ABSTRACT
Transcriptional activation of Notch signaling targets requires the formation of a ternary complex that involves the intracellular domain of the Notch receptor (NICD), DNA-binding protein Suppressor of Hairless [Su(H), RPBJ in mammals] and coactivator Mastermind (Mam). Here, we report that E(y)1/TAF9, a component of the transcription factor TFIID complex, interacts specifically with the NICD–Su(H)–Mam complex to facilitate the transcriptional output of Notch signaling. We identified E(y)1/TAF9 in a large-scale in vivo RNA interference (RNAi) screen for genes that are involved in a Notch-dependent mitotic-to-endocycle transition in Drosophila follicle cells. Knockdown of e(y)1/TAF9 displayed Notch-mutant-like phenotypes and defects in target gene and activity reporter expression in both the follicle cells and wing imaginal discs. Epistatic analyses in these two tissues indicated that E(y)1/TAF9 functions downstream of Notch cleavage. Biochemical studies in S2 cells demonstrated that E(y)1/TAF9 physically interacts with the transcriptional effectors of Notch signaling Su(H) and NICD. Taken together, our data suggest that the association of the NICD–Su(H)–Mastermind complex with E(y)1/TAF9 in response to Notch activation recruits the transcription initiation complex to induce Notch target genes, coupling Notch signaling with the transcription machinery.
KEY WORDS: E(y)1, TAF9, Notch pathway, Drosophila, TFIID complex, Transcriptional regulation
INTRODUCTION
The eukaryotic transcription factor IID (TFIID) complex, comprising the TATA box-binding protein (TBP) and 13 or 14 TBP-associated factors (TAFs), plays an essential role in the transcriptional regulation of gene expression, and all components of this complex were generally thought to be required for RNA-polymerase-II-initiated transcription in all eukaryotic cells (Goodrich and Tjian, 2010; Thomas and Chiang, 2006). However, an increasing number of studies suggest that some TAFs found in different species are vital in specific events, such as apoptosis, spermatogenesis and adipogenesis (Goodrich and Tjian, 2010). For example, mouse TAF7L is specifically required for male germ-cell differentiation (Cheng et al., 2007; Pointud et al., 2003), and, in both zebrafish and mouse embryonic stem cells, interaction between TRF3 and TAF3 is essential for hematopoiesis (Bártfai et al., 2004; Hart et al., 2007; Hart et al., 2009). Kalogeropoulou et al. have demonstrated that TAF4b is specifically associated with c-Jun and other AP-1 family members to regulate the expression of Integrin α6 in the context of cancer progression (Kalogeropoulou et al., 2010). Although a recent report has shown that TAF4 interacts with Pygopus, a transcriptional activator of Wingless (Wg) signaling, to induce transcription of naked cuticle in Drosophila (Wright and Tjian, 2009), how other signaling pathways, such as Notch, are regulated by specific TAFs is poorly understood.
Notch signaling is an evolutionally conserved pathway across species that plays a pivotal role in many different developmental events, including cell fate determination, control of cell proliferation and apoptosis, and the maintenance of stem cells (Artavanis-Tsakonas and Muskavitch, 2010; Guruharsha et al., 2012; Tien et al., 2009). Dysregulation of Notch is implicated in a number of diseases, including cancer (Louvi and Artavanis-Tsakonas, 2012; Ntziachristos et al., 2014). Notch signaling activation is mediated by a direct interaction between the Notch receptor in one cell and its ligand on the neighboring cell (Diaz-Benjumea and Cohen, 1995; Doherty et al., 1996). Such an interaction induces two consecutive proteolytic processes that result in the release of the Notch intracellular domain (NICD) (Struhl and Adachi, 1998), which is then translocated to the nucleus and activates transcription of its target genes by interacting with the DNA-binding protein Suppressor of Hairless [Su(H)] and the coactivator Mastermind (Mam) to form a functional Su(H)–NICD–Mam ternary complex (Bailey and Posakony, 1995; Lecourtois and Schweisguth, 1995). Although numerous Notch target genes have been identified in different tissues or organs during different developmental stages, how the Su(H)–NICD–Mam ternary complex regulates their expression is largely unclear. Recent findings in different systems have suggested that chromatin-associated epigenetically-regulatory mechanisms are very important for proper expression of Notch target genes (Bray et al., 2005; Domanitskaya and Schüpbach, 2012; Endo et al., 2011; Kugler and Nagel, 2007; Mulligan et al., 2011; Yu et al., 2013; Zeng et al., 2013). In flies, histone chaperones Asf1 and Nap1 are differentially associated with LID-associated factor (LAF) and RPD3-LID-associated factor (RLAF) silencing complexes to mediate epigenetic silencing at the Notch target Enhancer of Split [E(spl)] cluster (Moshkin et al., 2009). A SIRT1–LSD1 co-repressor complex regulates Notch target gene expression in both mammalian and Drosophila cultured cells (Mulligan et al., 2011). Our very recent study has demonstrated that the histone chaperone CAF-1 complex epigenetically and positively regulates Notch target gene expression in Drosophila (Yu et al., 2013). But the most basic questions of how the Su(H)–NICD–Mam complex activates expression of Notch target genes and which cofactor(s) bring it to the general transcription machinery are unanswered.
The Drosophila egg chamber is an ideal system to study the regulation of Notch signaling and its developmental consequences (Bastock and St Johnston, 2008; Klusza and Deng, 2011). During mid-oogenesis (stages 7–10A), Notch signaling is activated in entire follicular epithelia by the germline-expressed ligand, Delta (Dl) (Deng et al., 2001; López-Schier and St Johnston, 2001). This activation induces the follicle cells from the mitotic cycle into endoreplication, which is mediated by the suppression of Cut and activation of Hindsight (Hnt; also known as Pebbled) through Notch signaling (Sun and Deng, 2007; Sun and Deng, 2005). As in other processes, this Notch-dependent cell cycle transition also requires Notch protein cleavage, which is achieved by γ-secretase components Presenilin and Nicastrin. In addition, the nuclear effector of Notch signaling Su(H) is needed for the switch. The precise timing of Notch signaling during mid-oogenesis is regulated through multiple mechanisms (Domanitskaya and Schüpbach, 2012; Heck et al., 2012; Poulton et al., 2011). Our recent finding demonstrated that the microRNA pathway regulates the temporal pattern of Notch signaling by repressing Delta-mediated inhibition of Notch in Drosophila follicle cells (Poulton et al., 2011). Interestingly, transcriptional cofactors are also involved in precise control of Notch signaling (Domanitskaya and Schüpbach, 2012; Heck et al., 2012) – the transcriptional co-repressor SMRTER inhibits Notch activity in a temporally restricted manner in follicular epithelium (Heck et al., 2012), whereas the transcriptional cofactor Corepressor for element-1-silencing transcription factor (CoREST) promotes Notch signaling in a spatially restricted manner by affecting histone H3 K27 tri-methylation and histone H4 K16 acetylation in the follicle cells (Domanitskaya and Schüpbach, 2012). In a genetic RNA interference (RNAi) screen for regulators of Notch signaling in follicle cells, we identified e(y)1 as being required for proper Notch signaling in mediating the mitotic-to-endoreplicating cycle transition of follicle cells. Further studies showed that e(y)1 is also required for the transcriptional regulation of Notch target genes during wing development and in cultured S2 Drosophila cells. Epistatic analyses in both follicle cells and adult wings suggested that E(y)1 functions downstream of the Notch cleavage step. Biochemical studies in S2 cells demonstrated that E(y)1 physically interacts with both transcriptional effectors of Notch signaling, Su(H) and NICD. Taken together, our data reveal that the association of the NICD–Su(H)–Mastermind complex with E(y)1 in response to Notch activation recruits the transcription initiation complex to induce Notch target genes, coupling Notch signaling with the transcription machinery.
RESULTS
e(y)1 is required for Notch-signaling-mediated follicle cell transition from mitotic cycle to endocycle during Drosophila oogenesis
To identify genes that modulate Notch signaling, we performed a large-scale in vivo RNAi screen for defects in the expression of Notch target genes in Drosophila melanogaster follicle cells. The follicle cell epithelium shows a temporal Notch activation pattern during stages 7–10A of oogenesis, with both negative and positive targets identified (Sun and Deng, 2007; Sun and Deng, 2005). We tested around two thousand Transgenic RNAi Project (TRiP) RNAi lines (Ni et al., 2009; Ni et al., 2008; Ni et al., 2011), which contain either long double-strand (ds) hairpin (Ni et al., 2009; Ni et al., 2008) or short hairpin RNAs (Ni et al., 2011) (both referred to as RNAi) under the control of the upstream activating site (UAS). These lines were crossed to the flip-out Gal4 driver (Ito et al., 1997; Pignoni and Zipursky, 1997) to generate random follicle-cell RNAi clones that were marked by the expression of green and red fluorescent proteins (GFP and RFP, respectively). The follicle-cell RNAi clones were then screened for defects in the expression pattern of the negative Notch target Cut, a homeodomain-containing transcription factor (Sun and Deng, 2005). Cut is normally expressed in early oogenesis (stages 1–6) and then downregulated at stage 7 upon Notch activation in follicle cells (Sun and Deng, 2005). Failure to downregulate Cut at stage 7 suggests a defect in Notch signaling. From this screen, we found that knockdown of the gene CG6474 [enhancer of yellow 1 (e(y)1)], which encodes a homolog of TAF9, a core component of the TFIID transcription initiation complex (Soldatov et al., 1999; Thomas and Chiang, 2006), resulted in prolonged Cut expression in 74% (n = 72) of follicle cell RNAi clones at stages 7–8 (Fig. 1A), suggesting a Notch defect in e(y)1-depleted follicle cells.
Fig. 1.
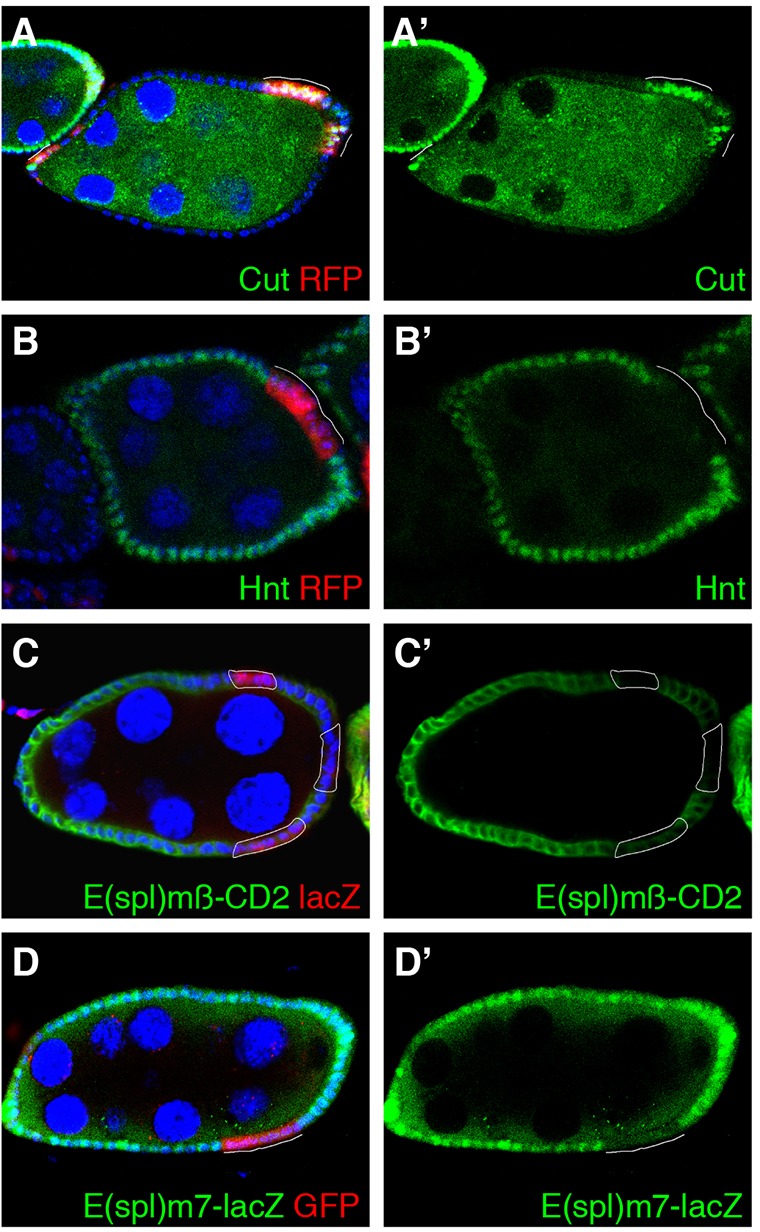
E(y)1 is required for the Notch signaling pathway in Drosophila follicle cells. (A,A′) Cut is upregulated in the e(y)1-RNAi follicle cells (expressing RFP) of a stage-7 egg chamber. (B,B′) Expression of Hnt is suppressed in the e(y)1-knockdown follicle cells (expressing RFP) of an egg chamber at stage 7. (C–D′) The expression of two direct reporters of Notch signaling, E(spl)mβ-CD2 and E(spl)m7-lacZ, labeled by the expression of lacZ in red (C) and of GFP in red (D), respectively, is markedly decreased in e(y)1-depleted follicle cells in mid-stage egg chambers. Nuclei were labeled with DAPI (blue).
To confirm that E(y)1 is required for Notch signaling in follicle cells, we examined the expression of zinc-finger protein Hnt, a positive follicle-cell-specific Notch target, which is normally expressed in the entire follicular epithelium from stages 7–10A (Sun and Deng, 2007). Downregulation of Hnt in mid-oogenesis (stages 7–10A) is an indication of disrupted Notch signaling in follicle cells (Sun and Deng, 2007). Indeed, Hnt downregulation was detected in 68% (n = 51) of e(y)1-knockdown follicle cells (Fig. 1B). Both Cut upregulation and Hnt downregulation were detected in an independent e(y)1-RNAi line (no. 6474R-1) from the National Institute of Genetics (NIG) that was used to knock down e(y)1 expression in mid-staged egg chambers (supplementary material Fig. S1A; data not shown).
To further exclude potential off-target effects on Notch signaling caused by e(y)1-RNAi, we set out to analyze the phenotypes of e(y)1 mutants. An allele named e(y)1190 was generated through imprecise excision of a P-element that had been inserted into the 5′-UTR of the e(y)1 locus (Fig. 2A; see Materials and Methods for details). This allele was both hemizygous and homozygous lethal during early larval stages and could be rescued to adulthood by a duplication [Dp(1;3)DC335 or Dp(1;3)DC336] harboring the e(y)1 genomic sequence (data not shown). Flippase-flippase recognition target (FLP-FRT)-induced follicle cell mitotic clones of e(y)1190 showed upregulated levels of Cut and downregulated levels of Hnt during stages 7–8 when compared with neighboring wild-type cells (Fig. 2B–C″), supporting our findings in e(y)1 knockdown cells.
Fig. 2.
Generation of an e(y)1 null mutant allele, e(y)1190, and characterization of its Notch defects in follicle cells and adult wings. (A) Genomic organization of the e(y)1 locus, with the p{GT}-BG00948 P-element insertion site (white triangle) and the deletion in e(y)1190 (black double arrows). The single black arrow denotes the transcription start site of e(y)1 and its direction. Black bars indicate the coding regions of e(y)1; white bars indicate 5′- and 3′-UTRs. Scale bar: 200 bps. (B–C″) Stage-7 egg chambers bearing e(y)1190 homozygous mutant follicle-cell clones labeled by the absence of RFP and outlined in white (B,C). Egg chambers were stained for Cut (B′) and Hnt (C′), both shown in green. Yellow arrows (B′,B″,C,C″) point to polar cells, in which Cut is normally expressed when Hnt is suppressed. Cell nuclei were stained with DAPI (blue) (B″,C″). (B″,C″) Merged images. (D,E) An e(y)1190 mosaic wing that was induced at larval stages has a defective wing edge (D), which can be fully restored to a normal wing margin by a duplication line carrying the e(y)1 genomic region (E).
Notch-induced transcription activation can be assessed in a more direct manner by using transgenic reporters. Both E(spl)mβ-CD2 (de Celis et al., 1998; Furriols and Bray, 2001) and E(spl)m7-lacZ (Assa-Kunik et al., 2007; Pines et al., 2010), which contain the Su(H) binding sites, are expressed in follicle cells during mid-oogenesis upon Notch activation. Indeed, the expression of these two reporters was substantially reduced in e(y)1-RNAi follicle cells (Fig. 1C,D). In addition, the expression of another follicle-cell-specific Notch reporter broad-early-enhancer-lacZ (brE-lacZ), recently identified by our laboratory (Jia et al., 2014), was also decreased in e(y)1-depleted follicle cells (supplementary material Fig. S1B). These results suggest that e(y)1 is required for proper transcriptional regulation of the Notch target genes in follicle cells.
A developmental consequence of Notch activation in a follicle cell during stage 7 is the induction of a switch from the mitotic divisions of early oogenesis to endoreplication cycles (endocycles) (the M/E transition) during mid-oogenesis (Deng et al., 2001; López-Schier and St Johnston, 2001). To determine whether e(y)1 is required for this Notch-dependent event, we examined nuclei size and expression of mitotic markers in mosaic egg chambers containing follicle-cell clones that expressed RNAi against e(y)1. Knockdown of e(y)1 resulted in smaller and more densely distributed nuclei than those of the neighboring wild-type follicle cells, suggesting a failure in the M/E switch (supplementary material Fig. S2A′). To determine whether e(y)1-knockdown follicle cells remained in the mitotic cycle, we monitored two mitotic markers – phosphorylated histone H3 and Cyclin B (CycB) – by staining the mosaic egg chambers. In wild-type egg chambers, phosphorylated histone H3 and CycB oscillate in early mitotic cycles up to stage 6 and are absent during endoreplication stages (stages 7–10A) (Deng et al., 2001; Shcherbata et al., 2004), whereas in e(y)1-knockdown follicle cells, both markers showed random expression after stage 6 (supplementary material Fig. S2B,C). Taken together, these data indicate that e(y)1 is potentially required for the Notch-dependent M/E switch in Drosophila follicle cells.
Disruption of e(y)1 affects the expression of Notch target genes in the wing imaginal disc and cultured S2 cells
To assess whether e(y)1 also modulates Notch signaling in other tissues, we examined the expression of two well-documented Notch target genes, wg and cut, in the wing imaginal disc. During wing disc development, the Notch signaling pathway is activated at the dorsal–ventral boundary by an interaction between the Notch receptor on one cell and its ligand (Delta or Serrate) on the neighboring cell (Diaz-Benjumea and Cohen, 1995; Doherty et al., 1996). Because e(y)1 mutant cells in the wing disc generated by FLP-FRT mosaic analysis displayed very poor viability (supplementary material Fig. S3B,B′), we employed the RNAi line to study the role of e(y)1 in Notch signaling during wing development. To circumvent early larval lethality of engrailed-Gal4 (en-Gal4)-induced e(y)1-RNAi (data not shown), we used the temporally controlled Gal4-Gal80ts system (McGuire et al., 2003) to express UAS-e(y)1-RNAi in the posterior compartment of a wing disc marked by GFP expression. The control discs did not show detectable changes in Wg (Fig. 3I) or Cut expression (Fig. 3J) (compare the GFP-positive posterior region with the GFP-negative anterior region). However, in en-Gal4, UAS-GFP; tub-Gal80ts>e(y)1-RNAi discs, levels of Wg or Cut in the GFP-expressing posterior compartment were decreased as compared with those in the anterior compartment (Fig. 3K,L). These results demonstrate that e(y)1 is involved in the expression of Notch target genes in the wing disc.
Fig. 3.
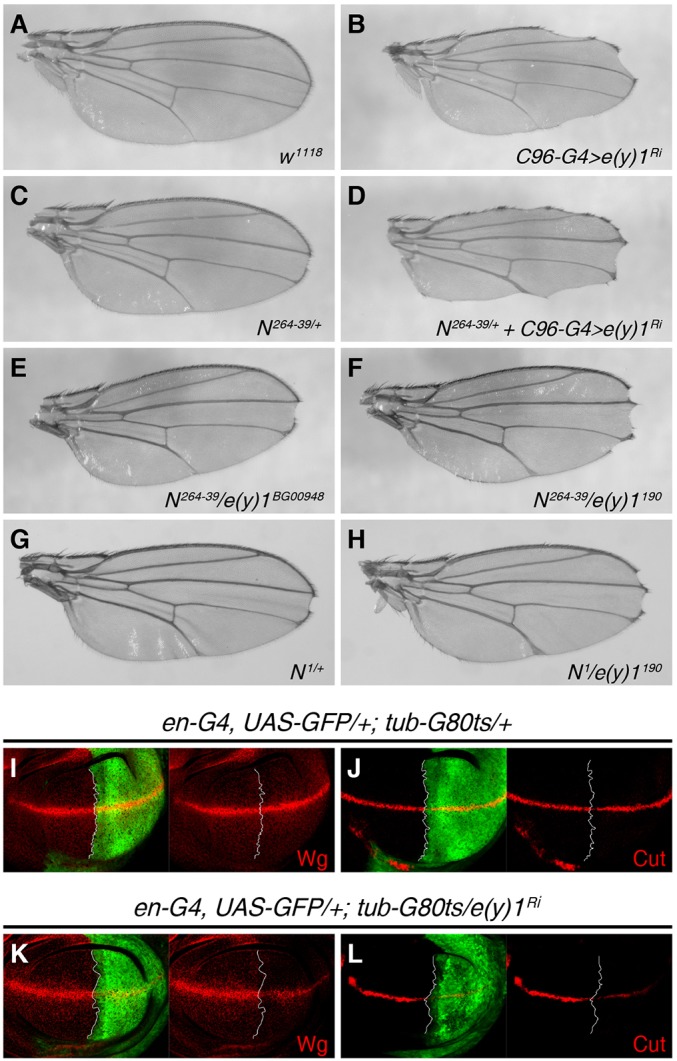
Genetic interaction between e(y)1 and Notch in wing development. (A) A wild-type adult wing with a normal wing margin. (B) Knockdown of e(y)1 under the C96-Gal4 driver caused partial wing margin loss, and Notch heterozygous flies (N264-39/+) showed very mild wing margin notching in the distal region (C). Combination of [C96-Gal4>e(y)1-RNAi+N264-39/+], however, resulted in nearly complete wing margin defects (D). An e(y)1 mutant allele [e(y)1190] was generated; see Materials and Methods for details. Trans-heterozygotes of N264/39/e(y)1BG00948 (E) showed a similar phenotype to Notch heterozygotes (C), with the same penetrance (64 out of 132 in E versus 49 out of 101 in C). e(y)1190 heterozygous flies showed no visible wing defects (data not shown), whereas introducing one copy of e(y)1190 into N264-39/+ flies substantially enhanced the wing margin defects of N264-39/+ (F). (G,H) Synergistic enhancement of notching of the wing margin between N1/+ and e(y)1190/+. N1 is a hypomorphic Notch allele, with 12 out of 81 wings showing mild notching when heterozygous (G, compared with 49 out of 101 of N264/39). When a copy of e(y)1190 was incorporated, the notched wing margin phenotype was visibly enhanced, and penetrance was remarkably increased to 50 out of 82 (H). (I–L) Knockdown of e(y)1 in wing discs compromised the expression of Notch target genes. Anterior and posterior compartments of wing discs are separated by white lines; anterior is to the left and posterior to the right. (I,J) Wing discs from wild-type control flies showed normal expression patterns of Wg (red, I) and of Cut (red, J) in the third-instar larvae. (K,L) Wg level was obviously reduced in the e(y)1-depleted posterior compartment marked by the expression of GFP (green) (K, compared with internal control level in the anterior compartment). The level of Cut was also dramatically decreased in the posterior e(y)1-RNAi area (L). Superscript Ri after a gene denotes RNAi against that gene.
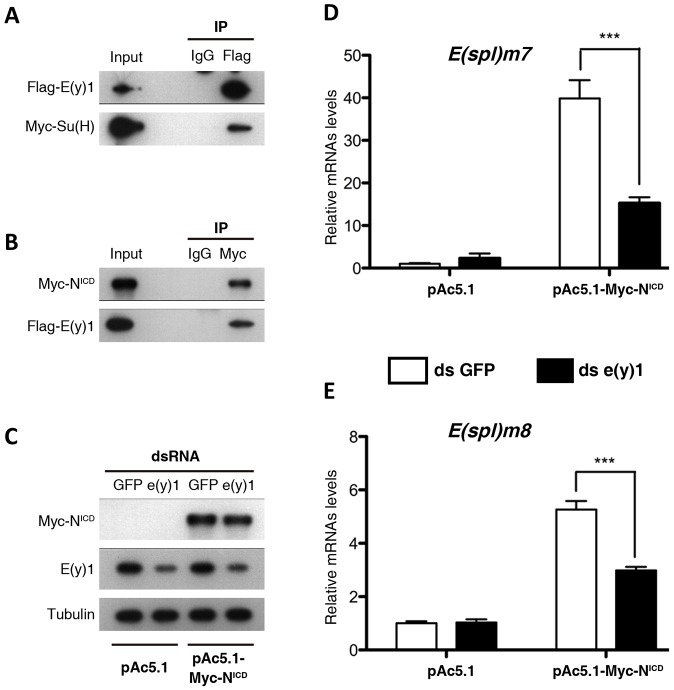
To determine whether E(y)1 is necessary for the transcription of Notch target genes when Notch signaling is artificially induced, we employed a well-documented transient Notch activation system in cultured S2 cells by transfecting a Notch-expressing plasmid, pMT-Notch (Krejcí and Bray, 2007; Yu et al., 2013). In this assay, the overexpressed full-length Notch can be cleaved to produce a constitutively active form of NICD (denoted by NICD) upon treatment with EDTA. NICD translocates into the nucleus and physically interacts with Su(H), which activates the transcription of Notch target genes. In this transient Notch activation system, assuming that E(y)1 is required for regulating Notch target gene expression, the induced expression of Notch target genes by pMT-Notch should be compromised by a perturbation of e(y)1. As expected, the introduction of interfering dsRNAs against e(y)1 [dse(y)1], but not against GFP (dsGFP), into the Notch-activating S2 cells led to a significant reduction in expression of the primary endogenous Notch target genes E(spl)m7 and E(spl)m8 (also known as E(spl)m7-HLH and E(spl)m8-HLH, respectively) (Fig. 5D,E). These results suggest that E(y)1 is also required for artificially induced Notch signaling in cultured S2 cells.
Fig. 5.
E(y)1 is physically associated with NICD and Su(H) and required for Notch target gene expression in S2 cells. (A) Flag-tagged E(y)1 interacts with Myc-tagged Su(H) in Drosophila S2 cells. (B) Myc-tagged NICD is associated with Flag-tagged E(y)1 in S2 cells. Flag-tagged E(y)1 and Myc-tagged Su(H) (A), and Myc-tagged NICD and Flag-tagged E(y)1 (B), were co-transfected into cultured S2 cells. Total cellular extracts were prepared for the co-immunoprecipitation assay with IgG (control) or the indicated antibodies. Antibodies used for immunoprecipitation (IP) in A,B are indicated at the top, and the proteins detected by western blotting after immunoprecipitation are indicated to the left. Input lanes represent 5% of the S2 cell extracts that were used for immunoprecipitation. (C) E(y)1 protein levels are greatly reduced by treatment with dse(y)1 but not dsGFP in S2 cells, whereas NICD and Tubulin protein levels were unchanged by treatment with dse(y)1. (D,E) Expression of two Notch target genes, E(spl)m7 (D) and E(spl)m8 (E), strongly induced by transfected Myc-tagged NICD in S2 cells, was significantly suppressed by e(y)1 knockdown. The basal expression of E(spl)m7 and E(spl)m8 is very low, indicated by transfection of the pAc5.1 empty vector, shown in the left side of each chart, and e(y)1-RNAi manipulation had little effect on their expression. E(spl)m7 and E(spl)m8 levels were increased 39-fold (D) and fivefold (E), respectively, when Notch signaling is induced by transfection of pAc5.1-Myc-NICD in normal S2 cells. Knockdown of e(y)1 significantly suppressed this activation induced by the expression of NICD. ***P<0.001.
e(y)1 genetically interacts with components of the Notch pathway
To determine whether e(y)1 is functionally involved in Notch signaling in the wing imaginal disc, we knocked down e(y)1 through use of a tissue-specific Gal4 driver, C96-Gal4, which is expressed in the cells of the dorsal–ventral boundary of the wing disc, the precursor of the adult wing margin (Gustafson and Boulianne, 1996; Saj et al., 2010). Defective Notch signaling in the dorsal–ventral boundary leads to loss of wing margins (de Celis et al., 1996). Depletion of e(y)1 under the C96-Gal4 driver also led to partial wing margin loss in 96.9% (n = 64) of wings (Fig. 3B), similar to the Notch loss-of-function phenotype. This notched wing margin phenotype was also observed in mosaic adult flies in which e(y)1190 random clones were induced at larval stages by heat-shock-induced Flp (hsFLP) (Fig. 2D). Notch heterozygous animals (N264-39/+) showed a mild wing margin notching in the distal region in 48.5% (n = 101) of wings (Fig. 3C). Combination of C96-Gal4>e(y)1-RNAi and N264-39/+ resulted in a synergistically enhanced notched wing phenotype in terms of both penetrance and expressivity (100%, n = 32; Fig. 3D). Furthermore, although e(y)1 heterozygous flies [e(y)1190/+] did not show any visible wing defects (data not shown), incorporation of a copy of the e(y)1190 mutation into Notch heterozygotes greatly enhanced the wing margin notching phenotype in both expressivity (Fig. 3F compared with Fig. 3E) and penetrance [increasing from 48.5% (n = 132; Fig. 3E) to 90% (n = 60; Fig. 3F)]. Similarly, e(y)1190 also displayed a synergistic interaction with another Notch allele, N1 (Fig. 3G,H). In addition to dorsal–ventral boundary formation in the wing disc, Notch signaling also plays an important role in cell fate determination through lateral inhibition (Artavanis-Tsakonas and Muskavitch, 2010). Indeed, we observed lateral specification defects in bristle and wing vein development when e(y)1 was depleted (supplementary material Fig. S3C–H). These genetic experiments suggest that e(y)1 functionally interacts with the Notch pathway during Drosophila wing development.
E(y)1 mediates the transcriptional output of Notch signaling through association with NICD and Su(H)
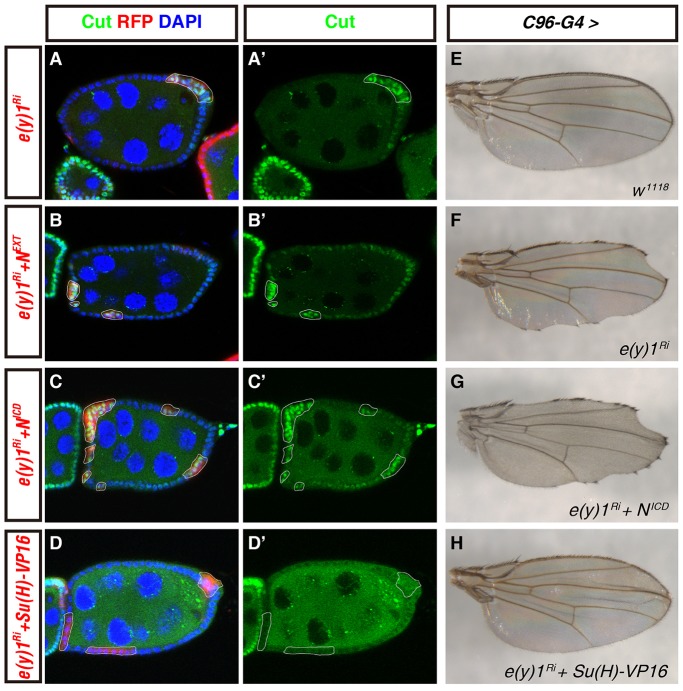
The Notch signaling pathway is delicately regulated at multiple levels, including ligand and receptor post-translational modification, endocytosis and vesicle trafficking, and epigenetic regulation of target gene expression (Fischer et al., 2006; Fortini and Bilder, 2009; Tien et al., 2009; Yu et al., 2013; Zeng et al., 2013). Interaction between Notch ligand and receptor induces two consecutive proteolytic cleavages of the Notch receptor, S2 and S3, which results in the release of NICD (Guruharsha et al., 2012). Translocation of the cleaved NICD to the nucleus activates transcription of the target genes through formation of a ternary complex with the DNA-binding protein Su(H) and the coactivator Mam (Guruharsha et al., 2012). e(y)1 putatively encodes a core component of the TFIID transcription initiation complex, which is required for polymerase-II-mediated gene expression (Dynlacht et al., 1991). Thus, two possibilities for its role in Notch signaling exist – first, E(y)1 is required for the transcriptional expression of a core component or a regulator of the Notch pathway; second, E(y)1 is directly engaged in the transcriptional control of Notch target genes that are mediated by the core NICD–Su(H)–Mam complex. To distinguish between these two possibilities, we conducted epistatic analyses in follicle cells by expressing transgenes that encode components acting in different steps of the Notch pathway. These transgenic constructs include a membrane-tethered active form of Notch, NEXT; a constitutively active form of Notch, NICD (Rebay et al., 1993); and a fusion protein Su(H)–VP16 comprising the DNA-binding domain of Su(H) and the strong activation domain of viral transcription-factor protein VP16 (Kidd et al., 1998; Shyu et al., 2009). NEXT is produced after S2 cleavage and NICD after S3 cleavage. Because the majority of Notch regulators and core components function upstream of either S2 cleavage or S3 cleavage, if E(y)1 is required for the expression of one of the necessary proteins, expression of NEXT and/or NICD would rescue the defects caused by e(y)1 depletion. However, if E(y)1 is directly involved in the expression of Notch target genes, NEXT and/or NICD would have no effect, but Su(H)–VP16 might activate Notch target gene expression in the absence of e(y)1, because Su(H)–VP16 is a more potent transcription activator than Su(H) and can activate a Notch target independently of Notch (Kidd et al., 1998). Because, in addition, the VP16 activation domain can directly interact with multiple components of different general transcription factor complexes (Hall and Struhl, 2002), we speculate that expression of certain Notch targets by Su(H)–VP16 might bypass E(y)1. Expression of these constructs can therefore distinguish whether the involvement of E(y)1 is at the level of target gene expression or component expression. Cut upregulation in e(y)1-knockdown follicle cells was employed as a readout in this epistatic analysis. During stages 7–8 of oogenesis, 76% (n = 66) of e(y)1-RNAi follicle cell clones showed Cut upregulation (Fig. 4A). Similar percentages of Cut upregulation were detected when NEXT (79%, n = 46; Fig. 4B) or NICD (70%, n = 82; Fig. 4C) was co-expressed with e(y)1-RNAi. By contrast, Cut upregulation resulting from e(y)1-RNAi was dramatically reduced by co-expression of Su(H)–VP16 (24%, n = 57; Fig. 4D). These results suggest that the involvement of E(y)1 in Notch signaling is probably not related to the expression of Notch regulators or core components upstream of the release of NICD. We employed similar epistatic analysis for the function of E(y)1 in the adult wing. As seen in Fig. 3B, C96-Gal4>e(y)1-RNAi adults had notched wing margins (Fig. 4F). Although co-expression of NICD did not alleviate this defect (Fig. 4G), C96-Gal4>e(y)1-RNAi animals expressing Su(H)–VP16 showed greatly alleviated wing margin defects (Fig. 4H). C96-Gal4>Su(H)–VP16 alone displayed typical Notch gain-of-function phenotypes during Drosophila wing development (supplementary material Fig. S4). Taken together, these epistatic results in both follicle cells and developing wings suggest that E(y)1 acts downstream of NICD and is engaged in transcriptional activation of Notch target genes.
Fig. 4.
Epistatic analyses of E(y)1 in the Notch pathway in endocycling follicle cells and adult wings. (A–D′) E(y)1 regulates Notch signaling downstream of Notch proteolytic cleavage in mid-stage follicle cells. Cell nuclei were stained with DAPI (blue; A,B,C,D). Flip-out clones were marked by the expression of RFP (red; A,B,C,D) and outlined by white lines. Mid-stage egg chambers were stained for Cut in green (A–D′). Because a stau:GFP transgene was recombined in the hsFLP-carrying X chromosome, punctate green signals were detected within the germ cells. (A,B,C,D) Merged pictures of three channels; (A′,B′,C′,D′) Single channel of Cut staining. (A,A′) Follicle cell clones of e(y)1-RNAi in a mid-stage egg chamber showed Cut upregulation. (B–C′) Expression of NEXT (B,B′) or NICD (C,C′) failed to stop Cut upregulation caused by e(y)1 depletion. (D,D′) Induction of the strong Su(H)–VP16 activator prevented Cut expression in mid-stage e(y)1-RNAi follicle cells. (E–H) Ectopic expression of Su(H)–VP16 suppressed the notched wing margin defect caused by C96-Gal4-driven e(y)1 RNAi. (E) C96-Gal4 control adult flies had normal wing margins. (F) A typical loss of wing margin in C96-Gal4>e(y)1-RNAi flies. (G) Co-expression of NICD under the same C96-Gal4 driver did not suppress wing margin defects caused by e(y)1 RNAi. (H) Co-expression of Su(H)–VP16 substantially suppressed the wing margin loss resulting from C96-Gal4>e(y)1-RNAi. All wings were from male flies. Superscript Ri after a gene denotes RNAi against that gene.
Previous studies have shown that specific transcription factors can directly interact with specific TAFs to recruit the transcriptional machinery to their target genes (Kalogeropoulou et al., 2010; Wright and Tjian, 2009). Su(H) is the executive core transcription factor of Notch signaling. Genetic analyses encouraged us to hypothesize that Su(H) physically interacts with E(y)1 to recruit the transcriptional machinery to initiate Notch target gene expression. To test this hypothesis, we turned to the Drosophila S2 cell system again. We co-transfected two constructs to express Flag-tagged E(y)1 and Myc-tagged Su(H), respectively, both under the control of the Actin5C promoter in S2 cells. As expected, Flag-tagged E(y)1 was able to immunoprecipitate Myc-tagged Su(H) (Fig. 5A). The NICD–Su(H)–Mam ternary complex has been reported to regulate Notch target gene activation in many organisms, and we therefore asked whether E(y)1 is also physically associated with NICD to promote Notch activation. Indeed, the association between E(y)1 and NICD was detected in a co-immunoprecipitation assay (Fig. 5B). Thus, E(y)1 mediates the transcriptional output of Notch signaling by interacting with NICD and Su(H) biochemically.
Requirement of other TAFs in Notch signaling
The results described thus far indicate that E(y)1 is required for the NICD–Su(H)–Mam-complex-mediated transcriptional program of Notch signaling. E(y)1, the Drosophila homolog of human TAF9, was initially identified as one of the core components of the TFIID complex (Dynlacht et al., 1991; Goodrich et al., 1993). To determine whether E(y)1 is a specific factor of the TFIID complex for Notch signaling and/or whether all components of the TFIID complex are required for Notch target gene activation, we examined Notch activity during Drosophila wing development after depleting other components of the TFIID complex through RNAi obtained from the TRiP. Knockdown of TAF12 by the en-Gal4 driver did not obviously affect Notch activity, as revealed by the expression of Wg (Fig. 6A), and consistently, the adult wings of C96- Gal4>TAF12-RNAi animals had normal wing margins (Fig. 6D). However, ablation of TAF1 under the en-Gal4 driver visibly decreased the level of Wg in the posterior compartment of the wing disc (Fig. 6B), and expectedly, overexpression of TAF1-RNAi induced by C96-Gal4 caused notched wing margins in adult flies (Fig. 6E). Furthermore, we tested several RNAi lines corresponding to specific components of the TFIID complex (TAF2, TAF5 and TAF6) available at the Vienna Drosophila RNAi Center under the same C96-Gal4 driver, and all of them showed notched wing margin defects with different expressivity (data not shown). These results suggest that all components of the TFIID complex we tested, except TAF12, are required for Notch signaling, at least during Drosophila wing development. The e(y)1, e(y)2 and e(y)3 genes were genetically identified as the respective mutations enhance the phenotype of the y2 mutation (Georgiev and Gerasimova, 1989; Georgiev et al., 1990), but only E(y)1 is believed to be a component of the TFIID complex. To investigate whether e(y)2 or e(y)3 plays a similar role in Notch signaling as that of e(y)1, we compromised e(y)2 function by expression of e(y)2-RNAi and found that it was not required for Notch activity during wing development (Fig. 6C,F), implying that unlike e(y)2, e(y)1 is specifically required for Notch signaling.
Fig. 6.
Effects of depleting e(y)2 or other TAF-encoding genes on Notch signaling. (A–C) Confocal images of wandering third-instar wing discs with en-Gal4 driving GFP expression plus the indicated constructs. Wing discs were stained for Wg (red). GFP (green) marks the posterior compartment of wing discs in which en-Gal4 is expressed. (D–F) Pictures of adult wings from flies expressing the indicated constructs induced by the C96-Gal4 driver. Superscript Ri after a gene denotes RNAi against that gene.
DISCUSSION
The Notch signaling pathway has been well documented in a variety of tissues and species, ranging from Drosophila to humans (Artavanis-Tsakonas and Muskavitch, 2010; Fortini and Bilder, 2009; Tien et al., 2009). The regulation of this pathway occurs at different levels and is especially well-characterized for the stages before nuclear entry of NICD (Andersson et al., 2011; Guruharsha et al., 2012; Tien et al., 2009). Recent findings from our laboratory and others have shown that Notch signaling can be regulated epigenetically by the CAF1 chromatin chaperone complex or by the SWI/SNF chromatin remodeling complex in different tissues (Yu et al., 2013; Zeng et al., 2013). Our studies address how the Su(H)–NICD–Mam transcriptional complex of the Notch pathway interacts with the general transcription factor complexes to initiate expression of Notch target genes. The results suggest that E(y)1/TAF9, a component of the TAFIID transcription initiation complex, directly interacts with Su(H)–NICD–Mam complex and recruits the TFIID complex to mediate the transcriptional activation of Notch signaling in Drosophila.
Drosophila e(y)1 was initially phenotypically characterized by enhancing the phenotype of the y2 allele when mutated (Georgiev et al., 1990). A following study demonstrated that e(y)1 is highly expressed in follicle cells and oocytes during Drosophila oogenesis and that decreased e(y)1 transcription causes dramatic underdevelopment of the ovaries and sterility of female flies (Soldatov et al., 1999). Consistent with this, our previous studies indicate that the effect of e(y)1 loss in follicle cells is stronger than that in the wing disc, suggesting that the intensity of e(y)1 involvement in Notch signaling is probably context dependent. A protein–protein interaction assay has revealed direct binding between E(y)1/TAF9 and the activation domains of VP16 and p53 (Goodrich et al., 1993; Thut et al., 1995). The human homolog of E(y)1 TAF9 has also been identified as a crucial protein that is required for p53- and VP16-dependent activation of transcription (Uesugi et al., 1997). A recent report has shown that TAF4 interacts with Pygopus, a transcriptional activator of Wg signaling, to induce transcription of naked cuticle (Wright and Tjian, 2009). Our study suggests that TAF9 mediates the transcriptional output of Notch signaling. It is very likely that, for a given signaling pathway, a specific TAF in the TFIID complex is required for transcriptional output of this pathway, which is mediated through direct interaction between the TAF and a transcriptional effector.
Genome-wide transcriptome studies in different cell types from a variety of species have revealed a considerable diversity in the expression of Notch-induced target genes (Aoyagi-Ikeda et al., 2011; Chadwick et al., 2009; Krejcí et al., 2009; Meier-Stiegen et al., 2010; Weerkamp et al., 2006), which might explain the function of Notch signaling in so many different cellular contexts. However, the basis for this transcriptome diversity is only partially understood. The conventional view is that Su(H) is statically associated with its target enhancers and that it represses transcription when Notch is not activated (Andersson et al., 2011). Upon Notch activation, NICD, together with Mam, then displaces co-repressors and brings co-activators to the Su(H)–NICD–Mam complex, which leads to transcriptional activation of target genes, but a systemic analysis of Su(H) binding at 11 E(spl) Notch target genes has revealed that in Drosophila, Su(H) occupancy at target loci is largely different before and after Notch activation (Krejcí and Bray, 2007). Prevailing models imply that the general TFIID complex binds to core promoters even in the absence of a specific transcription activator (Goodrich and Tjian, 2010). Our biochemical study shows that E(y)1/TAF9 can interact with both NICD and Su(H). We propose that, after Notch-activation-induced cleavage, the TFIID complex mediates the change of Su(H) occupancy on Notch target gene enhancers through the association between E(y)1/TAF9 and the Su(H)–NICD–Mam ternary complex, leading to transcriptional activation of Notch target genes.
Many Notch target genes have been found in different tissues, and their activation by Notch signaling is controlled in a spatiotemporal manner (Borggrefe and Oswald, 2009). In our studies, although many TAFs of the TFIID complex that we tested were required for Notch signaling during Drosophila wing development, TAF12 seemed to be dispensable for Notch signaling during wing margin formation, consistent with studies showing that some TAFs are not required for activation of certain genes in specific tissues (Goodrich and Tjian, 2010). In addition, the effect of e(y)1 loss in the wing disc appears to be weaker than that in follicle cells, suggesting that the intensity of e(y)1 involvement in Notch signaling is probably context dependent. Whether E(y)1 is required for Notch target gene expression in all tissues remains unclear. It is plausible that certain TAFs are involved in regulating the output and intensity of the expression of Notch target genes in a spatial- and temporal-specific manner. Our study suggests that, upon Notch signaling activation, Su(H) is released from the Su(H)–Hairless complex to form a transcriptional activator complex with NICD and Mam and this Su(H)–NICD–Mam complex is then recruited by E(y)1/TAF9 to the TFIID transcription initiation complex to activate expression of Notch target genes.
MATERIALS AND METHODS
Generation and characterization of the e(y)1190 allele
Fly P element w1118 P{GT1}e(y)1BG00948 was inserted 78 base pairs (bps) upstream of the start codon of the e(y)1 gene. Isogenized P-element flies that are both homozygous and hemizygous viable and fertile were used for generation of the e(y)1190 allele by a standard P-element-mediated imprecise jump-out strategy. Excisions of P{GT1}e(y)1BG00948 that were hemizygous lethal were sequenced by the use of a pair of primers (5′-CATAAGCTCACCGATTTC-3′ and 5′-CCTCCATCTTGAGATCTC-3′) that flanked the genomic region of the P{GT1}e(y)1BG00948 insertion site. The e(y)1190 mutation is a deletion of 339 bps including part of the 5′-UTR, exon 1, intron 1 and part of exon 2 of the e(y)1 locus (see Fig. 2A).
Fly stocks and genetics
For clonal analyses, the mutation of e(y)1190 was recombined with hsFLP122 and FRT19A on the X chromosome. Two independent UAS-e(y)1-RNAi lines were used to knock down e(y)1 levels in this paper – e(y)1TRiP#HMS00336 from the Bloomington Drosophila Stock Center and e(y)16474R-1 from the National Institute of Genetics, Japan. To induce random clones in follicle cells, the flip-out Gal4 driver [hsFLP; act>CD2 (or y+)>Gal4] was crossed to flies carrying an RNAi or an overexpression construct under the control of the UAS promoter or combination of interest. Their adult progeny were heat-shocked twice at 37°C for 30 minutes and then cultured in a wet-yeast-pasted vial at 29°C for 2–3 days before dissection. w ubi-GFP M(1)osp FRT19A was a gift from Richard S. Mann (Estella and Mann, 2010). Other fly stocks and genotypes related to the figures listed are listed in supplementary material Table S1.
Immunocytochemistry
Antibody staining was performed as previously described (Deng et al., 2001). Antibodies from the Developmental Studies Hybridoma Bank (University of Iowa, Iowa City, IA) were against Cut (1∶20), CycB (1∶10), Wg (1∶20) and Hnt (1∶15). Other antibodies used were against phosphorylated histone H3 (rabbit, 1∶400; Upstate), CD2 (mouse, 1∶50; Serotec) and β-Galactosidase (rabbit, 1∶2000; Sigma). Nuclei were co-stained with DAPI (1∶1000, Invitrogen) for 15 minutes at room temperature. Images were captured on a Zeiss LSM-510 confocal microscope in the Biological Science Imaging Resource Facility at Florida State University. Figures were processed and arranged in Adobe Illustrator.
S2 cell culture, transfection and RNAi assays
Drosophila S2 cells were cultured at room temperature in Hyclone serum-free insect cell culture media (Roche). Transfection of S2 cells was performed using FuGENE HD transfection kit reagents (Roche) following the manufacturer's instructions. The constructs used for transfections are available upon request. The S2 cells were normally harvested 48 hours after transfection for further experiments. Full-length Notch expression was induced by using 500 µM CuSO4 for 24 hours after transfection with pMT-Notch (Fehon et al., 1990). dsRNA was prepared with the RiboMAX large-scale RNA production system-T7 kit (Promega) as previously described (Huang et al., 2011). Primers used were – e(y)1 forward 5′-TTAATACGACTCACTATAGGGGAGATCATGTCCATCCTGAAGGAG-3′ and e(y)1 reverse 5′-TTAATACGACTCACTATAGGGGAGACGCTAGTTGGTCACAAACTC-3′; control GFP forward 5′-TTAATACGACTCACTATAGGGGAGAATGGTGAGCAAGGGCGAGGAGCTG-3′ and GFP reverse 5′-TTAATACGACTCACTATAGGGGAGACTTGTACAGCTCGTCCATGCCGAGAG-3′ (bases in bold indicate T7 promoter sequences). For a 6-well plate, cells in 2 ml of medium in each well were treated with 15 µg dsRNA for 4 days before plasmid transfection or induction with CuSO4.
Co-immunoprecipitation assay
Total protein extracts from S2 cells were prepared in immunoprecipitation lysis buffer (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 1 mM EDTA pH 7.4, 1% Triton X-100, 0.1% SDS) in the presence of protease inhibitors [1 mM PMSF; protease inhibitor cocktail (Calbiochem)]. For co-immunoprecipitation assays, extracts were incubated with specific antibodies and protein A/G agarose beads (Abmart) at 4°C overnight before washes and elution. Immunoprecipitates were boiled in 2×SDS loading buffer for elution from the beads. Rabbit anti-Flag (1∶100, Sigma) and rabbit anti-Myc (1∶200, Sigma) antibodies were used for co-immunoprecipitation experiments; mouse anti-E(y)1 (1∶1000, Sigma), rabbit anti-Flag (1∶1000, Sigma), rabbit anti-Myc (1∶2000, Sigma) and mouse anti-Tubulin (1∶2000, Sigma) antibodies were used for western blots.
Supplementary Material
Acknowledgments
We thank Trudi Schüpbach (Princeton University, Princeton, NJ), Richard S. Mann (Columbia University, New York, NY), the Bloomington Drosophila Stock Center, the Vienna Drosophila RNAi Center, the National Institute of Genetics and the TRiP at Harvard Medical School for fly stocks; and the Developmental Studies Hybridoma Bank for antibodies. We also thank Thomas J. Fellers from the Biological Science Imaging Resource of Florida State University for technical support; Jen Kennedy, William Palmer and Gabriel Calvin (Florida State University, Tallahassee, FL) for critical reading and helpful comments on the manuscript; Zhiqiang Shu (Florida State University, Tallahassee, FL) for assisting screen for e(y)1 mutant alleles; and members of the Deng and Jiao laboratories for feedback and suggestions.
Footnotes
Competing interests
The authors declare no competing interests.
Author contributions
W.-M.D., G.X. and R.J. conceived and designed the experiments. G.X., Z.Y. and D.J. performed the experiments. G.X., Z.Y., R.J. and W.-M.D. analyzed the data. G.X., W.-M.D. and R.J. prepared and wrote the paper with input from Z.Y. and D.J.
Funding
This work was supported by grants from the National Natural Science Foundation of China [grant number 31271573 to R.J. and 31228015 to W.-M.D.]; and a grant from the 973 program [grant number 2012CB825504 to R.J.]. W.-M.D. is supported by the National Science Foundation [grant number IOS-1052333]; and the National Institutes of Health [grant number R01 GM072562]. Deposited in PMC for release after 12 months.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.154583/-/DC1
References
- Andersson E. R., Sandberg R., Lendahl U. (2011). Notch signaling: simplicity in design, versatility in function. Development 138, 3593–3612 10.1242/dev.063610 [DOI] [PubMed] [Google Scholar]
- Aoyagi-Ikeda K., Maeno T., Matsui H., Ueno M., Hara K., Aoki Y., Aoki F., Shimizu T., Doi H., Kawai-Kowase K. et al. (2011). Notch induces myofibroblast differentiation of alveolar epithelial cells via transforming growth factor-beta-Smad3 pathway. Am. J. Respir. Cell Mol. Biol. 45, 136–144 [DOI] [PubMed] [Google Scholar]
- Artavanis-Tsakonas S., Muskavitch M. A. (2010). Notch: the past, the present, and the future. Curr. Top. Dev. Biol. 92, 1–29 10.1016/S0070-2153(10)92001-2 [DOI] [PubMed] [Google Scholar]
- Assa-Kunik E., Torres I. L., Schejter E. D., Johnston D. S., Shilo B. Z. (2007). Drosophila follicle cells are patterned by multiple levels of Notch signaling and antagonism between the Notch and JAK/STAT pathways. Development 134, 1161–1169 10.1242/dev.02800 [DOI] [PubMed] [Google Scholar]
- Bailey A. M., Posakony J. W. (1995). Suppressor of hairless directly activates transcription of enhancer of split complex genes in response to Notch receptor activity. Genes Dev. 9, 2609–2622 10.1101/gad.9.21.2609 [DOI] [PubMed] [Google Scholar]
- Bártfai R., Balduf C., Hilton T., Rathmann Y., Hadzhiev Y., Tora L., Orbán L., Müller F. (2004). TBP2, a vertebrate-specific member of the TBP family, is required in embryonic development of zebrafish. Curr. Biol. 14, 593–598 10.1016/j.cub.2004.03.034 [DOI] [PubMed] [Google Scholar]
- Bastock R., St Johnston D. (2008). Drosophila oogenesis. Curr. Biol. 18, R1082–R1087 10.1016/j.cub.2008.09.011 [DOI] [PubMed] [Google Scholar]
- Borggrefe T., Oswald F. (2009). The Notch signaling pathway: transcriptional regulation at Notch target genes. Cell. Mol. Life Sci. 66, 1631–1646 10.1007/s00018-009-8668-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray S., Musisi H., Bienz M. (2005). Bre1 is required for Notch signaling and histone modification. Dev. Cell 8, 279–286 10.1016/j.devcel.2004.11.020 [DOI] [PubMed] [Google Scholar]
- Chadwick N., Zeef L., Portillo V., Fennessy C., Warrander F., Hoyle S., Buckle A. M. (2009). Identification of novel Notch target genes in T cell leukaemia. Mol. Cancer 8, 35 10.1186/1476-4598-8-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Buffone M. G., Kouadio M., Goodheart M., Page D. C., Gerton G. L., Davidson I., Wang P. J. (2007). Abnormal sperm in mice lacking the Taf7l gene. Mol. Cell. Biol. 27, 2582–2589 10.1128/MCB.01722-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Celis J. F., Garcia-Bellido A., Bray S. J. (1996). Activation and function of Notch at the dorsal-ventral boundary of the wing imaginal disc. Development 122, 359–369 [DOI] [PubMed] [Google Scholar]
- de Celis J. F., Tyler D. M., de Celis J., Bray S. J. (1998). Notch signalling mediates segmentation of the Drosophila leg. Development 125, 4617–4626 [DOI] [PubMed] [Google Scholar]
- Deng W. M., Althauser C., Ruohola-Baker H. (2001). Notch-Delta signaling induces a transition from mitotic cell cycle to endocycle in Drosophila follicle cells. Development 128, 4737–4746 [DOI] [PubMed] [Google Scholar]
- Diaz-Benjumea F. J., Cohen S. M. (1995). Serrate signals through Notch to establish a Wingless-dependent organizer at the dorsal/ventral compartment boundary of the Drosophila wing. Development 121, 4215–4225 [DOI] [PubMed] [Google Scholar]
- Doherty D., Feger G., Younger-Shepherd S., Jan L. Y., Jan Y. N. (1996). Delta is a ventral to dorsal signal complementary to Serrate, another Notch ligand, in Drosophila wing formation. Genes Dev. 10, 421–434 10.1101/gad.10.4.421 [DOI] [PubMed] [Google Scholar]
- Domanitskaya E., Schüpbach T. (2012). CoREST acts as a positive regulator of Notch signaling in the follicle cells of Drosophila melanogaster. J. Cell Sci. 125, 399–410 10.1242/jcs.089797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dynlacht B. D., Hoey T., Tjian R. (1991). Isolation of coactivators associated with the TATA-binding protein that mediate transcriptional activation. Cell 66, 563–576 10.1016/0092-8674(81)90019-2 [DOI] [PubMed] [Google Scholar]
- Endo K., Karim M. R., Taniguchi H., Krejci A., Kinameri E., Siebert M., Ito K., Bray S. J., Moore A. W. (2011). Chromatin modification of Notch targets in olfactory receptor neuron diversification. Nat. Neurosci. 15, 224–233 10.1038/nn.2998 [DOI] [PubMed] [Google Scholar]
- Estella C., Mann R. S. (2010). Non-redundant selector and growth-promoting functions of two sister genes, buttonhead and Sp1, in Drosophila leg development. PLoS Genet. 6, e1001001 10.1371/journal.pgen.1001001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehon R. G., Kooh R. J., Rebay I., Regan C. L., Xu T., Muskavitch M. A., Artavanis-Tsakonas S. (1990). Molecular interactions between the protein products of the neurogenic loci Notch and Delta, two EGF-homologous genes in Drosophila. Cell 61, 523–534 [DOI] [PubMed] [Google Scholar]
- Fischer J. A., Eun S. H., Doolan B. T. (2006). Endocytosis, endosome trafficking, and the regulation of Drosophila development. Annu. Rev. Cell Dev. Biol. 22, 181–206 10.1146/annurev.cellbio.22.010605.093205 [DOI] [PubMed] [Google Scholar]
- Fortini M. E., Bilder D. (2009). Endocytic regulation of Notch signaling. Curr. Opin. Genet. Dev. 19, 323–328 10.1016/j.gde.2009.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furriols M., Bray S. (2001). A model Notch response element detects Suppressor of Hairless-dependent molecular switch. Curr. Biol. 11, 60–64 10.1016/S0960-9822(00)00044-0 [DOI] [PubMed] [Google Scholar]
- Georgiev P. G., Gerasimova T. I. (1989). Novel genes influencing the expression of the yellow locus and mdg4 (gypsy) in Drosophila melanogaster. Mol. Gen. Genet. 220, 121–126 10.1007/BF00260865 [DOI] [PubMed] [Google Scholar]
- Georgiev P. G., Kiselev S. L., Simonova O. B., Gerasimova T. I. (1990). A novel transposition system in Drosophila melanogaster depending on the Stalker mobile genetic element. EMBO J. 9, 2037–2044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich J. A., Tjian R. (2010). Unexpected roles for core promoter recognition factors in cell-type-specific transcription and gene regulation. Nat. Rev. Genet. 11, 549–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich J. A., Hoey T., Thut C. J., Admon A., Tjian R. (1993). Drosophila TAFII40 interacts with both a VP16 activation domain and the basal transcription factor TFIIB. Cell 75, 519–530 10.1016/0092-8674(93)90386-5 [DOI] [PubMed] [Google Scholar]
- Guruharsha K. G., Kankel M. W., Artavanis-Tsakonas S. (2012). The Notch signalling system: recent insights into the complexity of a conserved pathway. Nat. Rev. Genet. 13, 654–666 10.1038/nrg3272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson K., Boulianne G. L. (1996). Distinct expression patterns detected within individual tissues by the GAL4 enhancer trap technique. Genome 39, 174–182 10.1139/g96-023 [DOI] [PubMed] [Google Scholar]
- Hall D. B., Struhl K. (2002). The VP16 activation domain interacts with multiple transcriptional components as determined by protein-protein cross-linking in vivo. J. Biol. Chem. 277, 46043–46050 10.1074/jbc.M208911200 [DOI] [PubMed] [Google Scholar]
- Hart D. O., Raha T., Lawson N. D., Green M. R. (2007). Initiation of zebrafish haematopoiesis by the TATA-box-binding protein-related factor Trf3. Nature 450, 1082–1085 10.1038/nature06349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart D. O., Santra M. K., Raha T., Green M. R. (2009). Selective interaction between Trf3 and Taf3 required for early development and hematopoiesis. Dev. Dyn. 238, 2540–2549 10.1002/dvdy.22083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck B. W., Zhang B., Tong X., Pan Z., Deng W. M., Tsai C. C. (2012). The transcriptional corepressor SMRTER influences both Notch and ecdysone signaling during Drosophila development. Biol. Open 1, 182–196 10.1242/bio.2011047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H., Du G., Chen H., Liang X., Li C., Zhu N., Xue L., Ma J., Jiao R. (2011). Drosophila Smt3 negatively regulates JNK signaling through sequestering Hipk in the nucleus. Development 138, 2477–2485 10.1242/dev.061770 [DOI] [PubMed] [Google Scholar]
- Ito K., Awano W., Suzuki K., Hiromi Y., Yamamoto D. (1997). The Drosophila mushroom body is a quadruple structure of clonal units each of which contains a virtually identical set of neurones and glial cells. Development 124, 761–771 [DOI] [PubMed] [Google Scholar]
- Jia D., Tamori Y., Pyrowolakis G., Deng W. M. (2014). Regulation of broad by the Notch pathway affects timing of follicle cell development. Dev. Biol. 392, 52–61 10.1016/j.ydbio.2014.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalogeropoulou M., Voulgari A., Kostourou V., Sandaltzopoulos R., Dikstein R., Davidson I., Tora L., Pintzas A. (2010). TAF4b and Jun/activating protein-1 collaborate to regulate the expression of integrin alpha6 and cancer cell migration properties. Mol. Cancer Res. 8, 554–568 10.1158/1541-7786.MCR-09-0159 [DOI] [PubMed] [Google Scholar]
- Kidd S., Lieber T., Young M. W. (1998). Ligand-induced cleavage and regulation of nuclear entry of Notch in Drosophila melanogaster embryos. Genes Dev. 12, 3728–3740 10.1101/gad.12.23.3728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klusza S., Deng W. M. (2011). At the crossroads of differentiation and proliferation: precise control of cell-cycle changes by multiple signaling pathways in Drosophila follicle cells. Bioessays 33, 124–134 10.1002/bies.201000089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krejcí A., Bray S. (2007). Notch activation stimulates transient and selective binding of Su(H)/CSL to target enhancers. Genes Dev. 21, 1322–1327 10.1101/gad.424607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krejcí A., Bernard F., Housden B. E., Collins S., Bray S. J. (2009). Direct response to Notch activation: signaling crosstalk and incoherent logic. Sci. Signal. 2, ra1 10.1126/scisignal.2000140 [DOI] [PubMed] [Google Scholar]
- Kugler S. J., Nagel A. C. (2007). putzig is required for cell proliferation and regulates notch activity in Drosophila. Mol. Biol. Cell 18, 3733–3740 10.1091/mbc.E07-03-0263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecourtois M., Schweisguth F. (1995). The neurogenic suppressor of hairless DNA-binding protein mediates the transcriptional activation of the enhancer of split complex genes triggered by Notch signaling. Genes Dev. 9, 2598–2608 10.1101/gad.9.21.2598 [DOI] [PubMed] [Google Scholar]
- López-Schier H., St Johnston D. (2001). Delta signaling from the germ line controls the proliferation and differentiation of the somatic follicle cells during Drosophila oogenesis. Genes Dev. 15, 1393–1405 10.1101/gad.200901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louvi A., Artavanis-Tsakonas S. (2012). Notch and disease: a growing field. Semin. Cell Dev. Biol. 23, 473–480 10.1016/j.semcdb.2012.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire S. E., Le P. T., Osborn A. J., Matsumoto K., Davis R. L. (2003). Spatiotemporal rescue of memory dysfunction in Drosophila. Science 302, 1765–1768 10.1126/science.1089035 [DOI] [PubMed] [Google Scholar]
- Meier-Stiegen F., Schwanbeck R., Bernoth K., Martini S., Hieronymus T., Ruau D., Zenke M., Just U. (2010). Activated Notch1 target genes during embryonic cell differentiation depend on the cellular context and include lineage determinants and inhibitors. PLoS ONE 5, e11481 10.1371/journal.pone.0011481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshkin Y. M., Kan T. W., Goodfellow H., Bezstarosti K., Maeda R. K., Pilyugin M., Karch F., Bray S. J., Demmers J. A., Verrijzer C. P. (2009). Histone chaperones ASF1 and NAP1 differentially modulate removal of active histone marks by LID-RPD3 complexes during NOTCH silencing. Mol. Cell 35, 782–793 10.1016/j.molcel.2009.07.020 [DOI] [PubMed] [Google Scholar]
- Mulligan P., Yang F., Di Stefano L., Ji J. Y., Ouyang J., Nishikawa J. L., Toiber D., Kulkarni M., Wang Q., Najafi-Shoushtari S. H. et al. (2011). A SIRT1-LSD1 corepressor complex regulates Notch target gene expression and development. Mol. Cell 42, 689–699 10.1016/j.molcel.2011.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni J. Q., Markstein M., Binari R., Pfeiffer B., Liu L. P., Villalta C., Booker M., Perkins L., Perrimon N. (2008). Vector and parameters for targeted transgenic RNA interference in Drosophila melanogaster. Nat. Methods 5, 49–51 10.1038/nmeth1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni J. Q., Liu L. P., Binari R., Hardy R., Shim H. S., Cavallaro A., Booker M., Pfeiffer B. D., Markstein M., Wang H. et al. (2009). A Drosophila resource of transgenic RNAi lines for neurogenetics. Genetics 182, 1089–1100 10.1534/genetics.109.103630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni J. Q., Zhou R., Czech B., Liu L. P., Holderbaum L., Yang-Zhou D., Shim H. S., Tao R., Handler D., Karpowicz P. et al. (2011). A genome-scale shRNA resource for transgenic RNAi in Drosophila. Nat. Methods 8, 405–407 10.1038/nmeth.1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ntziachristos P., Lim J. S., Sage J., Aifantis I. (2014). From fly wings to targeted cancer therapies: a centennial for notch signaling. Cancer Cell 25, 318–334 10.1016/j.ccr.2014.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignoni F., Zipursky S. L. (1997). Induction of Drosophila eye development by decapentaplegic. Development 124, 271–278 [DOI] [PubMed] [Google Scholar]
- Pines M. K., Housden B. E., Bernard F., Bray S. J., Röper K. (2010). The cytolinker Pigs is a direct target and a negative regulator of Notch signalling. Development 137, 913–922 10.1242/dev.043224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pointud J. C., Mengus G., Brancorsini S., Monaco L., Parvinen M., Sassone-Corsi P., Davidson I. (2003). The intracellular localisation of TAF7L, a paralogue of transcription factor TFIID subunit TAF7, is developmentally regulated during male germ-cell differentiation. J. Cell Sci. 116, 1847–1858 10.1242/jcs.00391 [DOI] [PubMed] [Google Scholar]
- Poulton J. S., Huang Y. C., Smith L., Sun J., Leake N., Schleede J., Stevens L. M., Deng W. M. (2011). The microRNA pathway regulates the temporal pattern of Notch signaling in Drosophila follicle cells. Development 138, 1737–1745 10.1242/dev.059352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebay I., Fehon R. G., Artavanis-Tsakonas S. (1993). Specific truncations of Drosophila Notch define dominant activated and dominant negative forms of the receptor. Cell 74, 319–329 10.1016/0092-8674(93)90423-N [DOI] [PubMed] [Google Scholar]
- Saj A., Arziman Z., Stempfle D., van Belle W., Sauder U., Horn T., Dürrenberger M., Paro R., Boutros M., Merdes G. (2010). A combined ex vivo and in vivo RNAi screen for notch regulators in Drosophila reveals an extensive notch interaction network. Dev. Cell 18, 862–876 10.1016/j.devcel.2010.03.013 [DOI] [PubMed] [Google Scholar]
- Shcherbata H. R., Althauser C., Findley S. D., Ruohola-Baker H. (2004). The mitotic-to-endocycle switch in Drosophila follicle cells is executed by Notch-dependent regulation of G1/S, G2/M and M/G1 cell-cycle transitions. Development 131, 3169–3181 10.1242/dev.01172 [DOI] [PubMed] [Google Scholar]
- Shyu L. F., Sun J., Chung H. M., Huang Y. C., Deng W. M. (2009). Notch signaling and developmental cell-cycle arrest in Drosophila polar follicle cells. Mol. Biol. Cell 20, 5064–5073 10.1091/mbc.E09-01-0004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldatov A., Nabirochkina E., Georgieva S., Belenkaja T., Georgiev P. (1999). TAFII40 protein is encoded by the e(y)1 gene: biological consequences of mutations. Mol. Cell. Biol. 19, 3769–3778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl G., Adachi A. (1998). Nuclear access and action of notch in vivo. Cell 93, 649–660 10.1016/S0092-8674(00)81193-9 [DOI] [PubMed] [Google Scholar]
- Sun J., Deng W. M. (2005). Notch-dependent downregulation of the homeodomain gene cut is required for the mitotic cycle/endocycle switch and cell differentiation in Drosophila follicle cells. Development 132, 4299–4308 10.1242/dev.02015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Deng W. M. (2007). Hindsight mediates the role of notch in suppressing hedgehog signaling and cell proliferation. Dev. Cell 12, 431–442 10.1016/j.devcel.2007.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M. C., Chiang C. M. (2006). The general transcription machinery and general cofactors. Crit. Rev. Biochem. Mol. Biol. 41, 105–178 10.1080/10409230600648736 [DOI] [PubMed] [Google Scholar]
- Thut C. J., Chen J. L., Klemm R., Tjian R. (1995). p53 transcriptional activation mediated by coactivators TAFII40 and TAFII60. Science 267, 100–104 10.1126/science.7809597 [DOI] [PubMed] [Google Scholar]
- Tien A. C., Rajan A., Bellen H. J. (2009). A Notch updated. J. Cell Biol. 184, 621–629 10.1083/jcb.200811141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uesugi M., Nyanguile O., Lu H., Levine A. J., Verdine G. L. (1997). Induced alpha helix in the VP16 activation domain upon binding to a human TAF. Science 277, 1310–1313 10.1126/science.277.5330.1310 [DOI] [PubMed] [Google Scholar]
- Weerkamp F., Luis T. C., Naber B. A., Koster E. E., Jeannotte L., van Dongen J. J., Staal F. J. (2006). Identification of Notch target genes in uncommitted T-cell progenitors: No direct induction of a T-cell specific gene program. Leukemia 20, 1967–1977 10.1038/sj.leu.2404396 [DOI] [PubMed] [Google Scholar]
- Wright K. J., Tjian R. (2009). Wnt signaling targets ETO coactivation domain of TAF4/TFIID in vivo. Proc. Natl. Acad. Sci. USA 106, 55–60 10.1073/pnas.0811914106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z., Wu H., Chen H., Wang R., Liang X., Liu J., Li C., Deng W. M., Jiao R. (2013). CAF-1 promotes Notch signaling through epigenetic control of target gene expression during Drosophila development. Development 140, 3635–3644 10.1242/dev.094599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X., Lin X., Hou S. X. (2013). The Osa-containing SWI/SNF chromatin-remodeling complex regulates stem cell commitment in the adult Drosophila intestine. Development 140, 3532–3540 10.1242/dev.096891 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.