| PMC full text: | Published online 2021 Sep 16. doi: 10.1093/ibd/izab207
|
Figure 3.
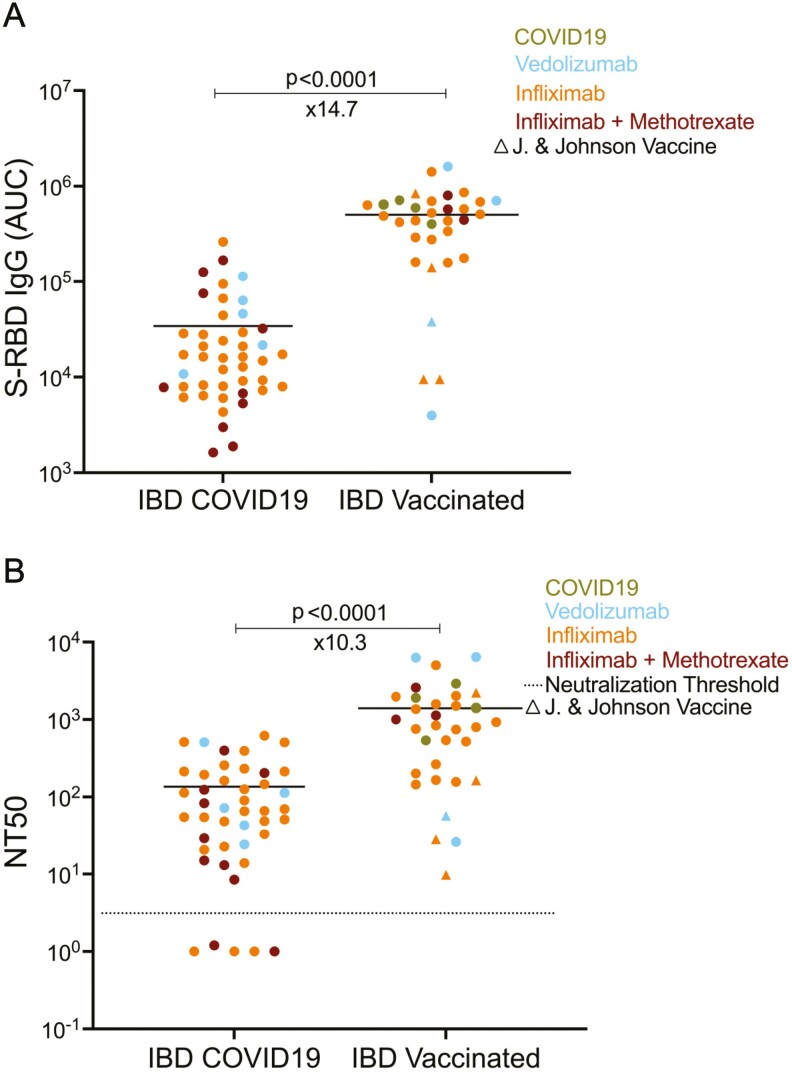
Comparing the antibody levels and neutralization titers of COVID-19-seropositive and vaccinated IBD pediatric subjects. A, S-RBD IgG levels of COVID-19-seropositive pediatric IBD subjects (n = 44) and vaccinated pediatric IBD subjects (n = 33), respectively. B, Neutralization titers (NT50) post-COVID19 and vaccination in IBD subjects. The average durations were 4.0 weeks after the first PCR+ test for IBD COVID-19 subjects and 3.2 weeks after the fully vaccination date for IBD vaccinated subjects. Blue, orange, and red dots indicate the subjects with vedolizumab monotherapy, infliximab monotherapy, and infliximab + methotrexate cotherapy, respectively. Green dots represent the COVID-19-seropositive subjects among the vaccinated group. Circles indicate RNA vaccines with 2 doses in the vaccinated group whereas triangles show Johnson & Johnson adenovirus vaccine, which was administered once. Horizontal bars show the mean value; x values under the significance bars represent the fold changes between the mean values of groups. Dotted lines indicate the neutralization threshold which was NT50 of 5. Two-tailed Mann-Whitney U test was used to determine the statistical significance.