Abstract
Background
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) affects tens of millions worldwide; the causes of exertional intolerance are poorly understood. The ME/CFS label overlaps with postural orthostatic tachycardia (POTS) and fibromyalgia, and objective evidence of small fiber neuropathy (SFN) is reported in approximately 50% of POTS and fibromyalgia patients.Research question
Can invasive cardiopulmonary exercise testing (iCPET) and PGP9.5-immunolabeled lower-leg skin biopsies inform the pathophysiology of ME/CFS exertional intolerance and potential relationships with SFN?Study design and methods
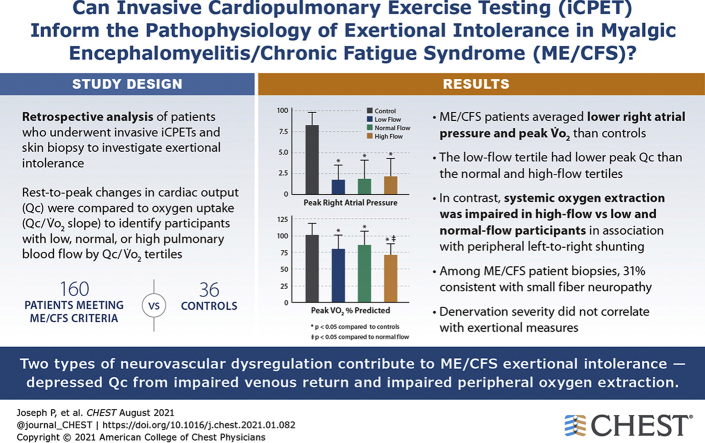
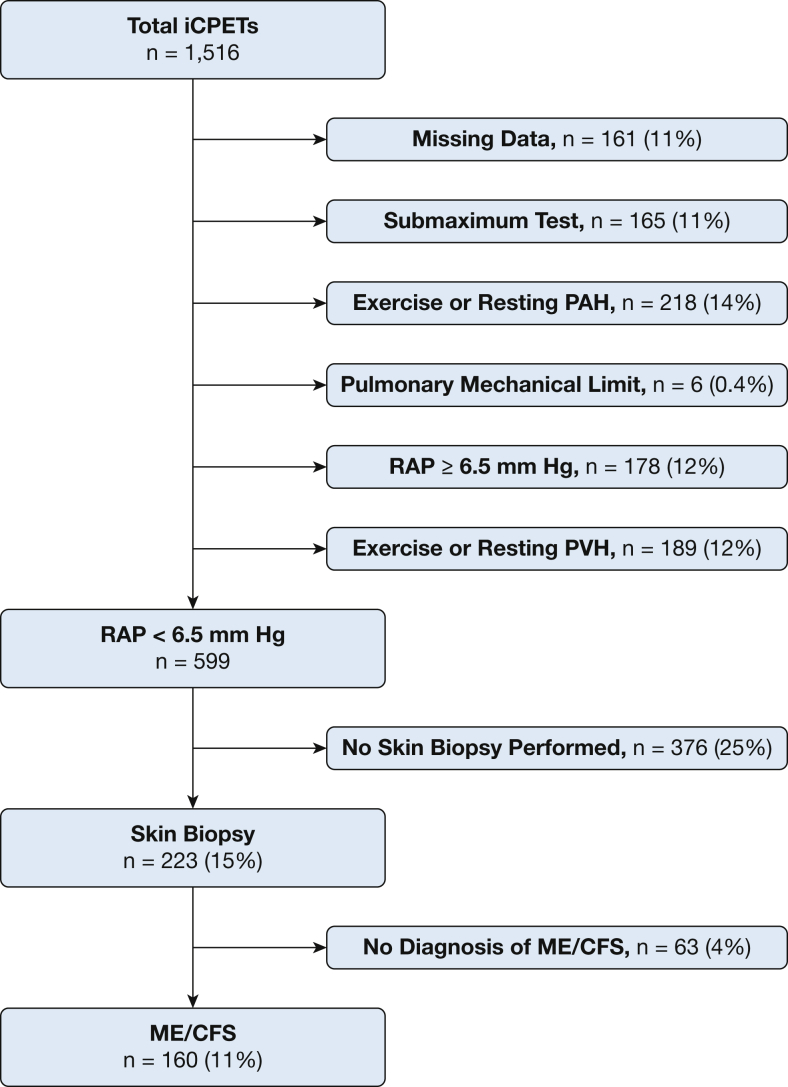
We analyzed 1,516 upright invasive iCPETs performed to investigate exertional intolerance. After excluding patients with intrinsic heart or lung disease and selecting those with right atrial pressures (RAP) <6.5 mm Hg, results from 160 patients meeting ME/CFS criteria who had skin biopsy test results were compared with 36 control subjects. Rest-to-peak changes in cardiac output (Qc) were compared with oxygen uptake (Qc/VO2 slope) to identify participants with low, normal, or high pulmonary blood flow by Qc/VO2 tertiles.Results
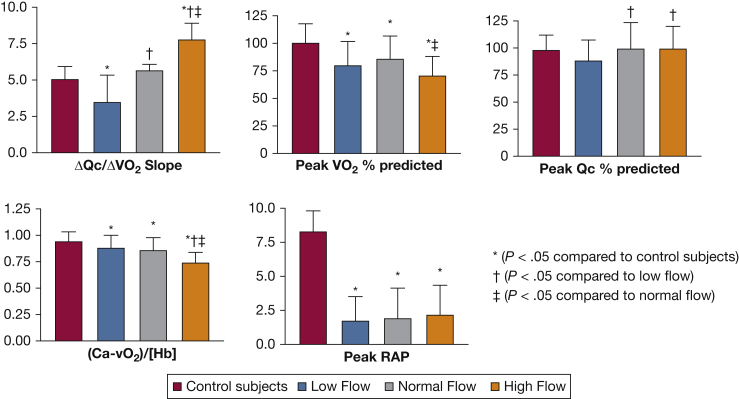
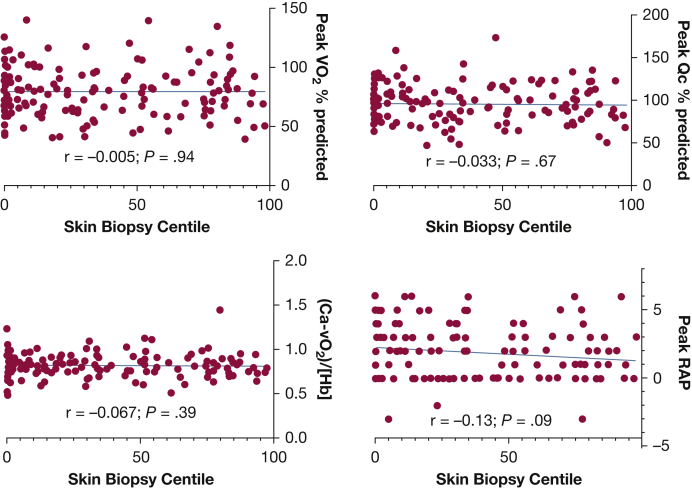
During exercise, the 160 ME/CFS patients averaged lower RAP (1.9 ± 2 vs 8.3 ± 1.5; P < .0001) and peak VO2 (80% ± 21% vs 101.4% ± 17%; P < .0001) than control subjects. The low-flow tertile had lower peak Qc than the normal and high-flow tertiles (88.4% ± 19% vs 99.5% ± 23.8% vs 99.9% ± 19.5% predicted; P < .01). In contrast, systemic oxygen extraction was impaired in high-flow vs low- and normal-flow participants (0.74% ± 0.1% vs 0.88 ± 0.11 vs 0.86 ± 0.1; P < .0001) in association with peripheral left-to-right shunting. Among the 160 ME/CFS patient biopsies, 31% were consistent with SFN (epidermal innervation ≤5.0% of predicted; P < .0001). Denervation severity did not correlate with exertional measures.Interpretation
These results identify two types of peripheral neurovascular dysregulation that are biologically plausible contributors to ME/CFS exertional intolerance-depressed Qc from impaired venous return, and impaired peripheral oxygen extraction. In patients with small-fiber pathology, neuropathic dysregulation causing microvascular dilation may limit exertion by shunting oxygenated blood from capillary beds and reducing cardiac return.Free full text

Insights From Invasive Cardiopulmonary Exercise Testing of Patients With Myalgic Encephalomyelitis/Chronic Fatigue Syndrome
Abstract
Background
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) affects tens of millions worldwide; the causes of exertional intolerance are poorly understood. The ME/CFS label overlaps with postural orthostatic tachycardia (POTS) and fibromyalgia, and objective evidence of small fiber neuropathy (SFN) is reported in approximately 50% of POTS and fibromyalgia patients.
Research Question
Can invasive cardiopulmonary exercise testing (iCPET) and PGP9.5-immunolabeled lower-leg skin biopsies inform the pathophysiology of ME/CFS exertional intolerance and potential relationships with SFN?
Study Design and Methods
We analyzed 1,516 upright invasive iCPETs performed to investigate exertional intolerance. After excluding patients with intrinsic heart or lung disease and selecting those with right atrial pressures (RAP) <6.5 mm Hg, results from 160 patients meeting ME/CFS criteria who had skin biopsy test results were compared with 36 control subjects. Rest-to-peak changes in cardiac output (Qc) were compared with oxygen uptake (Qc/VO2 slope) to identify participants with low, normal, or high pulmonary blood flow by Qc/VO2 tertiles.
Results
During exercise, the 160 ME/CFS patients averaged lower RAP (1.9 ± 2 vs 8.3 ± 1.5; P < .0001) and peak VO2 (80% ± 21% vs 101.4% ± 17%; P < .0001) than control subjects. The low-flow tertile had lower peak Qc than the normal and high-flow tertiles (88.4% ± 19% vs 99.5% ± 23.8% vs 99.9% ± 19.5% predicted; P < .01). In contrast, systemic oxygen extraction was impaired in high-flow vs low- and normal-flow participants (0.74% ± 0.1% vs 0.88 ± 0.11 vs 0.86 ± 0.1; P < .0001) in association with peripheral left-to-right shunting. Among the 160 ME/CFS patient biopsies, 31% were consistent with SFN (epidermal innervation ≤5.0% of predicted; P < .0001). Denervation severity did not correlate with exertional measures.
Interpretation
These results identify two types of peripheral neurovascular dysregulation that are biologically plausible contributors to ME/CFS exertional intolerance—depressed Qc from impaired venous return, and impaired peripheral oxygen extraction. In patients with small-fiber pathology, neuropathic dysregulation causing microvascular dilation may limit exertion by shunting oxygenated blood from capillary beds and reducing cardiac return.
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is a debilitating and common disorder that affects up to 2.5 million Americans. Its causes are unknown, but infections, especially viruses, and immunologic and endocrine disorders have been proposed. The National Academy of Medicine (formerly the Institute of Medicine) requires three major criteria for diagnosis; substantial impairment from fatigue for >6 months, post-exertional malaise, and unrefreshing sleep, plus either cognitive impairment or orthostatic intolerance.1
ME/CFS overlaps extensively with two other syndromic labels—postural orthostatic tachycardia syndrome (POTS) and fibromyalgia.2, 3, 4 Increasing evidence implicates small fiber neuropathy (SFN) in approximately 50% of adult and juvenile fibromyalgia5, 6, 7, 8 and 38% of POTS patients,9 generating suggestions of neuropathic and non-neuropathic subtypes in these syndromes.9,10
To elucidate potential mechanisms underlying ME/CFS symptoms, we had used invasive cardiopulmonary exercise testing (iCPET) to demonstrate that up to 20% of patients with unexplained exertional intolerance have low aerobic capacity attributable solely to low biventricular filling pressures, or “preload failure” (PLF).11,12 In accord, most PLF patients report chronic fatigue, and 20% have documented peripheral dysautonomia.11
Given the data suggesting hemodynamic phenotypes in POTS13 and neurovascular dysregulation in fibromyalgia,14 we hypothesized that the abnormal exercise phenotypes of ME/CFS can, in some patients, reflect SFN. We are not aware of prior iCPET or skin biopsy studies in ME/CFS despite their potential to identify mechanisms underlying exercise intolerance. The goal of this study was to use them to gain insights into contributors to acute exertional intolerance in ME/CFS, and to gather initial data on associations with SFN.
Study Design and Methods
The Partners Human Research Committee approved the review of clinical data (Protocol 2018P001903) and the telephone questionnaire (Protocol 2019P000039) containing the ME/CFS symptoms required for research diagnosis. Patients were given 2 weeks to decline telephone participation.
Study Population
Data from 1,516 clinically indicated iCPETs performed between 2011 to 2018 at the Brigham and Women’s Hospital Dyspnea Clinic (Boston, MA) were analyzed. To eliminate potentially confounding submaximal effort and intrinsic heart and lung disease, we excluded the following:
- 1.
Submaximal testing: Maximum heart rate achieved <80% predicted for age or a peak respiratory exchange ratio < 1.05
- 2.
Primary pulmonary mechanical limitation to exercise: Minute ventilation/maximum voluntary ventilation >0.7 at the anaerobic threshold
- 3.
Resting or exercise precapillary pulmonary hypertension: Patients ≤50 years of age: mean pulmonary artery pressure >30 mm Hg and pulmonary vascular resistance >1.34 Woods Units; patients >50 years of age: mean pulmonary artery pressure >33 mm Hg and pulmonary vascular resistance >2.10 Woods Units15
- 4.
Resting or exercise postcapillary pulmonary hypertension: Patients ≤50 years of age: pulmonary arterial wedge pressure (PAWP) >19 mm Hg; patients >50 years of age: PAWP >17 mm Hg15
- 5.
RAP ≥6.5 mm Hg, which defines our lower limit of normal11
- 6.
Incomplete data including lack of skin biopsy results
All then underwent additional medical record review to confirm presence of ME/CFS, using the National Academy of Medicine requirement of documentation of three major criteria (ie, chronic fatigue for ≥6 months, post-exertional malaise, unrefreshing sleep) plus one minor criteria (ie, either cognitive impairment, orthostatic intolerance).1 For those with uncertain documentation on medical record review, a short telephone questionnaire was used to finalize inclusion or exclusion.
A control cohort assembled for comparison purposes comprised 36 patients who had undergone iCPET for exertional intolerance but who had normal results. Specifically, there was no exercise or resting pulmonary hypertension, heart failure, or decreased aerobic capacity, defined as oxygen uptake (VO2), cardiac output (Qc), and arterial-mixed venous oxygen content difference (Ca-vO2/[Hb]) >80% of predicted, along with a Qc-VO2 percent predicted difference <10% of population-predicted.16,17
Invasive Cardiopulmonary Exercise Testing Protocol
Protocols for iCPET, hemodynamic measurements, and gas exchange measurements have been described previously.18,19 Briefly, the pulmonary and radial arteries are catheterized with ultrasound and fluoroscopic guidance, then standard right-heart catheterization performed with oxygen saturation measurements to assess for intracardiac shunting.20 Patients are then transported to the cardiopulmonary stress laboratory for incrementally increasing upright exercise to maximum on a cycle ergometer as ventilation and pulmonary gas exchange is measured (MGC Diagnostics). Hemodynamics, including right atrial pressure (RAP), mean pulmonary artery pressure, and mean arterial pressure are continuously recorded (Koninklijke Philips N.V., Amsterdam, Netherlands). PAWP and arterial and mixed-venous blood gases and pH are measured once per 60 seconds, and Qc calculated using the direct Fick principle. Carboxyhemoglobin levels are also returned from both compartments, and none were elevated (data not shown).
Skin Biopsy Measurements of SFN
At the discretion of the referring physician, 3-mm punch biopsy specimens were removed from lidocaine-anesthetized skin 10 cm proximal to the lateral malleolus using standard clinical methods, overnight fixed (Zamboni’s), and transported to the Massachusetts General Hospital clinical diagnostic laboratory for standard processing and assessment.21 Vertical sections were immunolabeled against PGP9.5, then epidermal neurite density was measured by skilled morphologists unaware of patient designations. The standard diagnostic cutoff (≤5% of the laboratory’s sex-, age-, and race-adjusted normal distribution) was applied to interpret biopsy normality.21,22
Data Analysis
Predicted values for peak oxygen uptake (VO2) were derived from the Wasserman-Hansen equation.16 Based on the rest-to-peak change of cardiac output in relation to oxygen uptake (Qc/VO2 slope), participants were categorized as low, normal, or high pulmonary blood flow according to Qc/VO2 tertiles. Statistical analyses were performed with GraphPad Prism (v8.0.0 for Windows, GraphPad Software). Normally distributed data were reported as mean ± SD; non-normally distributed data as median ± interquartile range. Multiple group comparisons used one-way analysis of variance with Tukey post hoc test. Correlations between variables were calculated using the Pearson correlation coefficient or the Spearman rank correlation coefficient according to data distribution. Two-tailed P values < .05 were considered significant.
Results
Among 1,516 patients whose iCPET reports were screened for inclusion, 223 met criteria for PLF and had skin biopsy data. The 72% (160/223) meeting the ME/CFS diagnostic criteria yielded a 160-patient study sample (Fig 1) that was 78% female. Significant associated conditions were POTS, fibromyalgia, mast cell activation syndrome, and history of preceding infection (Table 1). Few patients used diuretics or vasoactive medications.
Table 1
Baseline Characteristics of ME/CFS Participants
| Characteristics | Subjects | Control Subjects | P |
|---|---|---|---|
| No. | 160 | 36 | |
| Age, y | 47 ± 16 | 56 ± 12 | .0001 |
| Female (%) | 125 (78) | 16 (44) | .0001 |
| White race (%) | 149 (93) | 34 (94) | .78 |
| Weight, kg | 73 ± 17 | 85 ± 22 | .0007 |
| Height, cm | 167 ± 9 | 170 ± 9 | .08 |
| BMI, kg/m2 | 25.6 ± 5.3 | 28 ± 6 | .0001 |
| Hb, g/dL | 14.1 ± 1.4 | 15.3 ± 1.5 | .0001 |
| Comorbidities (%) | |||
| Hypertension | 33 (21) | 15 (41) | .002 |
| Dyslipidemia | 33 (21) | 10 (28) | .25 |
| Obesity | 28 (18) | 10 (28) | .09 |
| CV family history | 10 (6) | 5 (14) | .06 |
| Diabetes mellitus | 6 (4) | 2 (5) | .73 |
| Previous myocardial infarction | 5 (3) | 0 (0) | .08 |
| Coronary artery disease | 3 (2) | 0 (0) | .15 |
| Medications (%) | |||
| Statins | 26 (16) | 11 (30) | .02 |
| Beta blockers | 25 (16) | 5 (14) | .69 |
| ASA | 25 (16) | 7 (19) | .58 |
| Calcium channel blockers | 14 (9) | 3 (8) | .8 |
| Diuretics | 14 (9) | 5 (14) | .27 |
| ACE inhibitors | 11 (7) | 5 (14) | .52 |
| Associated conditions (%) | |||
| Small fiber neuropathy | 49 (31) | 0 (0) | <.0001 |
| POTS | 52 (33) | 3 (8) | .0001 |
| Fibromyalgia | 35 (22) | 2 (6) | .001 |
| MCAS | 11 (22) | 0 (0) | <.0001 |
| Preceding infection | 39 (24) | 0 (0) | <.0001 |
Bold signifies significant P values. ACE = angiotensin-converting enzyme; ASA = aspirin; CV = cardiovascular; MCAS = mast cell activation syndrome; ME/CFS = myalgic encephalomyelitis/chronic fatigue syndrome; POTS = postural orthostatic tachycardia syndrome.
The mean peak VO2 was decreased for all ME/CFS patients as compared with control subjects (Table 2). Analyzing incremental exercise Qc/VO2 slopes revealed low (4 [3.2-4.4]), normal (5.6 [5.3-6]), and high (7.5 [7-8.3]) pulmonary blood flow based on Qc/VO2 tertiles. The tertile with low-flow had disproportionately low Qc at peak exercise as compared with those with normal- and high-flow. At peak exercise, the high-flow tertile had significantly less oxygen extraction and higher venous oxygen tension than the others. The low- and normal-flow tertiles had oxygen extraction and venous oxygen tension values in the normal range, albeit lower than control subjects (Table 2, Fig 2). No participants had evidence of intracardiac left-to-right shunting during resting right heart catheterization, defined as oximetric step-up >9% between superior vena cava and pulmonary artery.20 Thirty one percent (49/160) had skin biopsies that pathologically confirmed SFN (Table 1). Figure 3 demonstrates that within the entire ME/CFS cohort and the tertiles, there were no correlations between epidermal neurite density and peak VO2 and Qc as a percentage of predicted, systemic oxygen extraction corrected for hemoglobin concentration, and peak RAPs.
Table 2
Exercise Variables and Skin Biopsy Results in ME/CFS
| Variables | Control Subjects | Low Flow | Normal Flow | High Flow | ANOVA |
|---|---|---|---|---|---|
| ΔQc/ΔVO2 | 5.1 [4.6-5.5] | 4 [3.2-4.4]a | 5.6 [5.3-6.0]b | 7.5 [7-8.3]a,b,c | <.0001 |
| Peak VO2 % predicted, % | 101.4 ± 17 | 80.4 ± 20.9a | 86.1 ± 21a | 71.3 ± 16.7a,c | <.0001 |
| Peak Qc % predicted, % | 98.5 ± 13.3 | 88.4 ± 19 | 99.5 ± 23.8b | 99.9 ± 19.5b | <.01 |
| Peak (Ca-vO2)/[Hb] | 0.95 ± 0.08 | 0.88 ± 0.11a | 0.86 ± 0.1a | 0.74 ± 0.1a,b,c | <.0001 |
| Rest SBP, mm Hg | 147 ± 23 | 141 ± 23 | 141 ± 21 | 135 ± 19 | .10 |
| Rest DBP, mm Hg | 85 ± 12 | 78 ± 11a | 80 ± 11 | 76 ± 10a | <.005 |
| Rest MAP, mm Hg | 106 ± 15 | 99 ± 14 | 100 ± 13 | 96 ± 12a | <.013 |
| Peak SBP, mm Hg | 200 ± 32 | 177 ± 37a | 186 ± 28.5 | 176 ± 28a | <.0021 |
| Peak DBP, mm Hg | 91 ± 16 | 82 ± 14a | 86 ± 14.4 | 85 ±12 | <.03 |
| Peak MAP, mm Hg | 127 ± 20 | 113 ± 20a | 119 ± 17 | 115 ± 16a | <.01 |
| Peak SVR, dyn·s·cm-5 | 731 ± 182 | 838 ± 228 | 766 ± 179 | 784 ± 248 | .1189 |
| Peak vPO2, mm Hg | 25 [24-28] | 27 [25-28] | 28 [25-30]a | 30 [28-31]a,b,c | <.0001 |
| Peak SaO2, % | 97 ± 1.3 | 97 ± 1.2 | 98 ± 1 | 98 ± 1.5a | .0356 |
| Peak SvO2, % | 30 ± 7 | 35 ± 7a | 37 ± 8a | 44 ± 7a,b,c | <.0001 |
| Peak RAP, mm Hg | 8 [7-9.8] | 2 [0-3]a | 1 [0-4]a | 2 [0-3]a | <.0001 |
| Peak PAWP, mm Hg | 14 [11-16] | 7 [4-10]a | 8 [5-11]a | 8 [5-10]a | <.0001 |
| Rest lactate, mmol/L | 1 ± 0.3 | 0.9 ± 0.4 | 0.9 ± 0.5 | 0.8 ± 0.4 | .2 |
| Peak lactate, mmol/L | 7 ± 2.2 | 6.2 ± 2.2 | 6.5 ± 2.4 | 5.5 ± 2a | <.05 |
| Rest A-a gradient, mm Hg | 12 ± 16 | 5.2 ± 17 | 2.2 ± 12a | 0.6 ± 13a | <.001 |
| Peak A-a gradient, mm Hg | 23 ± 11 | 13.2 ± 17a | 7.7 ± 14a | 5.3 ± 9.3a,b | <.0001 |
| Epidermal neurite density, centile | … | 21 [1.2-50] | 25 [2.4-76] | 26 [0-64] | .52 |
| ≤5th centile | … | 18 (35.3%) | 15 (28.8%) | 16 (31.4%) | .61 |
| 5th < centile ≤ 15th centile | … | 6 (11.8%) | 8 (15.4%) | 6 (11.8%) | .68 |
| >15th centile | … | 27 (52.9%) | 29 (55.8%) | 29 (56.9%) | .84 |
Bold signifies significant P values. ANOVA = analysis of variance; DBP = diastolic BP; Hb = hemoglobin; MAP = mean arterial pressure; ME/CFS = myalgic encephalomyelitis/chronic fatigue syndrome; PAWP = pulmonary arterial wedge pressure; Qc = cardiac output; RAP = right arterial pressure; SaO2 = oxygen saturation; SvO2 = mixed venous oxygen saturation; SVR = systemic vascular resistance; VO2 = oxygen uptake; VPO2 = venous oxygen tension.
Discussion
ME/CFS is a common and often disabling disorder of unknown pathogenesis reported to affect 10% to 25% of patients in primary care practices,23 75 to 267/100,000 people,24 or 836,000 to 2.5 million people in the United States.1 Annual productivity losses are estimated at $20,000 per patient, or $9.1 billion overall.25 Because nonspecific symptoms affecting multiple organ systems lead to evaluations by disparate medical specialties, this number is proposed to be an underestimate, with direct and indirect US costs actually approaching $23 billion per year.26 Hence, insights into pathogenesis are urgently needed because these can translate into improving diagnosis and identifying evidence-based treatment options to ameliorate symptom causes.
ME/CFS has significant overlap with the POTS and fibromyalgia syndrome labels2, 3, 4 where skin biopsy and other biomarker studies report high prevalence of abnormalities consistent with SFN.5, 6, 7,9 SFN is a common disorder caused by excess firing or axonal degeneration in the thinly myelinated and unmyelinated sensory/trophic/autonomic peripheral neurons. Common symptoms include distal-predominant sensory disturbances, including pain, exertional intolerance, nausea, postural orthostatic tachycardia, and hypotension.21 The SFN diagnosis is confirmed by abnormally low densities of PGP9.5-immunolabeled epidermal neurites in lower-leg skin biopsies (≤5% of predicted), which is recommended for research inclusion.27
Given the personal disability and high medical and societal costs of ME/CFS, the identification of potential pathophysiological contributors is of major importance.26 Studies of varying quality link ME/CFS to preceding infections, immune system disorders, adrenal insufficiency, and various causes of autonomic nervous system function. Noninvasive CPET has been previously used in the evaluation and investigation of ME/CFS,28,29 but this is the first direct measurement of patient hemodynamics and pulmonary and systemic gas exchange, and the first objective testing for SFN in ME/CFS patients.
ME/CFS Exercise Phenotypes
A major finding was the strong association of impaired aerobic capacity in ME/CFS with two types of systemic vascular dysregulation during upright cycling. Our methods and analyses, which metabolically correct Qc (the Qc/VO2 slope), identified two groups with abnormally low and high Qc during exercise.
In low-flow patients, there was no evidence that depressed peak VO2 and low Qc were attributable to intrinsic heart disease (eg, heart failure) or pulmonary hypertension. Rather, the sole causal mechanism identified was low biventricular filling pressures during upright cycling, as reported in undifferentiated exertional intolerance.11
In high-flow patients, low peak VO2 was associated with impaired systemic oxygen extraction. During exercise, high pulmonary blood flow plus impaired systemic oxygen extraction implicates either left-to-right blood shunting or mitochondrial myopathy (MM), where blood is delivered but muscle use impaired.30,31 It has been hypothesized that MM underlies exercise intolerance in a subset of patients with ME/CFS,32,33 whose hallmark during incremental exercise is an increased Qc/VO2 slope.31 Possibly the inclusion criteria of PLF undermined our ability to detect patients with MM and an increased Qc/VO2 slope. There was no evidence of intracardiac left-to-right shunting at the time of the resting right heart catheterization. Together these exclusions leave systemic microcirculatory dysfunction (impaired oxygen delivery to muscles) as the presumptive mechanism. Indeed, skin biopsies from patients with focal and small-fiber neuropathies and fibromyalgia reveal arteriovenous blood flow dysregulation, including abnormal innervation and cross-sectional area of arterio-venous shunts.14,34
Future studies of the high-flow phenotype are needed to determine how pulmonary blood flow can be exaggerated in the face of low biventricular filling pressures. The current conclusions are also consistent with those from noninvasive exercise studies in ME/CFS patients that also yielded low estimated systemic oxygen extraction.35 High-flow and low-flow phenotypes have previously been described for POTS,13 supporting the current results in ME/CFS. In both conditions, the high-flow profile features high Qc and low systemic vascular resistance, and the low-flow phenotype the opposite.
ME/CFS and SFN
The second major finding was very high prevalence of small fiber neuropathology; in one third of ME/CFS patients, slightly lower than the approximately 50% prevalences reported in fibromyalgia and POTS.5,9,36 Our iCPET data associate SFN with both low-flow and high-flow ME/CFS profiles. Immunohistochemical studies show that small fibers regulate microvascular tone, primarily by sympathetic and parasympathetic cholinergic synapses on perivascular myocytes.37 Distal degeneration (axonopathy) can impair venoconstriction with resultant peripheral blood pooling decreasing cardiac return, consistent with the “low-flow preload failure” we identified. Abnormal venous pooling in the legs on standing is demonstrated in POTS,38 and the excess peripheral venoconstriction to experimentally infused norepinephrine or phenylephrine documented is consistent with classic post-denervation adrenergic receptor upregulation.39,40 This is further supported by low norepinephrine release after sympathetic nervous system stimulation in POTS patients.41 Thus, we propose SFN as the major cause of preload failure in a substantial, not yet fully measured, proportion of ME/CFS patients.
In the “high-flow” group (high Qc/poor systemic oxygen extraction), SFN-associated tissue-level left-to-right shunting is biologically plausible, as demonstrated by pathological examination of cutaneous microvasculature in chronic regional pain syndrome,34 a regional small-fiber neuropathy.42 In fibromyalgia, there is similar evidence of abnormally innervated dilated cutaneous arteriole-venule shunts that permit oxygenated arteriolar blood to bypass capillary beds, returning unextracted to the venous circulation.14 In exercising muscles, such blood shunting and impaired exercise-associated vasodilation can cause premature anaerobic metabolism with lactic acidosis, premature muscle fatigue, and exertional intolerance. Neurogenic AV shunting in splanchnic microvessels is proposed to also contribute to SFN’s characteristic postprandial abdominal discomfort, dysmotility, and nausea in addition to denervation of enteric smooth muscles.21
Limitations
A common question is whether exertional intolerance in ME/CFS includes potentially confounding effects of deconditioning. However, aerobic capacity is lower in ME/CFS patients than in sedentary control subjects,29 and the longer CPET protocol of Keller et al29 documented significant decrease of peak VO2 only on day 2, both inconsistent with deconditioning.29 The current study definitively eliminates this possibility, because the hallmark of deconditioning is low peak-exercise Qc43 rather than the increased Qc discovered here, plus we saw abnormally low biventricular filling pressures rather than the higher pressures seen in detrained individuals attributable to cardiac atrophy and decreased ventricular compliance.44,45 Although training can increase arterial-venous oxygen content difference at peak exercise, deconditioning causes little or no change in maximum exercise systemic oxygen extraction.43,46 Although deconditioning does not explain the exertional intolerance in ME/CFS, many patients become deconditioned secondarily, adding disability. We address this in the clinic by encouraging fitness with a careful, graded exercise program to help compensate for disease-associated cardiovascular limitations.
Regarding study participants, because of the invasive nature of iCPET, this was a retrospective study cohort assembled by careful elimination of intrinsic cardiac and pulmonary disease. Peak RAP <6.5 was used as an inclusion criterion based on previous work showing low biventricular filling pressures depressed peak VO2 in the absence of intrinsic heart or lung disease.11 Many of these patients met clinical criteria for ME/CFS. However, this may have eliminated some ME/CFS patients with peak RAP >6.5. If so, this could have limited our ability to detect patients with MM and its characteristic increased Qc/VO2 slope. Similarly, because the risk-benefit ratio, ethical, and logistic concerns largely preclude recruiting healthy control subjects for iCPET, we used subjects who, despite referral for potential exertional intolerance, had no corroborating iCPET evidence of physiologic abnormality. Finally, the largely white study population, which reflects our institution’s referral patterns, limits applicability to other racial groups.
An important assumption underlying our conclusions is that the Fick principle variables are independent of each other at maximum exercise. If not, it would be possible for low Qc to be compensated for by increased systemic extraction and the reverse, as is known to happen during submaximum exercise. We have therefore chosen to look at tertiles of Qc/VO2 slopes, which represent the behavior of the Fick principle variables throughout the entire exercise epoch and are not limited to peak exercise.
Regarding small-fiber metrics, we used the very small single-site distal skin biopsies recommended as required for SFN research, given 91% specificity, ease of performance, and widespread accessibility to mail-in pathology interpretation.47 However, tradeoffs include lower sensitivity (58%)47 than more comprehensive testing such as autonomic function testing, which is not endorsed because of lower specificity, higher complexity of administration and interpretation, and limited availability.27 Another reason for low sensitivity is that axonal degeneration is an end-stage change, whereas earlier small fiber malfunction also contributes but is harder to quantify.48 Lower leg biopsies are less sensitive for patchy, proximal, or non-length-dependent SFN, and in fibromyalgia, wider areas used in biopsies identified proximal denervation as more prevalent (46% in upper-thigh biopsy specimens vs 32% in distal-leg biopsies).7 Although referral bias of ME/CFS patients referred for skin biopsy might skew our sample to inflate prevalence of SFN, the 32% to 50% prevalence widely reported in fibromyalgia corroborate our data.6, 7, 8 Our distal-only biopsies also may have contributed to lack of correlation between epidermal neurite density and exercise test physiologic variables, given the fibromyalgia study correlation between severity of cutaneous denervation and sensory symptoms.7 Although measuring SFN symptoms and signs is most important, future ME/CFS studies could consider expanded testing as well.49 We viewed it as unnecessary for our iCPET control subjects to have skin biopsies because our laboratory has far more comprehensive age-, sex-, and race-adjusted same-laboratory norms from more than 450 healthy control subjects.22
Interpretation
The findings of the first study to directly measure pulmonary and systemic blood flow and gas exchange and small-fiber metrics in ME/CFS generate the hypothesis that peripheral vascular dysregulation causes ME/CFS’s major symptom—exertional intolerance. As in POTS, there appear to be low and high pulmonary blood flow physiological subtypes, the latter associated with impaired peripheral oxygen extraction. We also report the first association of small-fiber pathology with ME/CFS, corresponding to reports in fibromyalgia and POTS, other syndromes that include exertional intolerance. More comprehensive evaluation is recommended to fully address SFN contribution to ME/CFS. Although neither ME/CFS nor any symptom-based syndrome is caused by only one single disease or pathophysiology, diagnosing established diseases when present shifts at least these patients to more effective clinical frameworks and facilitates detection of remaining contributors. This approach may become important given reports of ME/CFS-like syndrome after coronavirus disease 2019 infection, with questions of associated SFN.50
Acknowledgments
Financial/nonfinancial disclosures: None declared
Other contributions: We appreciate assistance with the invasive CPET database from Julie Tracy, MSc, Katherine Lewine, MSc, and Jon Hainer, BS, and Heather Downs’ contributions to skin biopsy ascertainment.
Footnotes
FUNDING/SUPPORT: D. M. S. received funding from the Solve ME/CFS Initiative and the Open Medicine Foundation (OMF). A. L. O. received funding from the National Institutes of Health [Grant R01NS093653] and private foundation grants.
References
Articles from Chest are provided here courtesy of American College of Chest Physicians
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.chest.2021.01.082
Read article for free, from open access legal sources, via Unpaywall:
http://journal.chestnet.org/article/S0012369221002567/pdf
Citations & impact
Impact metrics
Article citations
Untargeted Metabolomics and Quantitative Analysis of Tryptophan Metabolites in Myalgic Encephalomyelitis Patients and Healthy Volunteers: A Comparative Study Using High-Resolution Mass Spectrometry.
ACS Chem Neurosci, 15(19):3525-3534, 20 Sep 2024
Cited by: 0 articles | PMID: 39302151 | PMCID: PMC11450765
Towards an understanding of physical activity-induced post-exertional malaise: Insights into microvascular alterations and immunometabolic interactions in post-COVID condition and myalgic encephalomyelitis/chronic fatigue syndrome.
Infection, 06 Sep 2024
Cited by: 0 articles | PMID: 39240417
Review
Data-independent LC-MS/MS analysis of ME/CFS plasma reveals a dysregulated coagulation system, endothelial dysfunction, downregulation of complement machinery.
Cardiovasc Diabetol, 23(1):254, 16 Jul 2024
Cited by: 2 articles | PMID: 39014464 | PMCID: PMC11253362
SARS-CoV-2 infection decreases cardiorespiratory fitness and time-trial performance even two months after returning to regular training - Insights from a longitudinal case series of well-trained kayak athletes.
J Exerc Sci Fit, 22(4):350-358, 19 Jun 2024
Cited by: 0 articles | PMID: 39027081 | PMCID: PMC11255366
Cardiopulmonary and metabolic responses during a 2-day CPET in myalgic encephalomyelitis/chronic fatigue syndrome: translating reduced oxygen consumption to impairment status to treatment considerations.
J Transl Med, 22(1):627, 05 Jul 2024
Cited by: 0 articles | PMID: 38965566 | PMCID: PMC11229500
Go to all (47) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Neurovascular Dysregulation and Acute Exercise Intolerance in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Randomized, Placebo-Controlled Trial of Pyridostigmine.
Chest, 162(5):1116-1126, 06 May 2022
Cited by: 11 articles | PMID: 35526605
Deconditioning does not explain orthostatic intolerance in ME/CFS (myalgic encephalomyelitis/chronic fatigue syndrome).
J Transl Med, 19(1):193, 04 May 2021
Cited by: 11 articles | PMID: 33947430 | PMCID: PMC8097965
Cardiopulmonary and metabolic responses during a 2-day CPET in myalgic encephalomyelitis/chronic fatigue syndrome: translating reduced oxygen consumption to impairment status to treatment considerations.
J Transl Med, 22(1):627, 05 Jul 2024
Cited by: 0 articles | PMID: 38965566 | PMCID: PMC11229500
Exercise Pathophysiology in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome and Postacute Sequelae of SARS-CoV-2: More in Common Than Not?
Chest, 164(3):717-726, 11 Apr 2023
Cited by: 14 articles | PMID: 37054777 | PMCID: PMC10088277
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NINDS NIH HHS (3)
Grant ID: R01 NS042866
Grant ID: K24 NS059892
Grant ID: R01 NS093653
National Institutes of Health (1)
Grant ID: R01NS093653
Open Medicine Foundation (1)
Grant ID: R01NS093653