Abstract
Free full text

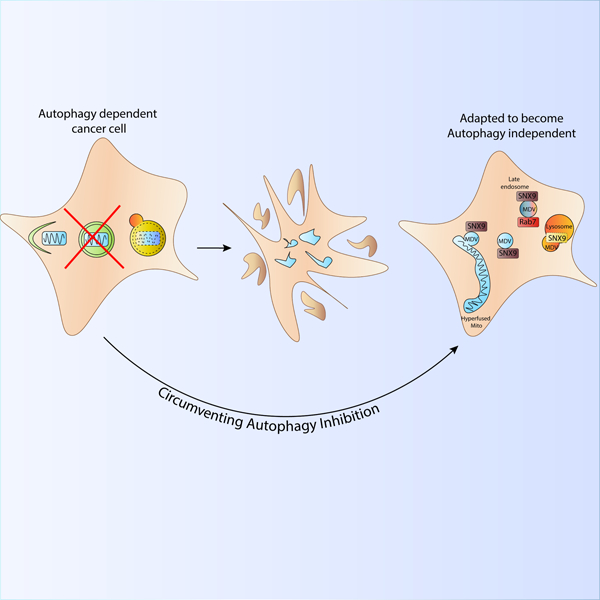
Mitochondrial derived vesicles compensate for loss of LC3-mediated mitophagy
Summary:
Mitochondria are critical metabolic and signaling hubs and dysregulated mitochondrial homeostasis is implicated in many diseases. Degradation of damaged mitochondria by selective GABARAP/LC3-dependent macro-autophagy (mitophagy) is critical for maintaining mitochondrial homeostasis. To identify alternate forms of mitochondrial quality control that functionally compensate if mitophagy is inactive, we selected for autophagy-dependent cancer cells that survived loss of LC3-dependent autophagosome formation caused by inactivation of ATG7 or RB1CC1/FIP200. We discovered rare surviving autophagy-deficient clones that adapted to maintain mitochondrial homeostasis after gene inactivation and identified two enhanced mechanisms affecting mitochondria including mitochondrial dynamics and mitochondrial derived vesicles (MDVs). To further understand these mechanisms, we quantified MDVs via flow cytometry and confirmed an SNX9-mediated mechanism necessary for flux of MDVs to lysosomes. We show that the autophagy-dependent cells acquire unique dependencies on these processes, indicating that these alternate forms of mitochondrial homeostasis compensate for loss of autophagy to maintain mitochondrial health.
eTOC blurb
Towers et al show that autophagy-dependent cancer cells can survive loss of canonical mitochondrial recycling pathways, known as mitophagy. Rare surviving autophagy deficient clones have upregulated mitochondrial dynamics to maintain mitochondrial homeostasis and become more dependent on mitochondrial derived vesicles to degrade damaged mitochondria independent of LC3-conjugation.
Introduction:
Mitochondria are critical organelles that regulate cellular metabolism, energy production, and cell death. Dysregulated mitochondrial homeostasis has been implicated in a variety of disease pathologies including cancer(Zong et al., 2016), neurodegeneration(Wilkins and Morris, 2017), and metabolic diseases(Bhatti et al., 2017). Moreover, harmful reactive oxygen species can accumulate from dysfunctional mitochondria increasing the mutational load of both nuclear and mitochondrial DNA exacerbating cellular abnormalities.
Cells have evolved highly regulated and complex mechanisms to maintain mitochondrial homeostasis involving mitochondrial biogenesis, dynamics and turnover. Fragmentation of mitochondria, otherwise known as mitochondrial fission, is dependent on post-translational modifications of the GTPase containing mechanoenzyme, DRP1(Smirnova et al., 2001, Ishihara et al., 2009). Mitochondrial fusion facilitates the reverse reaction and fusion of the outer mitochondrial membrane (OMM) is dependent on the GTPases, mitofusions 1 and 2 (MFN1 and MFN2), and inner mitochondrial membrane (IMM) fusion is dependent on optic atrophy 1 (OPA1)(Santel and Fuller, 2001, Chen et al., 2003, Griparic et al., 2004, Tilokani et al., 2018). While the two processes complement each other to maintain overall mitochondrial function, a highly fragmented phenotype is often associated with mitochondrial dysfunction while a hyperfused state is often associated with increased oxidative phosphorylation (OXPHOS) and cell survival(Chen et al., 2005, Chen et al., 2010).
Turnover of damaged mitochondria is dependent on double membrane organelles called autophagosomes that engulf mitochondria – either whole or in a piece-meal fashion – for subsequent lysosomal-mediated degradation(Pickles et al., 2018). Mitophagy is the selective form of autophagy that targets mitochondria and involves the initiation of a phagophore structure by an upstream signaling complex that includes the Unc-51 autophagy activating kinases 1 and 2 (ULK1 and ULK2) as well as FAK family kinase interacting protein of 200kDa (RB1CC1/FIP200) which together activate the BECLIN1 complex. Subsequent phagophore elongation, selective targeting of cargos including mitochondria, and ultimately phagophore closure are dependent on the incorporation of microtubule associated protein 1 light chain 3B (MAP1LC3B), which is part of the GABA type A associated proteins (GABARAP/LC3) family) onto the autophagosome membrane. Prior to GABARAP/LC3 incorporation, two separate ubiquitin-like conjugation reactions must occur, both of which are dependent on the E1-like enzyme ATG7, to facilitate the conjugation of phosphatidylethanolamine (PE) to GABARAP proteins(Dikic and Elazar, 2018). Autophagosomes go on to fuse with lysosomes via SNARE-containing proteins like STX17, a reaction that is also highly dependent on GABARAP/LC3(Tsuboyama et al., 2016).
Knock out of all 6 GABARAP family members causes a dramatic reduction in mitochondrial delivery to lysosomes(Nguyen et al., 2016) leading to the generally accepted conclusion that GABARAP-mediated autophagy is the only mechanism by which mitochondria are turned over by the lysosome. Nonetheless, select reports have also observed delivery of mitochondria to lysosomes in cells without genes critical for GABARAP/LC3 conjugation including ATG5 and ATG7(Hirota et al., 2015, Katayama et al., 2011). Recently mitochondrial derived vesicles (MDVs) have been discovered that can deliver pieces of damaged mitochondria directly to lysosomes(Sugiura et al., 2014, McLelland et al., 2016). MDVs have been identified by high resolution microscopy techniques and are approximately 50nM vesicles that preferentially contain either inner or outer mitochondrial material, but never material from both compartments. However, the mechanisms of LC3-independent mitochondrial delivery to lysosomes and particularly the physiological significance of such mechanisms in cancer cells have remained elusive.
Because autophagy plays such an integral role in maintaining mitochondrial homeostasis, we asked if cells can use alternate processes that compensate when canonical mitophagy is rendered inactive. Taking advantage of cancer cell plasticity, we utilized cancer cells to investigate alternate forms of mitochondrial quality control. We discovered that knock out of core autophagy genes kills autophagy-dependent cancer cells within 7 days of gene inactivation, however rare clones could survive and adapt to maintain mitochondrial homeostasis and normal growth under conditions where mitochondrial respiration is required. The surviving ATG7 and FIP200 KO clones maintained mitochondrial function due to increased mitochondrial dynamics and enhanced mitochondrial derived vesicles. Consequently, the autophagy deficient cells acquired a unique dependency on these processes for survival, indicating alternate forms of mitochondrial homeostasis can compensate for loss of autophagy and thus maintain efficient cancer cell growth.
Results:
Rare autophagy deficient cells can maintain functional mitochondria
We performed an acute CRISPR/Cas9 gene inactivation utilizing ribonucleic particles (RNPs) comprised of guide RNAs (gRNAs) targeting the core autophagy genes ATG7 or RB1CC1/FIP200 simultaneously with GFP (Figure 1A) in autophagy-dependent cancer cells. Live cell imaging confirmed loss of these genes caused a significant reduction in growth within the first 7 days of editing, similar to loss of the known essential gene, PCNA, which is required for DNA replication (Figure 1B, Figure S1A). Over a period of weeks, we then selected for the rare clones that were derived from the originally autophagy-dependent BT549 breast cancer line or the H292 lung cancer line that could survive loss of either ATG7 or FIP200(Towers et al., 2019). We identified multiple clones that despite complete inhibition of autophagic flux were still able to retain similar growth rates with WT cells (Figure 1C--D,D, Figure S1B-E). Previously, we have also shown the ATG7 KO cells can form tumors in mice and grow at equal rates compared to WT cells in immunocompromised animals(Towers et al., 2019). These data indicate autophagy-dependent cells can adapt to survive loss of the core autophagy machinery and, given the critical role autophagy plays in turnover of mitochondria, provide a model to test if and how such cells maintain mitochondrial function.
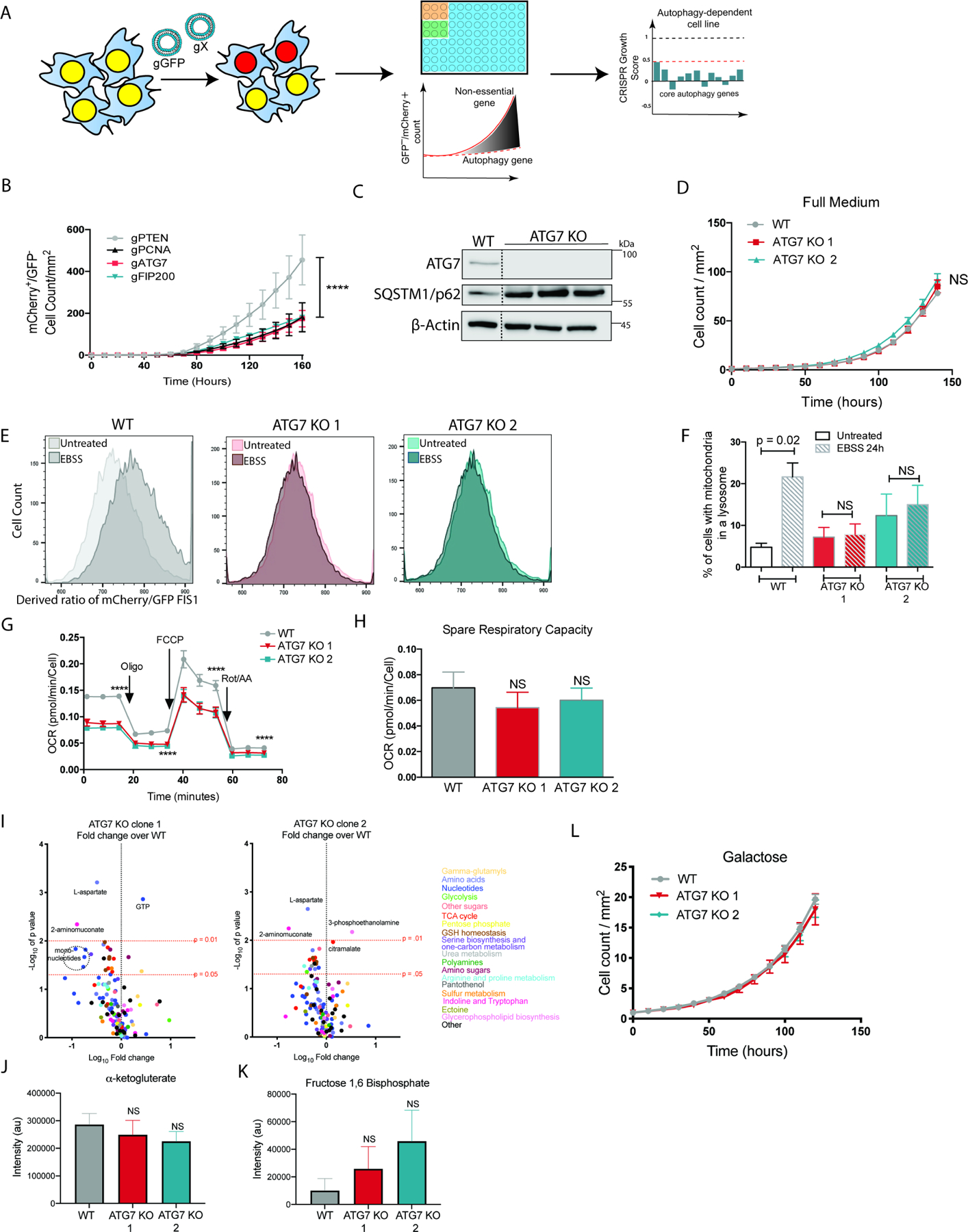
(A) Schematic describing the live-cell CRISPR assay using ribonucleic particles co-targeting a gene of interest and GFP arrayed in a 96 well plate and monitored by Incucyte live cell imaging. BT549 cells showing (B) Incucyte live cell imaging of the mCherry+/GFP- cell count/mm2 immediately after delivery of gRNAs targeting the indicated genes along with gRNAs targeting GFP in cells with stable expression of GFP-NLS and mCherry-NLS. Data are represented as mean ± SEM for technical replicates (N of 2–3). The graphs are representative of 3 experiments. Statistical analysis: two-way ANOVA and the significance at the last time point is shown. (C) Western blot analysis of rare clones that survived loss of ATG7. Blots are representative of 3 experiments. Unnecessary lanes were removed from the blot indicated by a dotted line. (D) Incucyte live cell imaging of mCherry+ cell count/mm2 normalized to time point 0. Data are represented as mean ± SEM for technical replicates (N of 3). The graphs are representative of 3 experiments. Statistical analysis: two-way ANOVA and the significance at the last time point is shown. (E-F) In cells with stable expression of mCh-GFP-Fis1 (E) representative histograms of ratiometric flow cytometry analysis before and after 24hours of starvation in EBSS medium. Graphs are representative of 3 experiments. (F) Quantitative analysis of ratiometric flow cytometry (mCherry/GFP). Graphs are represented as mean ± SEM for biological replicates (N of 4–5). Statistical analysis: one-way ANOVA. (G) Oxygen consumption rates and (H) spare respiratory capacity measured via a Seahorse mitochondrial stress test. Data are represented as the mean ± SD for technical replicates (N = 6) and are representative of 3 individual experiments. (I-K) Quantification of relative intensity of metabolite peaks determined by mass spectrometry and (I) displayed as a waterfall plot of the Log10 fold change of each clone compared to WT cells graphed relative to the p-value. Levels of significance are indicated with dotted red lines and metabolites are color coded according to pathway. The data is represented as the mean of 3 individual experiments. (J-K) Relative intensity (au) of the peaks corresponding to the indicated metabolites. The data is represented as the mean ± SEM of 3 individual experiments. Statistical analysis was performed with a one-way ANOVA. (L) Incucyte live cell imaging of mCherry+ cell count/mm2 normalized to time point 0 of cells grown in media where the glucose was substituted with 100mM galactose. Data are represented as mean ± SEM for technical replicates (N of 5). The graphs are representative of 3 experiments. Statistical analysis: two-way ANOVA and the significance at the last time point is shown. *p≤0.05, **p≤0.01, *** p≤0.01, **** p≤0.001. See also Figure S1, Figure S5, Tables S1 and Table S2.
To confirm that the autophagy deficient cells were incapable of performing canonical mitophagy, we employed the ratiometric tandem GFP-mCherry construct targeted to the outer-mitochondrial membrane by Fis1. An acidic environment quenches the pH sensitive GFP signal increasing the ratio of mCherry/GFP fluorescence for any mitochondria located within an acidic environment, namely a lysosome(Allen et al., 2013). Previous studies have shown that decreased nutrient availability increases mitophagy(Cheng et al., 2015, Allen et al., 2013, Mauro-Lizcano et al., 2015) and flow cytometry analysis confirmed that 24 hours of starvation induced an increase in the percent of cells delivering mitochondria to the lysosome in the WT cells that was not seen in the ATG7 KO clones (Figure 1E--F,F, Figure S1F). Similar results were seen with another pH sensitive mitophagy reporter construct mito-Keima(Katayama et al., 2011) targeted to the inner mitochondrial membrane via Cox8 (Figure S1G). These results are consistent with previous reports indicating that inactivation of all 6 GABARAP/LC3 family members causes a dramatic reduction in mitophagy(Nguyen et al., 2016).
Because the autophagy deficient clones could not perform canonical mitophagy, but could grow at normal rates, we tested mitochondrial function using the Seahorse mitochondrial stress test assay to monitor oxygen consumption rates after specific mitochondrial insult. The ATG7 KO clones displayed decreased basal oxygen consumption levels (Figure 1G). Interestingly however, mitochondria from autophagy deficient cells could still respond to each mitochondrial insult suggesting functional mitochondria remain (Figure 1G, Figure S1H). This is highlighted by comparable spare respiratory capacity levels between the clones and WT cells (Figure 1H). To further investigate global changes in the autophagy deficient clones, metabolomics analysis was performed. Overall there were very few (4/128) metabolites with consistently significant alterations in all of the autophagy deficient clones tested compared to WT cells (Figure 1I, Table S1). Moreover, specific mitochondrial related pathways were not consistently altered across the clones and there were no significant differences in the rate limiting steps for either the TCA cycle or glycolysis (Figure 1J--K,K, Table S1). The WT and ATG7 KO clones also maintained similar growth rates in galactose-containing medium indicating that, when forced to, their mitochondria can generate ATP through OXPHOS similarly to WT cells (Figure 1L, Figure S1I). Together these results indicate that autophagy-dependent cells forced to survive loss of core autophagy genes and canonical mitophagy still maintain mitochondrial function.
Autophagy deficient cells gain an acquired dependency on mitochondrial fusion
To examine the mitochondria, electron microscopy and structured illumination microscopy were performed in WT and ATG7 KO cells. Imaging revealed healthy structures in the ATG7 KO cells with intact cristae, however compared to WT cells the mitochondria appeared elongated (Figure 2A--B).B). This is in contrast to previous studies showing severely deformed mitochondrial cristae in autophagy dependent cancer cells that eventually lost their tumorigenic properties after KO of ATG7(Guo et al., 2013). This marked difference suggests that by selecting for ATG7 KO clones that can grow well, we allowed adaptation by activating alternate processes that maintain mitochondrial health. Western blot analysis revealed an increase in the GTPases necessary for fusion of the outer mitochondrial membrane (OMM) including both mitofusin proteins, MFN1 and MFN2(Chen et al., 2003), without a change in total mitochondrial mass, suggesting increased mitochondrial fusion (Figure 2C, Figure S2A).
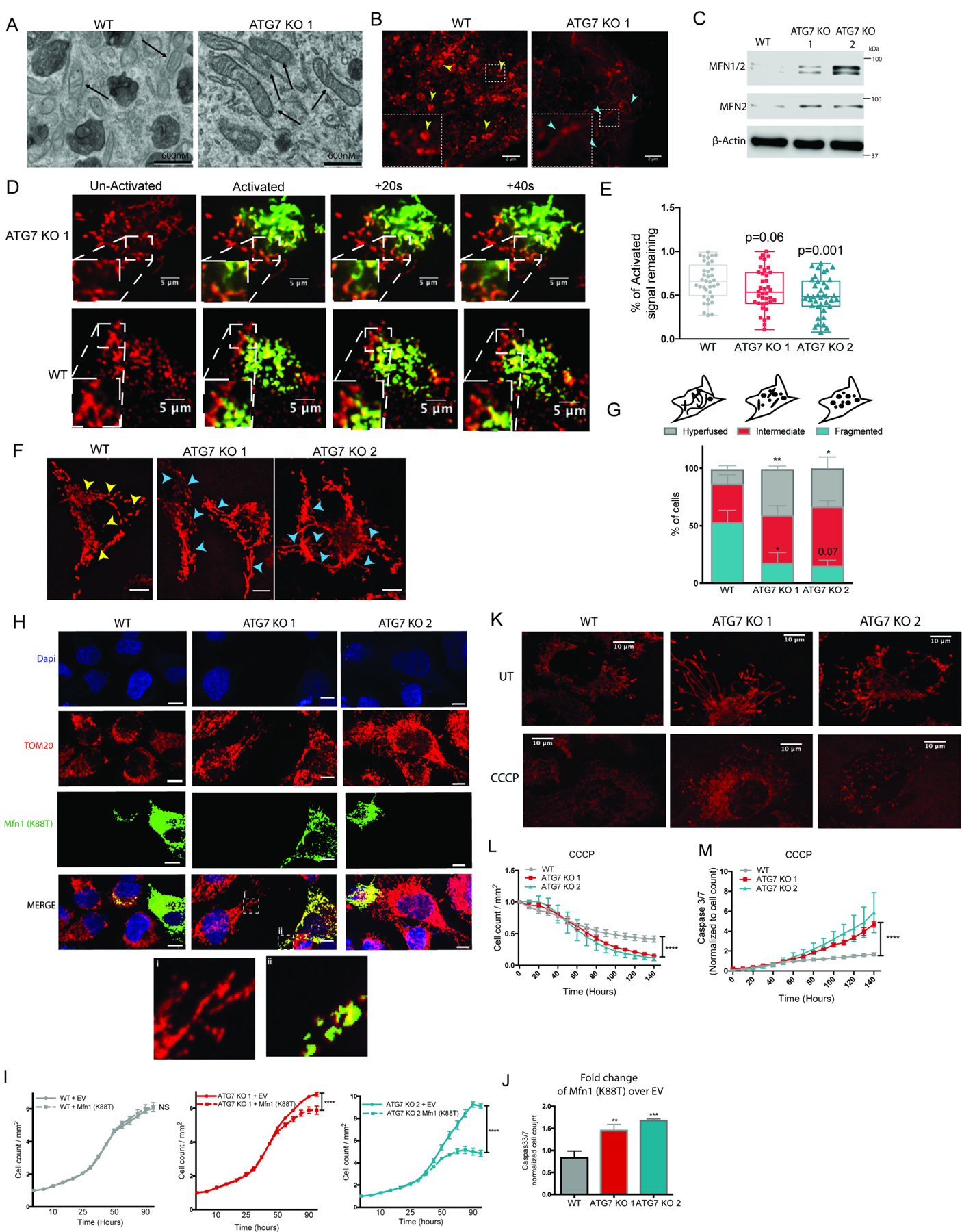
BT549 WT and ATG7 KO clones. (A) Representative electron microscopy images. Black arrows indicate mitochondria. (B) SIM images of mitotracker-red labeled mitochondria where yellow and red arrow heads indicate fragmented and hyperfused mitochondria, respectively. (C) Western blot for indicated proteins, representative blot shown (representative of 3 experiments). (D) Representative confocal images from movies of cells transfected with PA-GFP and co-labeled with mitotracker-red, images shown before and after 405nM light. (E) Quantification of loss of GFP signal 30 minutes after activation relative to the TP0. Data combined from 3 individual experiments, each point is a single cell. Statistical analysis: one-way ANOVA. (F) Representative confocal images of mitotracker-red labeled mitochondria. Yellow arrow heads: fractured mitochondria, red arrow heads: elongated/hyperfused mitochondria. Scale bars represent 10µm. (G) Top: Cartoon of how cells were categorized. Bottom: Quantification of categorized cells. Images from 4 individual experiments were combined and the data are represented as the mean± SEM and one-way ANOVA was performed to determine statistical significance. (H) Representative confocal imaging of immunofluorescent staining of 3X-MYC-MFN1(K88T) in mitotracker-red labeled cells with zoomed insets of merged images shown at the bottom. Scale bars represent 10µm. (I-J) Incucyte live cell imaging of mCherry+ cells transiently transfected with 3X-MYC-MFN1(K88T) and (I) the mCherry+ cell count/mm2 is shown normalized to TP0. Data are represented as mean ± SEM for technical replicates (N of 3). The graphs are representative of 2–3 experiments. Statistical analysis: two-way ANOVA and the significance at the last time point is shown. (J) CellEvent Caspase3/7 green count normalized to mCherry cell count after 4 days of imaging. The data is shown as the fold change of 3X-MYCMFN1(K88T) transfected cells compared to cells transfected with an EV and a one-way ANOVA was performed to assess statistical analysis. (K) Representative confocal live cell imaging of mitotracker-red labeled mitochondria 24hr after treatment with CCCP (20µM). (L-M) Incucyte live cell imaging of mCherry+ cells treated with CCCP (50µM) and CellEvent caspase 3/7 green. The data is shown as (L) mCherry+ cell count normalized to TP0 and (M) the Caspase3/7 green count normalized to the red count over time and represented as the mean ± SD for technical replicates (N of 3). The graphs are representative of 3 experiments. Statistical analysis: two-way ANOVA and the significance at the last time point is shown. *p≤0.05, **p≤0.01, *** p≤0.01, **** p≤0.001. See also Figure S2 and Figure S5.
To test if active mitochondrial fusion was increased in the ATG7 KO cells, time-lapse microscopy was performed on WT and ATG7 KO cells expressing mitochondrial-localized photo-activated-GFP(Karbowski et al., 2004) and mitotracker red. A region of interest (ROI) was activated with 405nm light and the interactions between activated GFP+ and adjacent nonactivated mitochondria were monitored. Active fusion events were observed in the ATG7 KO cells within a minute after activation (Figure 2D, Movie S1,S2) and quantification revealed decreased signal remaining in the ROI indicating the GFP signal had dissipated due to increased mitochondrial fusion. Decreased GFP signal remaining in the ROI was observed in both ATG7 KO clones compared to WT cells confirming the autophagy deficient cells have increased mitochondrial fusion (Figure 2E). For more quantitative analysis of the whole cell population, confocal microscopy of mitotracker red labeled mitochondria was used to calculate the percentage of cells with a hyperfused phenotype. Populations of the autophagy deficient clones had more cells displaying a hyperfused phenotype and less displaying a fragmented phenotype compared to WT populations (Figure 2F--G,G, Figure S2B-C).
To test if the ATG7 KO clones are more dependent on increased mitochondrial fusion for survival, we blocked fusion with a dominant negative construct harboring a mutation in the GTPase domain of mouse Mfn1 (Mfn1-K88T). Transfection conditions were chosen to allow expression of 3XMyc-Mfn1-K88T in only a small percentage of the cells, which showed severely fragmented phenotypes compared to adjacent untransfected cells (Figure 2H) indicating the mutant mouse protein could act as a dominant negative against the endogenous human MFN1. Live cell imaging after transfection of 3XMyc-Mfn1-K88T showed inhibition of mitochondrial fusion had no effect on WT cells but significantly decreased growth and increased caspase3/7 mediated apoptosis of the ATG7 KO clones (Figure 2I--J,J, Figure S2D). Accordingly, both autophagy deficient clones showed increased sensitivity to the fragmentation-inducing mitochondrial insult, CCCP, indicated by decreased growth and increased caspase3/7 mediated apoptosis compared to WT cells (Figure 2K--M).M). Together these data indicate that the autophagy deficient cells generated from originally autophagy dependent cancer lines increased mitochondrial fusion as an adaptive mechanism to maintain mitochondrial function and that this adaptation is required for cell survival.
Autophagy deficient cells produce more mitochondrial derived vesicles
Interestingly, the autophagy deficient cells maintain a larger proportion of hyperfused mitochondria under basal states; however, this differential mitochondrial phenotype was not observed under different types of stress including starvation or mitochondrial insult, suggesting alternate mechanisms may facilitate the mitochondrial homeostasis under different contexts (Figure S2E). A few reports have observed delivery of mitochondria to lysosomes in cells without genes critical for GABARAP/LC3 conjugation including ATG5 and ATG7(Hirota et al., 2015, Katayama et al., 2011), although the mechanisms and functional consequences have remained elusive and it is therefore unclear if such mechanisms can compensate for conventional mitophagy. Mitochondrial derived vesicles (MDVs) are small single membrane structures (apparent size ~500nM, actual size ~70–150nM) that bud off of either the inner or outer membrane of mitochondria via a Parkin-dependent but DRP1-independent mechanism(Soubannier et al., 2012a, Sugiura et al., 2014). These structures were initially characterized by their size and co-localization with either inner mitochondrial proteins like pyruvate dehydrogenase (PDH) or outer mitochondrial proteins such as TOMM20, but never both. While little is known about the role and function of MDVs for mitochondrial homeostasis, previous studies have shown that they form independent of LC3-conjugation and eventually traffic to the lysosomes(McLelland et al., 2016).
Immunofluorescence (IF) for PDH and TOMM20 in BT549 WT and autophagy deficient clone showed that while the vast majority of the mitochondria, even the small fragmented mitochondria, co-stained for both proteins, we could readily identify vesicles with an apparent size of ~300–500nM by high resolution confocal microscopy that were either TOMM20+/PDH- or TOMM20-/PDH+ (Figure 3A). Quantification of these structures revealed a significant increase in both types of MDVs as well as the total number of MDVs in the clones that survived loss of ATG7 (Figure 3B). Interestingly, there was a more consistent increase in TOMM20+/PDH-MDVs across all of the clones more so than the TOMM20-/PDH+ structures. Treatment with either the iron chelator, deferiprone (DFP), or CCCP caused an increase in MDVs in WT cells and a significantly larger increase in the ATG7 KO clones (Figure 3C--D,D, Figure S2F-G). Moreover, the autophagy deficient cells had more mitochondrial-localized PARKIN consistent with previous studies indicating PARKIN positively regulates MDV formation (Figure S2H). Together these results suggest the autophagy deficient clones have increased the formation of LC3-independent MDVs in order to facilitate mitochondrial degradation and homeostasis when canonical mitophagy is not functional.
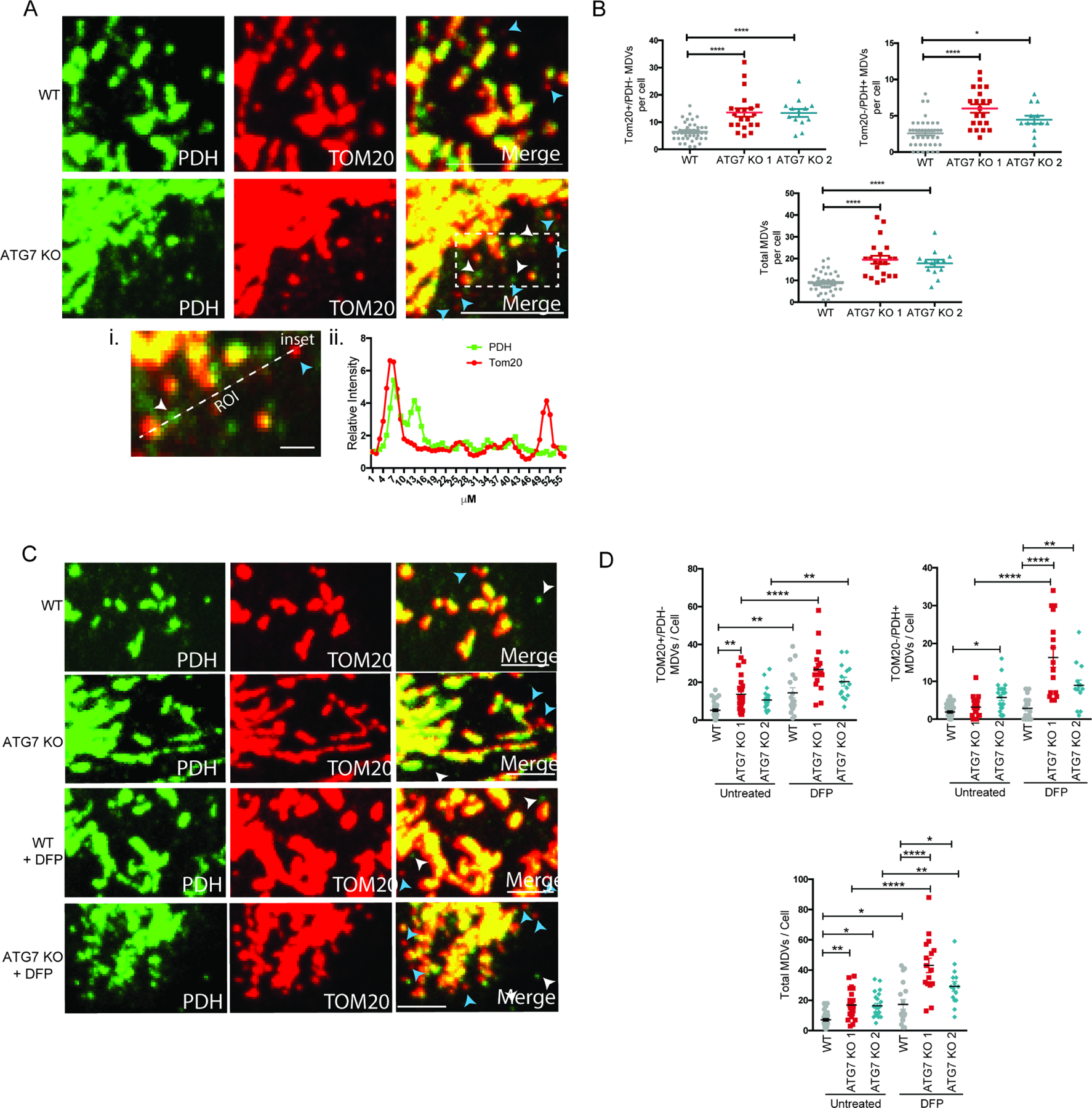
BT549 WT and ATG7 KO clones. (A) Representative confocal images from IF labeled cells with antibodies against TOM20 and PDH. Scale bar represents 10µm. Blue and white arrow heads point to examples of TOM20+/PDH- and TOM20-/PDH+, respectively. (A-i) Zoomed inset from merged image with ROI labeled and scale bar represents 2µM. (A-ii) Quantification of intensity along the ROI indicated in a-i inset. (B) Quantification of the number of MDVs identified per cell across multiple images combining two individual experiments. The data is represented as the mean ± SEM and each dot represents a cell. The total MDV count was determined by adding the TOM20+/PDH- count and the TOM20-/PDH+ count and a one-way ANOVA was performed to determine statistical analysis. (C) Representative confocal images from IF labeled cells with antibodies against TOM20 and PDH 24hr after treatment with DFP (1mM). Scale bar represents 5µm. Blue and white arrow heads point to examples of TOM20+/PDH- and TOM20-/PDH+, respectively. (D) Quantification of the number of MDVs identified per cell across multiple images and the data is representative of 2–3 experiments. The data is shown as the mean ± SEM and each dot represents a cell. The total MDV count was determined by adding the TOM20+/PDH- count and the TOM20-/PDH+ count and a one-way ANOVA was performed to determine statistical analysis. *p≤0.05, **p≤0.01, *** p≤0.01, **** p≤0.001. See also Figure S2 and Figure S6.
pH sensitive constructs can quantify the flux of MDVs to lysosomes
To investigate the flux of MDVs to lysosomes we tested if the pH sensitive mCh-GFP-Fis1 construct (targeted to the outer membrane) and the Cox8-Keima construct (targeted to the inner membrane) preferentially label the TOMM20+/PDH- and TOMM20-/PDH+ MDVs, respectively (Figure 4A). IF in WT and ATG7 KO cells with stable expression of either mCh-GFP-Fis1 or Cox8-keima showed that only TOMM20+/PDH- MDVs stained positive for the pH-stable mCherry fluorescence with reduced GFP fluorescence (Figure 4B), whereas TOMM20-/PDH+ MDVs preferentially stained positive for cox8-Keima (Figure S3A). Furthermore, mitochondrial insult with DFP caused an increase TOMM20+/PDH- MDVs with reduced GFP expression (Figure 4C). Together these data indicate the mCh-GFP-Fis1 construct exclusively labels the outer mitochondrial membrane derived-MDVs and can also monitor their delivery to acidic compartments like lysosomes. We confirmed that mitochondria were still delivered to lysosomes in the absence of LC3 conjugation by co-staining with the membrane potential independent mitotracker-green dye and lysotracker-red dye (Figure S3C). Since canonical mitophagy is not occurring in the autophagy deficient cells (Figure 1E--F,F, Figure S1F-G), we postulate any increased ratio of mCherry/GFP dictated by delivery of mCh-GFP-Fis1 to lysosomes is mediated by TOMM20+/PDH- MDVs. Quantitative assessment by ratiometric flow cytometry where a larger number of cells can be counted compared to microscopy techniques revealed that unlike bulk autophagy induction by starvation that could not induce delivery of mCh-GFP-Fis1 to lysosomes (Figure 1E--F,F, Figure S1F-G), direct mitochondrial insult with either DFP or CCCP did result in delivery of mCh-GFP-Fis1 to lysosomes in the autophagy deficient clones (Figure 4D--E,E, Figure S3B). Treatment with the lysosome inhibitor, Bafilomycin-A1, could reverse this phenotype, confirming the flux is mediated through the lysosomes (Figure S3D). These studies reveal that ratiometric flow cytometry provides a more quantitative assay to measure MDV delivery to lysosomes.
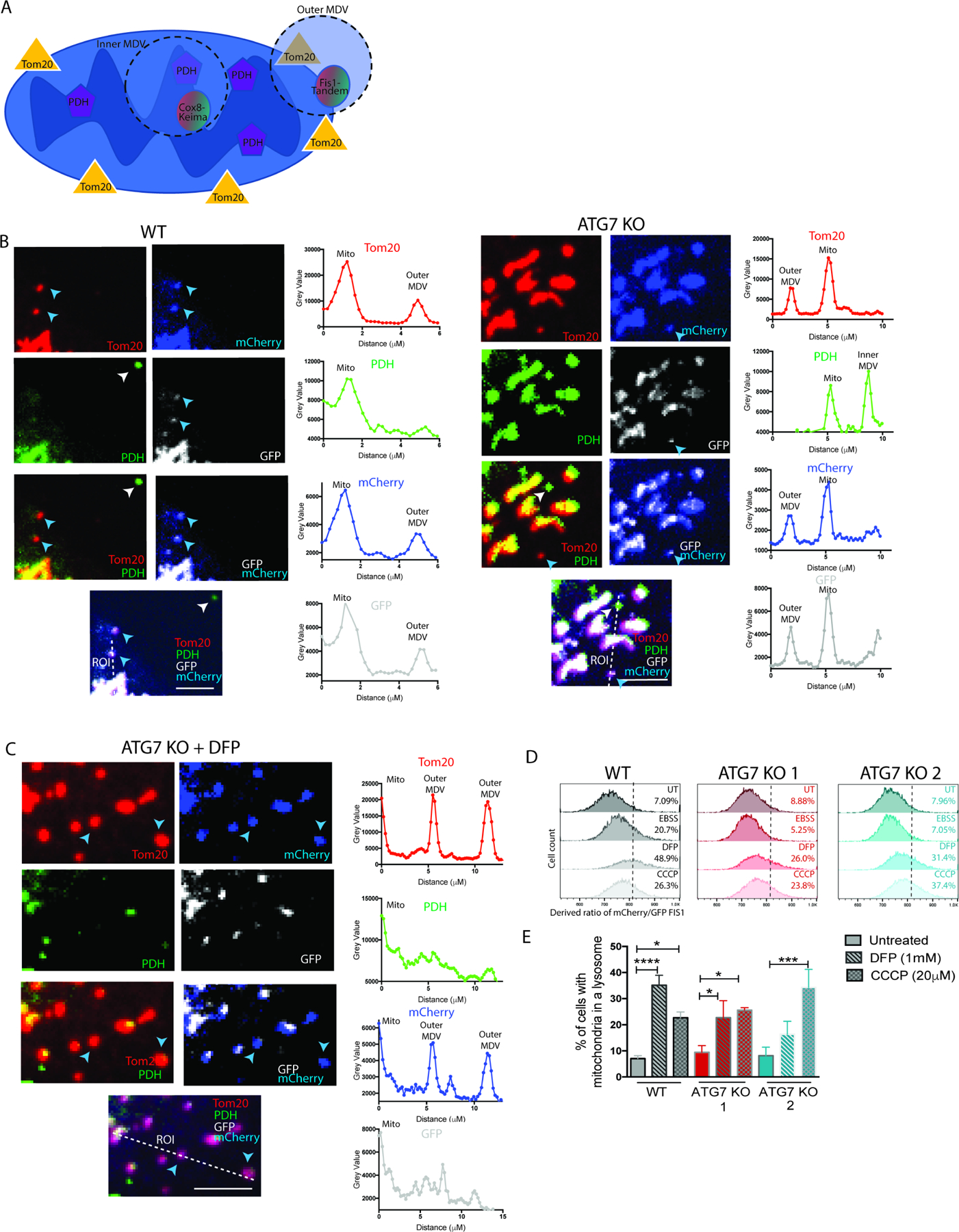
(A) Schematic representation of mCh-GFP-Fis1 localization to TOM20+/PDH- MDVs and Cox8Keima localization to TOM20-/PDH+ MDVs. BT549 WT and ATG7 KO clones with stable expression of mCh-GFP-Fis1. (B-C) Representative confocal images from IF labeled cells with antibodies against TOM20 and PDH either (B) untreated or (C) treated for 24hr with DFP (1mM). Scale bar represents 5µm. The mCherry is pseudo-colored blue and the GFP is pseudo-colored white. Blue and white arrow heads point to examples of TOM20+/PDH- and TOM20/PDH+, respectively. The merged image shows the ROI quantified for intensity in the graphs to the right where the bottom of the ROI line corresponds to the graph origin. (D-E) Quantitative analysis of ratiometric flow cytometry (mCherry/GFP) 24hr after indicated treatments. (D) Representative histograms with gate set to 5% in untreated WT cells and (E) shows multiple biological experiments combined for statistical analysis. Graphs are represented as mean ± SEM for biological replicates (N of 2–6). Statistical analysis: one-way ANOVA. *p≤0.05, **p≤0.01, *** p≤0.01, **** p≤0.001. See also Figure S3 and Figure S6.
Autophagy deficient cells are dependent on SNX9-mediated MDVs for mitochondrial function
To test the necessity of MDVs in mitochondrial homeostasis we performed shRNA mediated knock down of the endocytic protein, sorting nexin 9 (SNX9), which has been previously implicated in the formation of MDVs in immune cells(Matheoud et al., 2016). Knock down of SNX9 caused a significant reduction in both DFP and CCCP induced TOMM20+/PDH- MDVs in the ATG7 KO clones, with less effect on TOMM20-/PDH+ MDVs (Figure 5A--C).C). Ratiometric flow cytometry analysis of mCh-GFP-Fis1 confirmed that loss of SNX9 decreased the delivery of outer-mitochondria derived MDVs to lysosomes under both basal conditions and after mitochondrial insult with either DFP or CCCP (Figure 5D). While the ATG7 KO cells have more SNX9-mediated MDVs compared to WT cells, the autophagy deficient cells also have decreased protein expression of SNX9 (Figure 5B). Treatment with mitochondrial insults caused an even greater decrease in SNX9 expression, a phenotype that could be blocked with a lysosome inhibitor (data not show). These results lead us to hypothesize that SNX9 may act similar to an autophagy adaptor in order to traffic MDVs to lysosomes and in the process gets degraded itself. To test this hypothesis, we created a tandem tagged construct with mCherry and GFP fused to the N-terminus of full length SNX9 (Figure 5E). Ratiometric flow cytometry revealed a higher baseline mCherry/GFP ratio in the ATG7 KO cells (WT: 5.72%, ATG7 KO 1: 20.4%, ATG7 KO 2: 16.4%) consistent with the finding that the autophagy deficient cells have more SNX9-mediated MDVs. Moreover mitochondrial insult with either DFP or CCCP caused a robust increase in the derived ratio indicating that mitochondrial damage causes SNX9 to be degraded in lysosomes and to an even greater extent in cells that have adapted to loss of canonical autophagy.
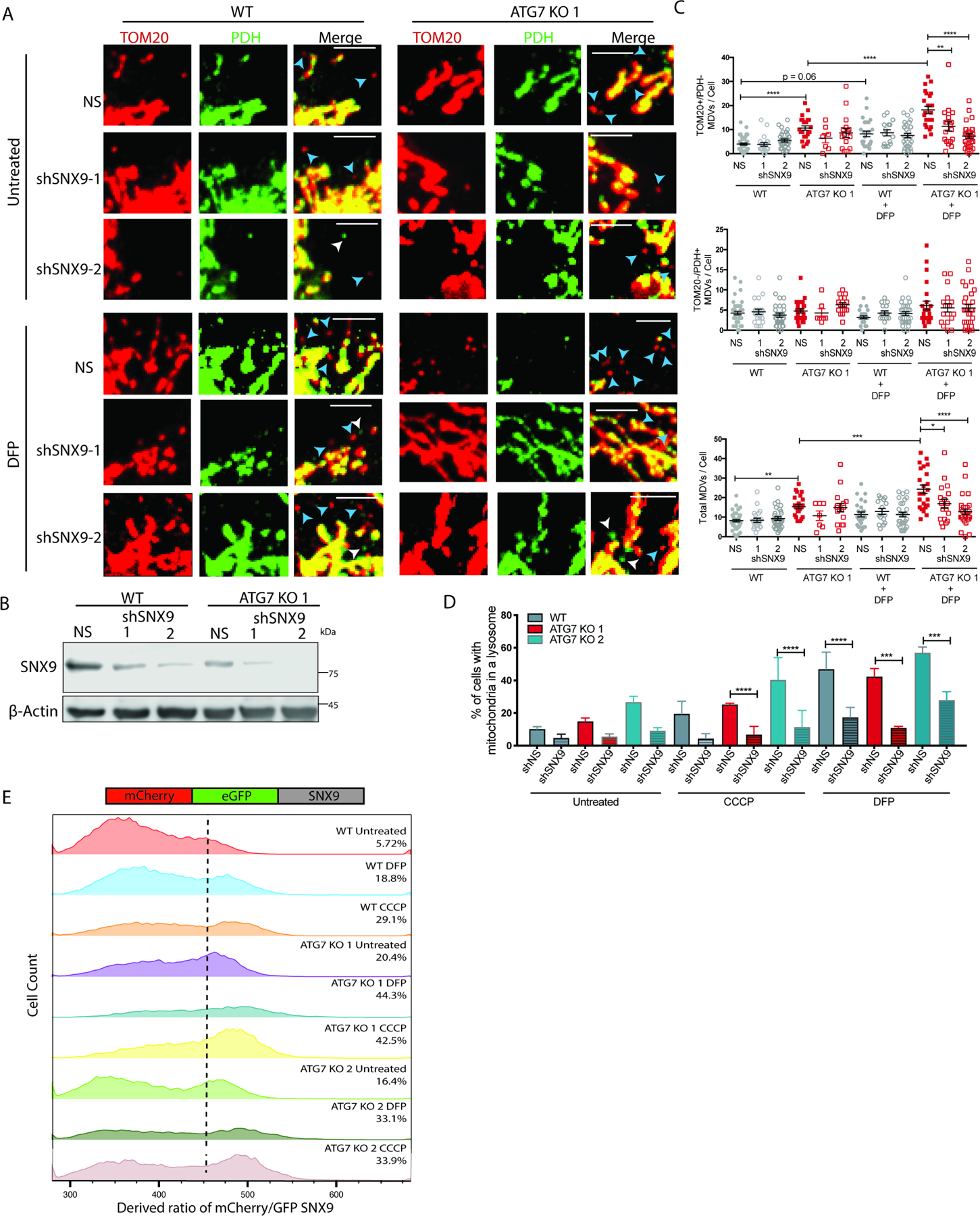
(A-D) BT549 WT and ATG7 KO cells 4 days after transduction with shRNAs targeting SNX9 or shNS. (A) Representative confocal images from IF labeled cells with antibodies against TOM20 and PDH 24hr after treatment with DFP (1mM). Scale bar represents 5µm. (B) Western blot to show knock down of SNX9. (C) Quantification of the number of MDVs identified per cell across multiple images combining two individual experiments. The data is represented as the mean ± SEM and each dot represents a cell. The total MDV count was determined by adding the TOM20+/PDH- count and the TOM20-/PDH+ count and a one-way ANOVA was performed to determine statistical analysis. (D) In cells with stable expression of mCh-GFP-Fis1, quantitative analysis of ratiometric flow cytometry (mCherry/GFP) 24 hr after indicated treatments. Graphs are represented as mean ± SEM for biological replicates (N of 2–4). (E) In BT549 WT and ATG7 KO cells with stable expression of mCh-GFP-SNX9, quantitative analysis of ratiometric flow cytometry (mCherry/GFP) 24hr after treatment with CCCP (50μM) or DFP (1mM). The histograms shown are representative of multiple experiments (N of 3). See also Figure S4.
MDVs have been previously shown to traffic through late endosomes. Consistent with this finding, shRNA mediated knock down of RAB7A – a member of the Ras family of GTPases necessary for the transfer of contents from late endosomes to lysosomes(Vanlandingham and Ceresa, 2009) – phenocopied loss of SNX9 and decreased the percentage of mitochondria that could be delivered to lysosomes via MDVs in the autophagy deficient cells after mitochondrial insult (Figure S4A-B). Loss of RAB7A did not cause a significant reduction in the number of MDVs, indicating RAB7A is important for trafficking MDVs to lysosomes, but not for their formation on mitochondria (Figure S4C). Importantly, knock down of the GTPase that regulates mitochondrial fission, DRP1, did not affect delivery of mCh-GFP-Fis1 to lysosomes, confirming the ratiometric flow cytometry assay is measuring lysosomal delivery of DRP1-independent mitochondrial derived vesicles, and not canonical mitophagy which requires DRP1- mediated mitochondrial fission. (Figure S4D-E).
We next assessed if the autophagy deficient cells have acquired a new dependency on SNX9-mediated MDVs for survival. Live cell imaging revealed that while WT cells could still grow after loss of SNX9, the ATG7 KO cells showed flat growth curves (Figure 6A--B).B). Moreover, loss of SNX9 caused a modest caspase3/7 activation in the WT cells, but the ATG7 KO cells underwent significantly more apoptosis indicating hypersensitivity to loss of SNX9 (Figure 6C,,H).H). Mitochondrial function assays confirmed that the SNX9-mediated effects were focused at the mitochondria, as knock down caused a significant reduction in oxygen consumption rates, spare respiratory capacity, and mitochondrial membrane potential in the autophagy deficient clones but not the WT cells (Figure 6D--E,E, Figure S4 F-G ). Consequently, shSNX9 caused the autophagy deficient cells to be even more sensitive to mitochondrial insult with CCCP (Figure 6F--HH ). Together these results highlight a role for MDVs in mitochondrial homeostasis and turnover of damaged mitochondria. Moreover, these LC3-independent vesicles can compensate for loss of canonical mitophagy.
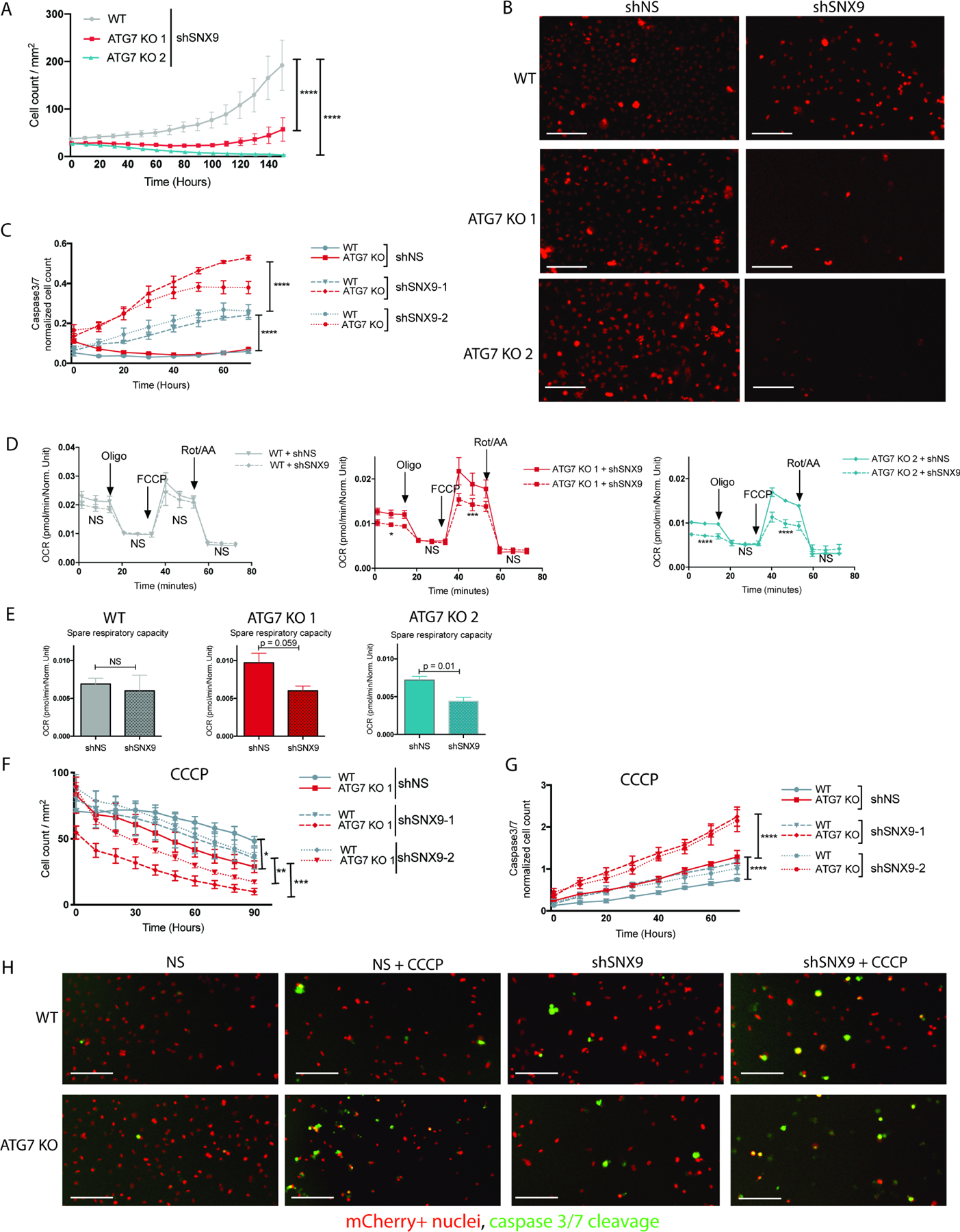
BT549 WT and ATG7 KO cells 4 days after transduction with shRNAs targeting SNX9 or shNS. (A-B) Incucyte live cell imaging of cells with stable mCherry-NLS where mCherry+ counts are graphed over time in cells with shSNX9 and representative images 4 days after plating are shown in B. The data in A is shown as mCherry+ count/mm2 and represented as the mean ± SEM for technical replicates (N of 3). The graphs are representative of 3 experiments. Statistical analysis: two-way ANOVA and the significance at the last time point is shown. Scale bar is 200μM. (C) Incucyte live cell imaging of mCherry+ cells and CellEvent caspase 3/7 green. The data is shown as the Caspase3/7 green count normalized to the mCherry+ red count over time and represented as the mean ± SD for technical replicates (N of 3). The graphs are representative of 3 experiments. Statistical analysis: two-way ANOVA and the significance at the last time point is shown. (D) Oxygen consumption rates and (E) spare respiratory capacity measured via a Seahorse mitochondrial stress test. Data are represented as the mean ± SD for technical replicates (N = 3) and are representative of 3 individual experiments (F-H) Incucyte live cell imaging of mCherry+ cells and CellEvent caspase 3/7 green treated with CCCP (20µM) where F shows the mCherry+ counts for viability curves and G shows the Caspase3/7 green count normalized to the mCherry+ red count over time and both F and G are represented as the mean ± SD for technical replicates (N of 3). The graphs are representative of 3 experiments. Statistical analysis: two-way ANOVA and the significance at the last time point is shown. (H) Representative images of mCherry-NLS and Caspase3/7 green 24hrs after treatment with CCCP. Scale bar is 200μM. *p≤0.05, **p≤0.01, *** p≤0.01, **** p≤0.001. See also Figure S6.
Adaptation to loss of RB1CC1/FIP200 also leads to increased mitochondrial fusion and MDVs
Interestingly, murine embryonic fibroblasts derived from ATG3−/− and ATG5−/− animals did not show increased MDVs compared to their WT counterparts (Figure S5A-B). These data suggest the adaptions may be enhanced or restricted to cancer cells with high demands on mitochondria rather than non-transformed cells. But, these adaptations were not exclusively restricted to ATG7 KO cancer cells since rare clones from autophagy dependent cancer cells could also survive and adapt to KO of the upstream autophagy regulator RB1CC1/FIP200 (Figure 1B, Figure S1D-E). Unlike the ATG7 KO clones which can still form deficient autophagosomes, FIP200 KO cells cannot form STX17-positive autophagosome structures, even in the context of autophagy inducing stimuli(Towers et al., 2020b, Tsuboyama et al., 2016). Despite a loss of canonical mitophagic flux (Figure S5C-D), metabolomics and oxygen consumption assays indicate that similar to ATG7 KO clones the FIP200 KO cells maintain mostly functional mitochondria without significant perturbations to OXPHOS or glycolysis (Figure S5E-I,, Table S1). The rare FIP200 KO clones utilized the same mechanisms as ATG7 KO clones to maintain mitochondrial homeostasis and overall cell survival including increased mitochondrial fusion and increased SNX9-mediated mitochondrial derived vesicles (Figure S5J-L, Figure S6A-C). Moreover, the FIP200 KO cells were also hypersensitive to knock down of SNX9 (Figure S6D-F). Together these results indicate that any defective autophagosomes still generated in ATG7 KO clones are not responsible for the LC3 conjugation-independent maintenance of mitochondrial homeostasis. Moreover, this suggests the mechanisms we have identified including mitochondrial fusion and MDVs are the preferred mechanism to compensate for loss of mitophagy in the absence of both canonical LC-3 mediated autophagy and any autophagosomes without conjugated GABARAP/LC3 proteins.
Discussion:
We have shown that cancer cells dependent on autophagy for survival can give rise to rare clonal populationsin the absence of core autophagy genes like ATG7 or RB1CC1/FIP200 (Figure 1, S1). Surprisingly however, despite the lack of canonical LC3-mediated mitophagy, the autophagy deficient cells maintain functional mitochondria that can adequately support energy needs when required to utilize OXPHOS (Figure 1). These data indicate that, when forced to, cancer cells can adapt to use alternate methods to maintain mitochondrial homeostasis. Previous studies have shown that tumor cell knock out of core autophagy genes that are critical for LC3-conjugation, including ATG5 and ATG7, severely disrupts mitochondrial homeostasis leading to disorganized cristae and reduced oxidative phosphorylation(Strohecker et al., 2013, Guo et al., 2016). In tumor bearing mice, loss of mitochondrial function caused by deletion of such ATGs resulted in extensive metabolic reprograming and a switch from malignant adenomas to benign oncocytomas(Guo et al., 2013). In agreement with these studies, we show that loss of ATG7 or FIP200 causes a significant portion of the cell population to die due, at least in part, to defective mitochondrial function. However, unlike previous studies, our work focused on the rare surviving cells instead of the majority of the population. In these rare cells that escaped loss of autophagy inhibition, we identified two distinct mechanisms that the cells co-opted to maintain functional mitochondria and overall homeostasis.
Under basal conditions and in the absence of overt mitochondrial damage, autophagy deficient cells gained an acquired dependency on mitochondrial fusion (Figure 2). While mitochondrial dynamics have been previously linked to mitophagy, particularly DRP1-mediated fission(Tanaka et al., 2010, Twig et al., 2008), our results are the first to suggest that one process can compensate for lack of the other. Our results further support a cytoprotective role for mitochondrial fusion that is associated with increased levels of MFNs in autophagy-deficient cells. However, the exact mechanisms leading to an increase in mitochondrial fusion GTPases still need to be elucidated.
However, while our data suggest that under basal conditions, increased fusion may be sufficient to compensate for loss of mitophagy, we found that altered mitochondrial dynamics were not sufficient to fully rescue loss of mitophagy in the context of direct mitochondrial insults. Instead, the autophagy deficient cells utilized mitochondrial derived vesicles to traffic severely damaged mitochondria to lysosomes via late endosomes (Figure 3 and and4).4). Even cells incapable of generating autophagosomes due to loss of the upstream autophagy regulator RB1CC1/FIP200(Towers et al., 2020a, Tsuboyama et al., 2016) can still deliver mitochondrial material from either the inner or outer membranes to lysosomes via MDVs (Figure S6). MDVs have only recently been discovered and biochemical assays indicate their ability to recycle damaged and oxidized mitochondrial proteins without disturbing an individual mitochondrion’s function(Soubannier et al., 2012b). However, their physiological role has remained elusive, particularly in cancer cells. Here we show that SNX9-mediated MDVs can compensate for loss of canonical mitophagy to facilitate degradation of damaged mitochondria (Figure 5–6). Our work shows that MDVs provide a form of quality control in the absence of LC3-dependent mitophagy, but it could not be formally proven that this is the only factor mediating mitochondrial quality control. Moreover, our work suggests that SNX9 acts as the adaptor protein that is necessary not only for the formation of MDVs on mitochondria but also for their delivery to lysosomes. Similar to other adaptor proteins, SNX9 is itself degraded via the lysosome in the process (Figure 5). While more studies are needed to better understand the key regulators of MDV formation, our studies suggest that co-targeting autophagy and MDV trafficking may be a more efficacious cancer therapy than autophagy inhibition alone. Previously these tiny vesicles have only been measured by high resolution microscopy, which limits the ability to assess large number of cells or to obtain reliable quantitative measurements, creating a barrier for researchers to understand their role in both normal physiology as well as their potential role in different disease pathologies. We have described a more quantitative way to measure MDV flux through lysosomes utilizing flow cytometry and pH sensitive probes that can preferentially label inner and outer derived MDVs (Figure 4). These techniques will be instrumental in future studies from diverse fields to further understand these still highly understudied vesicles.
Limitations of the study:
One limitation of these studies is the use of cancer cell lines grown on plastic and in RPMI growth media that has non-physiologic levels of many nutrients including amino acids. Future studies should involve more physiologically relevant growth conditions including GEMMs.
STAR Methods:
RESORCE AVAILABILITY
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Christina Towers (ude.klas@srewotc).
Materials Availability
During this study we generated the mCherry-GFP-SNX9 plasmid which has been deposited into Addgene (ID 170551).
Data and Code Availability
The published article includes all datasets generated or analyzed during this study in Table S1.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Experimental Models
All Cell lines were maintained at 37°C and 5 % CO2. BT549 (Female) cells were maintained in Roswell Park Memorial Institute medium (RPMI 1640) with 10 % FBS and Insulin (7.5 µg/ml), NCIH292 (Female) cells were maintained in RPMI 1640 with 10 % FBS, Mouse embryonic fibroblasts (MEFs) were obtained from Dr. Jay Debnath’s laboratory at UCSF and were maintained in Dulbecco’s Modified Eagle’s Medium with 10% FBS.. All cell lines were maintained in penicillin streptomycin while in culture and periodically monitored for mycoplasma contamination. Cells were transduced with either pBabe-mCh-GFP-LC3 retrovirus (generous gift from Dr. Jayanta Debnath (N’Diaye et al., 2009)), pBabe-mCh-GFP-Fis1 retrovirus (generous gift from Dr. Ian Ganley(Allen et al., 2013)), pCHAC-mt-Keima retrovirus (gift from Dr. Richard Youle Addgene plasmid # 72342(Lazarou et al., 2015)), or pBabe-mCh-GFP-SNX9 retrovirus (this construct was created by excising LC3 from the pBabe-mCh-GFP-LC3 vector using ECoRI and SalI and inserting full length SNX9 with the same restriction enzymes) and flow sorted for mCh+ cells. To transiently block mitochondrial fusion, a PCDNA3.1 vector containing the 3xMyc-Mfn1(K88T) mutation (generous gift from Dr. David Chan(Chen et al., 2003)) was transfected into 45,000 cells using TransIT LT1 transfection reagent (Mirus; #Mir2304). Assays were performed 48 hrs after transfection. Transient knock down of SNX9, RAB7A, or DRP1 was established using lentivirus generated in 293FTs transfected with PLKO plasmids (Sigma Aldrich) (Table S2). 45,000 cells were plated and treated with 2ml of virus-containing media harvested from 293FTs. The next day the media was changed to remove the virus and the following day the infected cells were re-plated for experimentation.
METHOD DETAILS
Live Cell CRISPR assay
The acute CRISPR assay was performed as previously described. A nested PCR was performed to generate a DNA template for each guide RNA that contains the T7 sequence adjacent to a 20 base pair gRNA target sequence (designed using crispr.mit.edu) and a tracer RNA region according to previous reports [15] using a T7 FWD primer (TAATACGACTCACTATAG) and TrcRNA REV primer (AAAAGCACCGACTCGGTGCCAC) amplifying off of a tracer RNA PCR product (itself amplified off of lentiCRISPRV2 plasmid (University of Colorado, FGF; Addgene, 62348) with primers: GTTTTAGAGC TAGAAATAGCAAG, AAAAGCACCGACTCGGTGCCAC). The gene specific primers are as provided in Table S2. Following in vitro transcription using the MEGAshortscript T7 transcription kit (Thermo Fisher, AM1354) and subsequent RNA clean-up using the MEGAclear transcription clean-up kit (Thermo Fisher, AM1908), ribonucleic particles (RNPs) were transfected into cells with 200 ng of recombinant Cas9 protein (PNAbio, CP01) and 5 ng of each guide RNA (two that target GFP and two that target the gene of interest), with Lipofectamine CRISPR Max kit transfection kit (Thermo Fisher, CMAX00003). The transfection mixture (20 μl) was added to each individual well of a 96 well plate where cells (mCherry+ GFP+) were seeded at 500 cells per well in 100 μl in triplicate wells the previous day and allowed to incubate for 4.5 h at 37°C and 5% CO2 before replacing the medium. The plates were monitored by Incucyte live-cell imaging for 7–10 d with images taken every 2–4 h. Medium was replaced as needed every 3–4 d. For each cell line, gRNAs targeting essential and non-essential genes were tested in parallel in triplicate wells on each 96 well plates and used for normalization.
Western Blotting
Cells were lysed in stringent RIPA buffer (150 mM NaCl, 1% NP-40 [IGEPAL; Sigma, I8896], 0.5% sodium deoxycholate [Sigma, D6750], 10% SDS, 50 mM Tris, 5 mM EDTA, 2% NaF) containing 1X protease inhibitor cocktail (Roche, 11836153001). For mitochondrial fractions, the reagent based protocol from the mitochondrial isolation kit (Thermo Scientific, 89874) was utilized. After loading 5–30 μg of lysate (depending on the antibody), proteins were separated on SDS-PAGE 1.5 mm mini gels (made with 8–10% acrylamide; Bio-Rad, 1610156) in running buffer at 100 V for 2 h, and then transfered to PVDF membranes (Immobiolon-p transfer membranes, PVH00010) using a semi-dry transfer apparatus (Biorad, SD1703940) at 15 V for 70 min. Membranes were then blocked in 5% milk (Labscientific, M0842) for 1 h at room temperature, washed in TBST (Tris-buffered saline with 1% Tween (Fisher bioreagents, 21359)), and then incubated overnight at 4°C with gentle rocking in primary antibodies including anti-ATG7 (Cell Signaling Technology, 8558), anti-SQSTM1 (Novus, H00008878-M01), anti-LC3 (Novus, NB100–2220), anti-RB1CC1/FIP200 (Novus, NBP1– 31583), anti-Snx9 (Proteintech 15720–1-AP), anti-Myc (From a Hybridoma cell line made through the UCDenver Tissue Culture Core), anti-MFN1/2 (Abcam Ab57602), anti-MFN2 (Cell Signaling: 9482), anti-PARKIN (Cell Signaling: 2132s), anti-COXIV (Abcam: AB14744) or anti-ACTB /β-actin (Sigma-Aldrich A5441). Membranes were washed three times in TBST and incubated for 1 h at room temperature with gently rocking in secondary antibodies (anti-rabbit [Cell Signaling Technologies, 7074], anti-mouse [Cell Signaling Technologies, 7076]), followed by 3 additional TBST washes. Membranes were developed with Immobilon western chemi- luminescent HRP substrate (Millipore, P90720) and analyzed on the OdysseyFc imaging system (Li-cor biosciences, 2800–03).
Incucyte live cell imaging
The Incucyte (dual color model 4459) at 4X magnification was used for live cell imaging and images in the red and green channel were taken every 2–4 hours (for figure presentation, quantification ever 8–12 hours is shown). For mCherry cell count, mCherry+ cells/mm2 were masked (and optimized for each cell type) and for caspase 3/7 activity: CellEvent Caspase 3/7 green reagent (Thermo Fisher C10423) was added (2µM) and green events were masked and counted. Green count/mm2 was then normalized to red count/mm2 to normalize for cell number at every time point. The overlap mask was used for quantification of GFP- cell count during the Live-cell CRISPR assay to quantify the number of double positive cells. At each time point the overlap/mm2 count was subtracted from the red count/mm2 to quantify the GFP- cell count.
Measurement of autophagic/mitophagic flux by ratiometric flow cytometry
Cells stably expressing mCherry-GFP-LC3 (to measure autophagy), mCherry-GFP-Fis1 (to measure mitophagy based on degradation of the outer mitochondrial membrane), mito-Keima (to measure mitophagy based on degradation of the inner mitochondrial membrane), or mCherry-GFP-SNX9 (to measure flux of SNX9 through lysosomes) were used for flow cytometric analysis. Flow cytometry was performed with a Gallios 561 (Beckman Coulter) using the 488 and 561 nM lasers. The appropriate side/forward scatter profile was used to exclude non-viable cells and doublets, and unstained cells were used to generate the “tandem+” gate. FlowJo software was then used to calculate the ratiometric analysis of mCherry/GFP within the “tandem+” gate. For quantification of mitochondrial delivery to lysosomes, the gate was set to 10% of tandem+ cells based on the mCherry/GFP ratio in untreated or vehicle treated conditions.
nM lasers. The appropriate side/forward scatter profile was used to exclude non-viable cells and doublets, and unstained cells were used to generate the “tandem+” gate. FlowJo software was then used to calculate the ratiometric analysis of mCherry/GFP within the “tandem+” gate. For quantification of mitochondrial delivery to lysosomes, the gate was set to 10% of tandem+ cells based on the mCherry/GFP ratio in untreated or vehicle treated conditions.
Measurement of Oxygen Consumption Rate
Between 5,000–8,000 mCherry+ cells (to match confluency on day of the assay) were plated in 96-well plates in technical replicates of 3–6 (XF96 plates) and incubated at 37 °C overnight. The next day, the medium was changed to XF Assay Medium (Agilent) containing 10 mM glucose, 1 mM pyruvate, and 2 mM glutamine and the cells were incubated at 37 °C for 1hr. The Seahorse XF mitochondrial stress test (Agilent) was performed according to manufacturer’s instructions and oxygen consumption rates (OCR) were measured with a Seahorse XFe96 extracellular flux analyzer (Agilent). Briefly, basal OCR was measured prior to the addition of oligomycin (0.5μM), followed by 3 more OCR measurements and then carbonilcyanide p-triflouromethoxyphenylhydrazone FCCP (0.5µM in BT549 and 1.0 µM in H226 cell lines) was added. Three more OCR measurements were taken before Rotenone/Antimycin (0.5µM) was added followed by three final OCR measurements. All data was analyzed in Wave software and normalized to mCherry cell count/mm2 (assessed by a scan-on-demand in the incucyte performed immediately after the mito stress test). The parameter values were calculated in Wave as follows: Basal respiration – Last rate measurement before the first injection subtract the non-mitochondrial respiration rate (minimum rate measurement after rotenone/Antimycin A injection), spare respiratory capacity – maximal respiration (maximum rate measurement after FCCCP injection subtract the non-mitochondrial respiration rate) subtract basal respiration.
Microscopy
Electron Microscopy
Cells were fixed on coverslips in 2.5% glutaraldehyde in 0.1 M Sodium Cacodylate buffer. Cells were first rinsed three times in 0.1 M Sodium Cacodylate buffer (pH 7.4), then post-fixed in 1% osmium tetroxide and 1.5% potassium ferrocyanide for 30 minutes. After three washes in water, the cells were immersed in 1% uranyl acetate in water for 30 minutes. Following 2 rinses in water, they were dehydrated through a graded ethanol series (50%, 70%, 95%, 100% x2) for 5 minutes each. The coverslips were quickly blotted on Whatman paper, being careful not to let the cells dry out, and a drop of Epon/Araldite resin was applied to the coverslips before pre-filled BEEM capsules were flipped over on top of them. Samples were polymerized for 24 hours at 60°C in an oven. After polymerization, the coverslips were removed by dipping the block in liquid nitrogen. Ultra-thin sections (60 nM) were cut on a Reichert Ultracut S from a small trapezoid positioned over the cells and were picked up on copper mesh grids (EMS). Sections were imaged on a FEI Tecnai G2 transmission electron microscope (Hillsboro, OR) with an AMT digital camera (Woburn, MA).
Structured illumination microscopy
75,000 cells were plated on 35mm2 glass bottom plates and the following day treated with mitotracker red at a 1:30,000 dilution for 30 minutes. Images were acquired with a Nikon N-SIM E Structured illumination microscope and reconstructed with Nikon Elements software as previously described(Hiester et al., 2017, Smith et al., 2014).
Confocal microscopy
For live cell imaging, live cells were plated and imaged in MatTek 35mm glass bottom culture dishes using a confocal laser scanning Olympus FV1000 with a 60X objective. Mitotracker (1:30,000–60,000) was added 5 minutes prior to imaging each dish. For quantification of fractionated, intermediate and hyperfused mitochondria: 5–10 images were taken per condition and 20–30 cells analyzed for each condition per experiment. For immunofluorescent imaging, cells were grown and treated on glass cover slips and then fixed in formalin (Fisher Scientific, Cat# SF93) at room temperature for 15 minutes, washed thrice in PBS, and then permeabilized with 0.1% Triton X-100/PBS for 5 min at RT, followed by another 3 PBS washes. After a 30 minute block in 5% normal goat serum (NSG)/0.3 M glycine/PBS at room temperature, primary antibodies were added to incubate over night at 4°C. Primary antibodies were diluted in blocking buffer and used at the following dilutions: anti-Myc (1:1000, From a Hybridoma cell line made through the UCDenver Tissue Culture Core), anti-TOMM20 (1:1000, Abcam: #Ab78547), anti-PDH (1:200, Abcam: #Ab110330) After 3 PBS washes, Alexa Fluor conjugated secondary antibodies were added at 1:1000 including Alexa Fluor 594 goat anti-rabbit IgG (Life Technologies: A11012), Alexa Fluor 488 goat- anti mouse IgG (Life Technologies: A11029), or in cells already expressing mCherry and GFP Alexa Fluor 633 goat anti-rabbit IgG (Life Technologies: A21070) and Alexa Fluor 405 goat- anti mouse IgG (Life Technologies: A31553) and incubated at room temperature for 45 minutes in the dark. After another 3 PBS washes, the cover slips were mounted onto glass slides with ProLong Diamond Antifade with DAP and allowed to dry overnight and then imaged with a confocal laser scanning Olympus FV1000 with a 60X objective. Z-stacked images were taken and then displayed as max-projections.
Mitochondrial Fusion Assay
75,000 live cells were plated on MatTek 35mm glass bottom culture dishes and the following day transfected with 2µg of mito-PA-GFP recombinant DNA(Karbowski et al., 2004) and 6µl TransIT LT1 transfection reagent according to manufactures instructions. After a 16hr incubation the transfection medium was washed off and 2ml of the appropriate full medium was added back to each dish. The following day mitotracker red was added at a 1:30,000 dilution 5 minutes prior to imaging on a confocal laser scanning Olympus FV1000 with a 60X objective. One small region of interest (ROI) per cell was activated with 405nm light at 2% power for 2ms. Z-stack images were taken in the red channel at 561nm and the green channel at 488nm immediately before photo-activation (pre-activation image), immediately following photo-activation (post-activation image) and 30 minutes after photo-activation (30 minute image). For quantification of the GFP signal in the 488 channel, an ROI with an area of 8–12 pixels was used to measure the region in the cell with the greatest mean intensity in the post-activation image. That ROI was copied into the pre-activation and 30 minute images to calculate the mean intensity. In the 30 minute image the ROI was adjusted slightly for cell drift and/or mitochondrial movement if needed. Cells with less than a 1.5 fold increase in activated GFP (measured by mean intensity) were discarded. The percent remaining of GFP was calculated as a ratio of the mean intensity in the 30 minute image over the mean intensity of the ROI in the post-activation image. 10–15 cells were analyzed per condition per experiment. For movies, images were taken every 20 seconds, and photo-activation occurred after the first image.
Metabolomics:
Metabolites from frozen cell pellets were extracted at 2 million cells per mL in ice cold 5:3:2 methanol:acetonitrile:water and resulting extracts analyzed using a 3 min isocratic C18 method on a Thermo Vanquish UHPLC coupled to a Thermo Q Exactive mass spectrometer as previously described(Nemkov et al., 2015). Samples were analyzed in positive and negative ion modes (separate runs); quality control and data analysis were performed as described(Nemkov et al., 2017).
QUANTIFICATION AND STATISTICAL ANALYSIS:
The correct statistical analysis test was performed within GraphPad PRISM as indicated within the figure legends for each figure. Depending on the number of variables considered either a one-way ANOVA (considering one variable) or a two-way ANOVA (considering two variables) was per- formed. Significance is noted within each figure as follows: *p 0.05, **p 0.01, *** p 0.01, **** p 0.001**** p 0.0001. For incucyte data, the significance corresponding to the last time point is shown.
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| P62 | Novus | Cat# H00008878-M01, RRID:AB_548364 |
| LC3 | Novus | Cat# NB100–2220, RRID:AB_10003146 |
| ATG5 | Cell Signaling Technology | Cat# 9980S, RRID:AB_10829153 |
| ATG7 | Cell Signaling Technology | Cat# 8558, RRID:AB_10831194 |
| ATG12 | Cell Signaling Technology | Cat # 2010s RRID:AB_2059086 |
| FIP200 | Novus | Cat# NBP1–31583, RRID:AB_2300812 |
| Parkin | Cell Signaling Technology | Cat# 2132s, RRID: AB_10693040 |
| SNX9 | Proteintech | Cat# 15721–1-AP, RRID:AB_2286415 |
| ATG 3 | Cell Signaling Technology | Cat# 3415, RRID:AB_2059244 |
| COXIV | Abcam | Cat# ab14744, RRID:AB_301443 |
| MFN1/2 | Abcam | Cat# ab57602, RRID:AB_2142624 |
| MFN2 | Cell Signaling Technology | Cat# 9482, RRID:AB_2716838 |
| TOMM20 (TOM20) | Abcaam | Abcam Cat# ab78547, RRID:AB_2043078) |
| Pyruvate Dehydrogenase (PDH) | Abcam | Cat# ab110330, RRID:AB_10858459 |
| β-Actin | Sigma-Aldrich | Cat# A5441, RRID:AB_476744 |
| β-Tubulin | Sigma-Aldrich | Cat# T5168, RRID:AB_477579 |
| Mouse-IgG | Cell Signaling Technology | Cat# 7076, RRID:AB_330924 |
| Rabbit-IgG | Cell Signaling Technology | Cat# 7074, RRID:AB_2099233 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Bafilomycin A1 | Sigma-Aldrich | B1792; CAS RN: 88899–55-2 |
| Protease Inhibitor Cocktail | Roche | 11836153001 |
| Mitotracker Green | ThermoFisher Scientific | M7512 |
| Mitotracker Red | ThermoFisher Scientific | M7514 |
| Critical Commercial Assays | ||
| MEGAscript T7 Transcription Kit | Thermo Fisher | AM1354 |
| MEGclear Transcription Clean Up Kit | Thermo Fisher | AM1908 |
| Wizard SV Gel PCR clean up kit | Promega | A9282 |
| Lipofectamine CRISPR Max Cas9 transfection reagent | Thermo Fisher | CMAX00003 |
| RNeasy RNA isolation kit | Qiagen | 74104 |
| Seahorse XF Mitochondrial Stress Test | Agilent | 103015–100 |
| Experimental Models: Cell Lines | ||
| NCIH292 | Kindly provided from Dr. Joaquin Espinosa | ECACC Cat# 91091815, RRID:CVCL_0455 |
| BT549 | ATCC | NCI-DTP Cat# BT-549, RRID:CVCL_1092 |
| ATG3−/− Mouse Embryonic Fibroblasts | Kindly provided from Dr. Jayanta Debnath | |
| ATG5−/− Mouse Embryonic Fibroblasts | Kindly provided from Dr. Jayanta Debnath | |
| Oligonucleotides: | ||
| Oligonucleotides for guide RNA design: Table S2 | This study | N/A |
| Recombinant DNA | ||
| pBabe-mCherry-GFP-LC3 | (N’Diaye et al., 2009) | N/A |
| pBabe-mCherry-GFP-Fis1 | (Allen et al., 2013) | N/A |
| pBabe-mCherry-GFP-SNX9 | This paper | N/A |
| PA-GFP | (Karbowski et al., 2004) | N/A |
| LentiCRISPR – ATG7 | (O’Prey et al., 2017) | N/A |
| PLJM1-GFP-3xNLS puromycin | (Thorburn et al., 2017) | N/A |
| PLJM1-mCherry-3xNLS blasticidin | (Thorburn et al., 2017) | N/A |
| PLKO.1 shRNA-SNX9 TRCN0000147006 | Sigma-Aldrich | N/A |
| PLKO.1 shRNA-SNX9 TRCN0000147249 | Sigma-Aldrich | N/A |
| PLKO.1-shRNA-RAB7 TRCN0000380577 | Sigma-Aldrich | N/A |
| PLKO.1-shRNA-DRP1 TRCN0000318424 | ||
| PLKO.1-shRNA-DRP1 TRCN0000318425 | Sigma-Aldrich | N/A |
| Other | ||
| Phusion High Fidelity DNA Polymerase | New England Biolabs | M0530L |
| CellEvent Caspase3/7 Green Detection Reagent | Thermo Fisher Scientific | C10423 |
| TransIT LT1 Transfection Reagent | Mirus | MIR 2304 |
| Cas9 recombinant protein | PNAbio | CP01 |
Supplementary Material
2
3
4
5
Acknowledgments:
This work was supported by NIH grants RO1CA150925 & RO1CA190170 (to A.T.), 5 T32 CA 190216–2 (C.G.T), the American Cancer Society Post-Doctoral fellowship AWD 183648 (C.G.T), K99CA245187 (C.G.T) and shared resources supported by the University of Colorado Cancer Center P30CA046934.
Funding:
This work was supported by NIH grants [RO1CA150925] (to A.T.), [RO1CA190170] (to A.T.), [5T32 CA 190216–2] (C.G.T), the American Cancer Society Post-Doctoral Fellowship AWD 183648 (C.G.T), and shared resources supported by the University of Colorado Cancer Center [P30CA046934].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests:
The authors declare no competing interests.
Inclusion and Diversity Statement:
One or more of the authors of this paper self-identifies as an underrepresented ethnic minority in science.
Disclosure statement:
The authors have nothing to disclose.
Supplemental Video Legends:
Movie S1: WT cells undergo less mitochondrial fusion. Related to Figure 2
WT BT549 cells transfected with PA-GFP and co-labeled with mitotracker-red, with frames taken before and after 405nM light. Scale bar represents 5μM.
Movie S2: ATG7 KO cells undergo more mitochondrial fusion. Related to Figure 2
ATG7 KO BT549 cells transfected with PA-GFP and co-labeled with mitotracker-red, with frames taken before and after 405nM light. Scale bar represents 5μM.
Supplementary Excel Table Title:
Table S1: Metabolomics analysis. Related to Figure 1 and S5.
References
- ALLEN GF, TOTH R, JAMES J & GANLEY IG 2013. Loss of iron triggers PINK1/Parkin-independent mitophagy. EMBO Rep, 14, 1127–35. [Europe PMC free article] [Abstract] [Google Scholar]
- BHATTI JS, BHATTI GK & REDDY PH 2017. Mitochondrial dysfunction and oxidative stress in metabolic disorders - A step towards mitochondria based therapeutic strategies. Biochim Biophys Acta Mol Basis Dis, 1863, 1066–1077. [Europe PMC free article] [Abstract] [Google Scholar]
- CHEN H, CHOMYN A & CHAN DC 2005. Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J Biol Chem, 280, 26185–92. [Abstract] [Google Scholar]
- CHEN H, DETMER SA, EWALD AJ, GRIFFIN EE, FRASER SE & CHAN DC 2003. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol, 160, 189–200. [Europe PMC free article] [Abstract] [Google Scholar]
- CHEN H, VERMULST M, WANG YE, CHOMYN A, PROLLA TA, MCCAFFERY JM & CHAN DC 2010. Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell, 141, 280–9. [Europe PMC free article] [Abstract] [Google Scholar]
- CHENG B, XU A, QIAO M, WU Q, WANG W, MEI Y & WU M 2015. BECN1s, a short splice variant of BECN1, functions in mitophagy. Autophagy, 11, 2048–2056. [Europe PMC free article] [Abstract] [Google Scholar]
- DIKIC I & ELAZAR Z 2018. Mechanism and medical implications of mammalian autophagy. Nat Rev Mol Cell Biol, 19, 349–364. [Abstract] [Google Scholar]
- GRIPARIC L, VAN DER WEL NN, OROZCO IJ, PETERS PJ & VAN DER BLIEK AM 2004. Loss of the intermembrane space protein Mgm1/OPA1 induces swelling and localized constrictions along the lengths of mitochondria. J Biol Chem, 279, 18792–8. [Abstract] [Google Scholar]
- GUO JY, KARSLI-UZUNBAS G, MATHEW R, AISNER SC, KAMPHORST JJ, STROHECKER AM, CHEN G, PRICE S, LU W, TENG X, SNYDER E, SANTANAM U, DIPAOLA RS, JACKS T, RABINOWITZ JD & WHITE E 2013. Autophagy suppresses progression of K-ras-induced lung tumors to oncocytomas and maintains lipid homeostasis. Genes Dev, 27, 1447–61. [Europe PMC free article] [Abstract] [Google Scholar]
- GUO JY, TENG X, LADDHA SV, MA S, VAN NOSTRAND SC, YANG Y, KHOR S, CHAN CS, RABINOWITZ JD & WHITE E 2016. Autophagy provides metabolic substrates to maintain energy charge and nucleotide pools in Ras-driven lung cancer cells. Genes Dev, 30, 1704–17. [Europe PMC free article] [Abstract] [Google Scholar]
- HIESTER BG, BOURKE AM, SINNEN BL, COOK SG, GIBSON ES, SMITH KR & KENNEDY MJ 2017. L-Type Voltage-Gated Ca(2+) Channels Regulate Synaptic-Activity-Triggered Recycling Endosome Fusion in Neuronal Dendrites. Cell Rep, 21, 2134–2146. [Europe PMC free article] [Abstract] [Google Scholar]
- HIROTA Y, YAMASHITA S, KURIHARA Y, JIN X, AIHARA M, SAIGUSA T, KANG D & KANKI T 2015. Mitophagy is primarily due to alternative autophagy and requires the MAPK1 and MAPK14 signaling pathways. Autophagy, 11, 332–43. [Europe PMC free article] [Abstract] [Google Scholar]
- ISHIHARA N, NOMURA M, JOFUKU A, KATO H, SUZUKI SO, MASUDA K, OTERA H, NAKANISHI Y, NONAKA I, GOTO Y, TAGUCHI N, MORINAGA H, MAEDA M, TAKAYANAGI R, YOKOTA S & MIHARA K 2009. Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat Cell Biol, 11, 958–66. [Abstract] [Google Scholar]
- KARBOWSKI M, ARNOULT D, CHEN H, CHAN DC, SMITH CL & YOULE RJ 2004. Quantitation of mitochondrial dynamics by photolabeling of individual organelles shows that mitochondrial fusion is blocked during the Bax activation phase of apoptosis. J Cell Biol, 164, 493–9. [Europe PMC free article] [Abstract] [Google Scholar]
- KATAYAMA H, KOGURE T, MIZUSHIMA N, YOSHIMORI T & MIYAWAKI A 2011. A sensitive and quantitative technique for detecting autophagic events based on lysosomal delivery. Chem Biol, 18, 1042–52. [Abstract] [Google Scholar]
- LAZAROU M, SLITER DA, KANE LA, SARRAF SA, WANG C, BURMAN JL, SIDERIS DP, FOGEL AI & YOULE RJ 2015. The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature, 524, 309–314. [Europe PMC free article] [Abstract] [Google Scholar]
- MATHEOUD D, SUGIURA A, BELLEMARE-PELLETIER A, LAPLANTE A, RONDEAU C, CHEMALI M, FAZEL A, BERGERON JJ, TRUDEAU LE, BURELLE Y, GAGNON E, MCBRIDE HM & DESJARDINS M 2016. Parkinson’s Disease-Related Proteins PINK1 and Parkin Repress Mitochondrial Antigen Presentation. Cell, 166, 314–327. [Abstract] [Google Scholar]
- MAURO-LIZCANO M, ESTEBAN-MARTINEZ L, SECO E, SERRANO-PUEBLA A, GARCIA-LEDO L, FIGUEIREDO-PEREIRA C, VIEIRA HL & BOYA P 2015. New method to assess mitophagy flux by flow cytometry. Autophagy, 11, 833–43. [Europe PMC free article] [Abstract] [Google Scholar]
- MCLELLAND GL, LEE SA, MCBRIDE HM & FON EA 2016. Syntaxin-17 delivers PINK1/parkin-dependent mitochondrial vesicles to the endolysosomal system. J Cell Biol, 214, 275–91. [Europe PMC free article] [Abstract] [Google Scholar]
- N’DIAYE EN, KAJIHARA KK, HSIEH I, MORISAKI H, DEBNATH J & BROWN EJ 2009. PLIC proteins or ubiquilins regulate autophagy-dependent cell survival during nutrient starvation. EMBO Rep, 10, 173–9. [Europe PMC free article] [Abstract] [Google Scholar]
- NEMKOV T, D’ALESSANDRO A & HANSEN KC 2015. Three-minute method for amino acid analysis by UHPLC and high-resolution quadrupole orbitrap mass spectrometry. Amino Acids, 47, 2345–57. [Europe PMC free article] [Abstract] [Google Scholar]
- NEMKOV T, HANSEN KC & D’ALESSANDRO A 2017. A three-minute method for high-throughput quantitative metabolomics and quantitative tracing experiments of central carbon and nitrogen pathways. Rapid Commun Mass Spectrom, 31, 663–673. [Europe PMC free article] [Abstract] [Google Scholar]
- NGUYEN TN, PADMAN BS, USHER J, OORSCHOT V, RAMM G & LAZAROU M 2016. Atg8 family LC3/GABARAP proteins are crucial for autophagosome-lysosome fusion but not autophagosome formation during PINK1/Parkin mitophagy and starvation. J Cell Biol, 215, 857–874. [Europe PMC free article] [Abstract] [Google Scholar]
- O’PREY J, SAKAMAKI J, BAUDOT AD, NEW M, VAN ACKER T, TOOZE SA, LONG JS & RYAN KM 2017. Application of CRISPR/Cas9 to Autophagy Research. Methods Enzymol, 588, 79–108. [Abstract] [Google Scholar]
- PICKLES S, VIGIE P & YOULE RJ 2018. Mitophagy and Quality Control Mechanisms in Mitochondrial Maintenance. Curr Biol, 28, R170–R185. [Europe PMC free article] [Abstract] [Google Scholar]
- SANTEL A & FULLER MT 2001. Control of mitochondrial morphology by a human mitofusin. J Cell Sci, 114, 867–74. [Abstract] [Google Scholar]
- SMIRNOVA E, GRIPARIC L, SHURLAND DL & VAN DER BLIEK AM 2001. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell, 12, 2245–56. [Europe PMC free article] [Abstract] [Google Scholar]
- SMITH KR, KOPEIKINA KJ, FAWCETT-PATEL JM, LEADERBRAND K, GAO R, SCHURMANN B, MYCZEK K, RADULOVIC J, SWANSON GT & PENZES P 2014. Psychiatric risk factor ANK3/ankyrin-G nanodomains regulate the structure and function of glutamatergic synapses. Neuron, 84, 399–415. [Europe PMC free article] [Abstract] [Google Scholar]
- SOUBANNIER V, MCLELLAND GL, ZUNINO R, BRASCHI E, RIPPSTEIN P, FON EA & MCBRIDE HM 2012a. A vesicular transport pathway shuttles cargo from mitochondria to lysosomes. Curr Biol, 22, 135–41. [Abstract] [Google Scholar]
- SOUBANNIER V, RIPPSTEIN P, KAUFMAN BA, SHOUBRIDGE EA & MCBRIDE HM 2012b. Reconstitution of mitochondria derived vesicle formation demonstrates selective enrichment of oxidized cargo. PLoS One, 7, e52830. [Europe PMC free article] [Abstract] [Google Scholar]
- STROHECKER AM, GUO JY, KARSLI-UZUNBAS G, PRICE SM, CHEN GJ, MATHEW R, MCMAHON M & WHITE E 2013. Autophagy sustains mitochondrial glutamine metabolism and growth of BrafV600E-driven lung tumors. Cancer Discov, 3, 1272–85. [Europe PMC free article] [Abstract] [Google Scholar]
- SUGIURA A, MCLELLAND GL, FON EA & MCBRIDE HM 2014. A new pathway for mitochondrial quality control: mitochondrial-derived vesicles. EMBO J, 33, 2142–56. [Europe PMC free article] [Abstract] [Google Scholar]
- TANAKA A, CLELAND MM, XU S, NARENDRA DP, SUEN DF, KARBOWSKI M & YOULE RJ 2010. Proteasome and p97 mediate mitophagy and degradation of mitofusins induced by Parkin. J Cell Biol, 191, 1367–80. [Europe PMC free article] [Abstract] [Google Scholar]
- TILOKANI L, NAGASHIMA S, PAUPE V & PRUDENT J 2018. Mitochondrial dynamics: overview of molecular mechanisms. Essays Biochem, 62, 341–360. [Europe PMC free article] [Abstract] [Google Scholar]
- TOWERS CG, FITZWALTER BE, REGAN D, GOODSPEED A, MORGAN MJ, LIU CW, GUSTAFSON DL & THORBURN A 2019. Cancer Cells Upregulate NRF2 Signaling to Adapt to Autophagy Inhibition. Dev Cell [Europe PMC free article] [Abstract]
- TOWERS CG, WODETZKI D & THORBURN A 2020a. Autophagy-dependent cancer cells circumvent loss of the upstream regulator RB1CC1/FIP200 and loss of LC3 conjugation by similar mechanisms. Autophagy, 1–9. [Europe PMC free article] [Abstract]
- TOWERS CG, WODETZKI D & THORBURN A 2020b. Autophagy-dependent cancer cells circumvent loss of the upstream regulator RB1CC1/FIP200 and loss of LC3 conjugation by similar mechanisms. Autophagy, 16, 1332–1340. [Europe PMC free article] [Abstract] [Google Scholar]
- TSUBOYAMA K, KOYAMA-HONDA I, SAKAMAKI Y, KOIKE M, MORISHITA H & MIZUSHIMA N 2016. The ATG conjugation systems are important for degradation of the inner autophagosomal membrane. Science, 354, 1036–1041. [Abstract] [Google Scholar]
- THORBURN J, STASKIEWICZ L, GOODALL ML, DIMBERG L, FRANKEL AE, FORD HL & THORBURN A 2017. Non-cell-autonomous Effects of Autophagy Inhibition in Tumor Cells Promote Growth of Drug-resistant Cells. Mol Pharmacol, 91, 58–64. [Europe PMC free article] [Abstract] [Google Scholar]
- TWIG G, ELORZA A, MOLINA AJ, MOHAMED H, WIKSTROM JD, WALZER G, STILES L, HAIGH SE, KATZ S, LAS G, ALROY J, WU M, PY BF, YUAN J, DEENEY JT, CORKEY BE & SHIRIHAI OS 2008. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J, 27, 433–46. [Europe PMC free article] [Abstract] [Google Scholar]
- VANLANDINGHAM PA & CERESA BP 2009. Rab7 regulates late endocytic trafficking downstream of multivesicular body biogenesis and cargo sequestration. J Biol Chem, 284, 12110–24. [Europe PMC free article] [Abstract] [Google Scholar]
- WILKINS HM & MORRIS JK 2017. New Therapeutics to Modulate Mitochondrial Function in Neurodegenerative Disorders. Curr Pharm Des, 23, 731–752. [Europe PMC free article] [Abstract] [Google Scholar]
- ZONG WX, RABINOWITZ JD & WHITE E 2016. Mitochondria and Cancer. Mol Cell, 61, 667–676. [Europe PMC free article] [Abstract] [Google Scholar]
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.devcel.2021.06.003
Read article for free, from open access legal sources, via Unpaywall:
http://www.cell.com/article/S1534580721004810/pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/108136575
Article citations
Molecular mechanisms of mitochondrial dynamics.
Nat Rev Mol Cell Biol, 17 Oct 2024
Cited by: 0 articles | PMID: 39420231
Review
The role of SIRT1 in autophagy and drug resistance: unveiling new targets and potential biomarkers in cancer therapy.
Front Pharmacol, 15:1469830, 30 Sep 2024
Cited by: 0 articles | PMID: 39403142 | PMCID: PMC11471651
Review Free full text in Europe PMC
Mitochondrial-derived compartments are multilamellar domains that encase membrane cargo and cytosol.
J Cell Biol, 223(11):e202307035, 13 Aug 2024
Cited by: 2 articles | PMID: 39136939
Vacuolar degradation of plant organelles.
Plant Cell, 36(9):3036-3056, 01 Sep 2024
Cited by: 2 articles | PMID: 38657116
Review
PFN1 Knockdown Aggravates Mitophagy to Retard Lung Adenocarcinoma Initiation and M2 Macrophage Polarization.
Mol Biotechnol, 09 Aug 2024
Cited by: 0 articles | PMID: 39120820
Go to all (59) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Nucleotide Sequences (2)
- (1 citation) ENA - PVH00010
- (1 citation) ENA - C10423
RRID - Resource Identification Portal (4)
- (1 citation) RRID - RRIDAB_10858459
- (1 citation) RRID - RRIDAB_10003146
- (1 citation) RRID - RRIDAB_10831194
- (1 citation) RRID - RRIDAB_10829153
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Beyond mitophagy: mitochondrial-derived vesicles can get the job done!
Autophagy, 18(2):449-451, 15 Nov 2021
Cited by: 6 articles | PMID: 34781816 | PMCID: PMC8942527
Receptor-mediated clustering of FIP200 bypasses the role of LC3 lipidation in autophagy.
EMBO J, 39(24):e104948, 23 Nov 2020
Cited by: 55 articles | PMID: 33226137 | PMCID: PMC7737610
Mitochondrial-derived vesicles: Gatekeepers of mitochondrial response to oxidative stress.
Free Radic Biol Med, 188:185-193, 21 Jun 2022
Cited by: 10 articles | PMID: 35750270
Review
Collapsin response mediator protein 5 (CRMP5) induces mitophagy, thereby regulating mitochondrion numbers in dendrites.
J Biol Chem, 289(4):2261-2276, 09 Dec 2013
Cited by: 17 articles | PMID: 24324268 | PMCID: PMC3900971
Funding
Funders who supported this work.
American Cancer Society
Cancer Center, University of Colorado (1)
Grant ID: P30CA046934
NCI NIH HHS (5)
Grant ID: T32 CA190216
Grant ID: P30 CA046934
Grant ID: R01 CA150925
Grant ID: R01 CA190170
Grant ID: K99 CA245187
NIMH NIH HHS (1)
Grant ID: R01 MH119154
National Cancer Institute (4)
Grant ID: RO1CA190170
Grant ID: RO1CA150925
Grant ID: K99CA245187
Grant ID: 5 T32 CA 190216-2