of the extant studies reviewed specifically examined clinical threshold pediatric PTSD, and interpretations should remain cautious given the overall paucity of work and the mixed findings. Many important questions remain. How do normative decreases in adolescent extinction learning contribute to PTSD risk, particularly at a time in development when trauma incidence increases? Which alterations in threat learning are specifically associated with pediatric PTSD and recovery, and how do these change over development? While further study is clearly needed, we pivot next to neurodevelopment in pediatric PTSD, which may offer additional insights into the circuits and networks underlying threat processing and regulation.
Neurodevelopment in Key Brain Networks
The interpretation of findings showing altered dynamics of threat learning in PTSD continues to be guided by corresponding investigations into the neural substrates of traumatic stress symptoms. Formative work in threat processing provided the necessary foundation to explore differences in the activation and circuit connectivity across a handful of brain regions linked to recognition and reactivity to threatening stimuli (e.g., amygdala) and modulation of the threat response (vmPFC, dlPFC, hippocampus) (73). Guided by a theoretical current driving modern neuroscience toward network science frameworks of brain function (and away from investigation of circumscribed regions or circuits), investigators are increasingly recognizing PTSD as a manifestation of changes in the flow of information across the networked brain. Although numerous networks are posited to exist, most investigations of PTSD (and pediatric PTSD) focus on connectivity within and between three canonical brain networks with well-established links to psychopathology. We review three pertinent areas of investigation in relation to pediatric PTSD and highlight the need to further consider the influence of biological change across development (Figure 1).
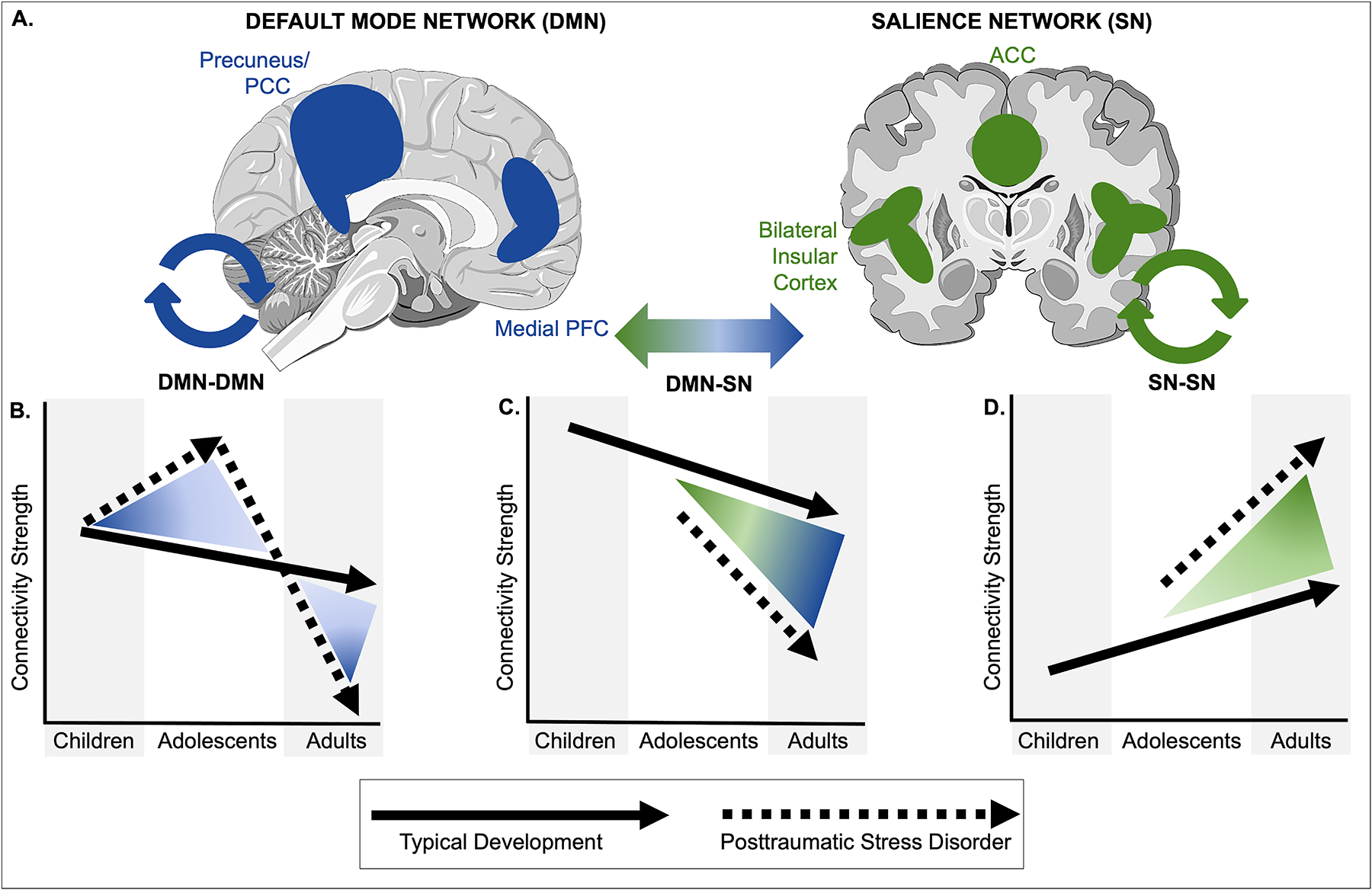
Canonical network development in health and pediatric PTSD (theoretical)a
aIn panel A, key brain structures of the default mode network (DMN) and the salience network (SN) are shown. Panels B–D show theoretical trajectories of intra-connectivity strength within the DMN (panel B), within the SN (panel D), and in DMN–SN inter-connectivity (panel C) in children, adolescents, and adults with PTSD, as compared to typical development. ACC=anterior cingulate cortex; PCC=posterior cingulate cortex; PFC=prefrontal cortex; PTSD=posttraumatic stress disorder.
The default mode network (DMN) is a well-identified pattern of coactivation (connectivity) across the ventral and dorsal medial prefrontal cortices, as well as the posterior cingulate cortex, precuneus, and angular gyrus. The DMN is broadly presumed to govern processes of internal mentation, and, accordingly, disturbance in DMN dynamics often is associated with cognitive symptoms of psychopathology (74), such as intrusive or negative thoughts in PTSD. In adults, PTSD is linked to decreased connectivity strength among regions within the DMN, an effect posited to manifest in impaired cognitive control and dysregulated affect (75). Conversely, our group has identified increased within-DMN connectivity among PTSD-afflicted youths relative to healthy control subjects, an effect further shown to predict the severity of intrusion symptoms (criterion B) (76). Moreover, participant age moderated group differences in connectivity, suggesting potential developmental declines in connectivity among typically developing youths, but increases among those with PTSD. These findings are consistent with work demonstrating increasing hippocampus activation (a key DMN node highly relevant to PTSD) over time in youths with persistent PTSD (77), a positive relationship between assault severity and within-DMN connectivity in adolescent girls (78), and a longitudinal study showing that PTSD symptoms predicted increased connectivity within the DMN over a 3-year period (79) (although see also 80). Various sources have linked aberrant DMN connectivity to impairments in autobiographical memory, cognitive reappraisal, and avoidant behaviors (81).
The salience network (SN) functions as a way station and network switch by assigning behavioral salience weights to information input from external or internal sources, alternately activating the DMN or the central executive network (underlying higher-order cognitive functions) in response (82). Within the SN, network switching is generally localized to the anterior insula and is performed in anticipation of engaging an appropriate behavioral response based on evaluation of the degree of threat or safety indicated by extrinsic or intrinsic inputs. Adults with PTSD exhibit increased connectivity within regions of the SN, thought to reflect hyperactivity within this network. Research in youths is limited, and extant findings are mixed, with reports suggesting increased as well as decreased activation and connectivity within the SN among youths with PTSD (80, 83, 84). This may be in part due to developmental shifts in SN activation in pediatric PTSD. For example, cross-sectional work suggests increasing amygdala activation with age in pediatric PTSD (opposite that of typically developing youths), and which may approach a more adult-like pattern by late adolescence (12). Insights about differences in the SN can also be extrapolated from the broader work examining effects of early-life adversity, where results link trauma exposure to increased activation of the anterior insula specifically, and greater intrinsic connectivity of the SN broadly (83, 85–89).
Cross-sectional investigations in youths with PTSD have variously shown increased or decreased levels of SN-DMN coupling compared with typically developing youths (90). As noted above, age and developmental effects may represent an important aspect of these discrepancies. In a recent two-wave study of adolescents with and without pediatric PTSD (91), our group found decreasing connectivity between the vmPFC and the amygdala (DMN and SN regions, respectively) among affected youths, and increasing connectivity among healthy control subjects. This is consistent with previous cross-sectional work demonstrating decreasing SN-DMN connectivity with age (92) as well as decreased (i.e., more anticorrelated) SN-DMN connectivity in pediatric PTSD (76).
Overall, the research on canonical network development in pediatric PTSD remains limited. However, based on current findings and normative developmental patterns, we posit that pediatric PTSD may be characterized by increasing within-DMN and within-SN connectivity, which may be accompanied by decreasing connectivity between SN and DMN (Figure 1). These patterns of altered network development could contribute to a constellation of symptoms in youths, specifically reexperiencing symptoms/intrusive thoughts (DMN), poor threat-safety discrimination (SN), and difficulty switching from internal mentation to goal-oriented behavior (SN-DMN). Further study will be required to fully test this model.
Treatment-Related Neural Change in Pediatric PTSD
Treatments for pediatric PTSD have not changed substantially over the past few decades, and largely mirror modalities used for adults, albeit with some developmental modifications, particularly for younger children. Evidence-based treatments for pediatric PTSD include trauma-focused cognitive-behavioral therapy (TF-CBT), prolonged exposure, eye-movement desensitization and reprocessing, and narrative exposure therapy (93). Incremental gains continue to emerge from pharmacological research evaluating treatments for PTSD in adults, yet these often fail to translate to youths. Notably, there are currently no evidence-based (or FDA-approved) pharmacological or somatic treatments available for pediatric PTSD.
Studies of potential biomechanisms of improvement in pediatric PTSD from therapeutic modalities are few at present. Neuroimaging studies of TF-CBT (with pre-post imaging) in adolescents have shown that treatment response is associated with increased or maintained insula volume (94), increased prefrontal, entorhinal, and cuneus volumes (95), reduced posterior cingulate/precuneus activation (96), and reduced amygdala–insula (97), dorsal anterior–posterior cingulate, and hippocampus–superior temporal gyrus functional connectivity (98). Additionally, naturalistic recovery from PTSD in youths has been associated with frontal pole surface area expansion as well as increased hippocampal–visual cortex connectivity over 1 year (77, 99). These initial findings suggest that recovery from PTSD in youths may involve decreased connectivity strength within the DMN and within the SN, as well as greater anticorrelation between the SN and DMN. If supported by larger studies, such findings could offer novel biomarkers and targets to be combined with current and novel treatments in a precision medicine approach.
Intergenerational Transmission of Traumatic Stress
A critical area of study in youths involves examination of caregiver transmission of traumatic stress, which may offer additional insights into pediatric PTSD risk and additional intervention strategies. Caregiver transmission of traumatic stress to the child involves both behaviorally modeled (socialization) and biological mechanisms such as epigenetic transfer. Particularly in preadolescence, caregivers are a critical source of information in areas of social cognition, emotion regulation, and threat-safety discrimination. Accordingly, caregiver modeling of behaviors and emotion is likely to influence PTSD risk in offspring after trauma exposure, albeit in ways that are moderated by attachment security and the caregiver-child relationship, as described below.
Previous research has shown that parental anxiety can be directly transmitted to offspring (100), though transmission can be mitigated by parent coaching (101). An anxious parenting style may also mediate the effects of stressful life events on youth anxiety well into early adolescence (102). Parental PTSD is associated with child distress, behavior problems, and altered hypothalamic-pituitary-adrenal axis functioning (103, 104), effects that may be mediated by parenting stress (105). Similarly, maternal emotion dysregulation increases risk for child PTSD symptoms (106), while lower levels of parent distress and PTSD following a child’s trauma predict more favorable outcomes for the child (107). These findings have relevance for clinical treatment. For example, in TF-CBT, which incorporates both caregiver and child, improvements in parent distress and symptoms mediate broad improvements in internalizing and externalizing symptoms in youths with PTSD (108, 109). The biological mechanisms underlying the behavioral transfer of risk and resilience are only beginning to be understood. For example, higher maternal trauma and PTSD symptoms are associated with poorer discrimination between threat and safety cues in children (ages 8–13 years) as measured by fear-potentiated startle (110), whereas maternal anxiety is associated with enhanced threat-safety discrimination in children and adolescents (ages 6–17 years) (111). Finally, maternal warmth has been shown to buffer the effects of violence exposure on amygdala sensitization in children (ages 8–14 years), with concomitant reductions in externalizing behaviors (112).
Recent studies have begun to explore the impacts of parental symptoms and the parent-child relationship on vicariously learned threat responses. For example, higher child anxiety sensitivity and lower father-child relationship security are associated with higher vicariously learned (via watching their parent) physiological threat responses in children (113). Relatedly, higher parent-child physiological synchrony during vicarious threat learning, but only in the context of a less secure parent-child relationship, is associated with higher vicariously learned threat responses in children (114). Finally, in a proof-of-concept study (Heyn et al. in revision), we examined vicarious extinction learning in youths with PTSD, where youths learned to extinguish a threat stimulus by watching their parent undergo extinction learning. The youths’ reexperiencing symptoms were associated with higher physiological responses, particularly to extinguished threat cues during recall. Additionally, higher physiological synchrony was associated with lower physiological response during extinction recall. Taken together, these studies point to behavioral and physiological mechanisms by which PTSD and parental trauma may induce risk for psychiatric problems, including PTSD, particularly in the context of caregiver-child relationship security. Further study will be needed to explore these pathways that are specific to pediatric PTSD.
While a full review is beyond the scope of this overview, the epigenetic transmission of traumatic stress has gained increasing recognition. The epigenetic transmission of stress (and other environmental exposures) may occur through various mechanisms in the germline (particularly sperm), including DNA methylation, histone modification, and noncoding RNA, as well as in utero influences (e.g., maternal stress hormones) on epigenetic modification in the fetus (115). These biological pathways may induce neural connectivity changes early in development, as evidenced by emerging work demonstrating relationships between maternal childhood maltreatment and offspring amygdala-prefrontal connectivity in the fetal to neonatal period (116, 117). Notably, studies in animal models to humans demonstrate epigenetic alterations, particularly in offspring cortisol and glucocorticoid pathways, beginning prenatally (118). While our initial work implicates epigenetic modifications in pediatric PTSD (119), it remains to be seen which of these modifications, if any, are intergenerationally transmitted, induced by trauma, or a result of PTSD itself. Overall, it remains unclear what the full extent of epigenetic modifications in pediatric PTSD may be, which modifications are intergenerationally transmitted, and how these might compare in risk to genetic (Mendelian) inheritance.
Treatment Implications and Future Directions
The findings summarized in this overview highlight the importance of considering pediatric PTSD as a neurodevelopmental disorder and placing it in contrast to adult PTSD in symptom expression, threat learning, and brain network development. Such strategies offer opportunities to develop novel preventive and intervention strategies for pediatric PTSD. For example, combining genetic, epigenetic, and neural markers in youths at risk (e.g., with trauma-exposed parents or caregivers) may offer biologically based markers of risk that could serve as an indicator for early intervention, such as support of caregiver needs, parent coaching strategies, and CBT-based interventions in youths prior to PTSD onset. Given the findings on caregiver transmission of stress, intervening in the family support system, either before or after child PTSD onset, may be particularly fruitful, and it is a strategy that has only been utilized to some extent in TF-CBT. With more precise characterization of biobehavioral markers, there is also the potential to use neuromodulatory therapies in pediatric PTSD (e.g., transcranial magnetic stimulation, direct current stimulation), although the longer-term effects on neurodevelopment remain to be clarified. Emerging studies in adults also suggest a potential benefit of neuroplastic agents such as ketamine and MDMA in adult PTSD (120, 121). Such agents could hold promise for youths, particularly in combination with psychotherapy, although research is clearly needed to characterize the safety profiles of such agents on the developing brain. Finally, youths may benefit from modalities that are more intuitively engaging for new generations, harnessing technology for therapeutic delivery. As one such example, we and collaborators have begun piloting a virtual-reality biofeedback platform for youths with trauma, which is highly immersive, engaging, and well-tolerated in youths, based on initial data. In summary, our hope is that this overview will stimulate further research in a population of youths that is sorely in need of novel, effective, and accessible treatments.
Acknowledgments
Dr. Russell is supported by a Young Investigator Grant from the Brain and Behavior Research Foundation. Dr. Herringa is supported by NIMH (grants R01MH115910, R01MH117141, R01MH128371, and R01MH124076) and by a Mind and Life Institute PEACE grant.
Dr. Herringa is a consultant for Jazz Pharmaceuticals. The other authors report no financial relationships with commercial interests.
Child and Adolescent Psychiatry, PTSD
References
Full text links
Read article at publisher's site: https://doi.org/10.1176/appi.ajp.20230523
Read article for free, from open access legal sources, via Unpaywall:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10636806
Citations & impact
Impact metrics
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/154117353
Article citations
Prospective Measurement of Skin Conductance Response during Trauma Interview Predicts Future PTSD Severity in Trauma Exposed Children.
J Mood Anxiety Disord, 7:100061, 02 Mar 2024
Cited by: 0 articles | PMID: 38559776
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Therapist-supported online cognitive therapy for post-traumatic stress disorder (PTSD) in young people: protocol for an early-stage, parallel-group, randomised controlled study (OPTYC trial).
BMJ Open, 12(3):e054852, 21 Mar 2022
Cited by: 1 article | PMID: 35314471 | PMCID: PMC8938692
Practice parameters for the assessment and treatment of children and adolescents with posttraumatic stress disorder.
J Am Acad Child Adolesc Psychiatry, 37(10 suppl):4S-26S, 01 Oct 1998
Cited by: 47 articles | PMID: 9785726
Review
[The post-traumatic stress disorder--PTSD--in psychiatry by children and teenagers: diagnostic and treatments].
Rev Med Brux, 31(2):111-115, 01 Mar 2010
Cited by: 1 article | PMID: 20677666
DECRYPT trial: study protocol for a phase II randomised controlled trial of cognitive therapy for post-traumatic stress disorder (PTSD) in youth exposed to multiple traumatic stressors.
BMJ Open, 11(7):e047600, 01 Jul 2021
Cited by: 0 articles | PMID: 34210731 | PMCID: PMC8252885
Funding
Funders who supported this work.
NIMH NIH HHS (4)
Grant ID: R01 MH124076
Grant ID: R01 MH115910
Grant ID: R01 MH128371
Grant ID: R01 MH117141




