Abstract
Rationale: The current outbreak of coronavirus disease (COVID-19) pneumonia caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in Wuhan, China, spreads across national and international borders. The overall death rate of COVID-19 pneumonia in the Chinese population was 4%.
Objectives: To describe the process of hospitalization and critical care of patients who died of COVID-19 pneumonia.
Methods: This was a multicenter observational study of 109 decedents with COVID-19 pneumonia from three hospitals in Wuhan. Demographic, clinical, laboratory, and treatment data were collected and analyzed, and the final date of follow-up was February 24, 2020.
Results: The mean age of 109 decedents with COVID-19 pneumonia was 70.7 years, 35 patients (32.1%) were female, and 85 patients (78.0%) suffered from one or more underlying comorbidities. Multiple organ failure, especially respiratory failure and heart failure, appeared in all patients even at the early stage of disease. Overall, the mean time from onset of symptoms to death was 22.3 days. All 109 hospitalized patients needed admission to an intensive care unit (ICU); however, because of limited availability, only 51 (46.8%) could be admitted. The period from hospitalization to death in the ICU group and non-ICU group was 15.9 days (standard deviation = 8.8 d) and 12.5 days (8.6 d, P = 0.044), respectively.
Conclusions: Mortality due to COVID-19 pneumonia was concentrated in patients above the age of 65 years, especially those with major comorbidities. Patients who were admitted to the ICU lived longer than those who were not. Our findings should aid in the recognition and clinical management of such infections, especially with regard to ICU resource allocation.
Keywords: COVID-19, mortality, pneumonia, SARS-CoV-2
The ongoing pandemic of coronavirus disease (COVID-19), caused by the novel coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is an emerging, rapidly evolving situation. As of March 30, 2020, a total of 735,560 confirmed cases and 34,830 deaths had been reported in at least 174 countries, indicating that the overall rate of death from COVID-19 pneumonia was 4.7% (1). In the first cohort of 41 patients with COVID-19 pneumonia from Wuhan, China, 13 patients (31.7%) were admitted to an intensive care unit (ICU) and 6 (14.6%) died (2). When the cohort size expanded to 99 cases, 11 patients (11.1%) worsened in a short period of time and died of multiple organ failure (3). Recent studies have demonstrated that older age, higher Sequential Organ Failure Assessment (SOFA) scores, and elevated D-dimer are risk factors for death in patients with COVID-19 pneumonia (4–6), and that high fever (≥39°C) is associated with a lower likelihood of death (6). However, these studies have not reported the issues faced around the world with regard to the rapid increase in cases potentially overwhelming healthcare resources. Between December 25, 2019, and February 15, 2020, a total of 1,017 patients with confirmed COVID-19 pneumonia were admitted to one special hospital for infectious diseases and two general hospitals in Wuhan. As of February 24, 2020, 114 patients had died in the hospitals, and data required for this study were available for 109 of these patients. This report describes the clinical course and critical care of these 109 decedents from the initial Wuhan experience to address the issues concerning ICU resources.
Methods
Patients
In response to a pandemic of COVID-19 pneumonia, all hospitals in Wuhan initiated active surveillance for the disease on December 31, 2019 at the requirement of the Wuhan Health Commission. Between December 25, 2019, and February 15, 2020, the numbers of patients with COVID-19 pneumonia who had been admitted to Wuhan Pulmonary Hospital (Pulmonary Hospital), Tianyou Hospital Affiliated with the Wuhan University of Science and Technology (Tianyou Hospital), and Central Hospital of Wuhan (Central Hospital) were 273, 301, and 443, respectively. A diagnosis of COVID-19 pneumonia was confirmed according to the case definition established by World Health Organization interim guidance with positive SARS-CoV-2 test results in throat-swab specimens (7).
Data Collection
We retrospectively collected the demographics, clinical symptoms or signs, laboratory findings on admission and during hospitalization, treatment, and date of death from electronic medical records of the decedents. Data were entered into a computerized database and cross-checked. Ethics approval was exempted by the institutional review boards of the hospitals because we collected and analyzed all data from the patients according to the policy for public health outbreak investigations of emerging infectious diseases issued by the National Health Commission of the People’s Republic of China. Two researchers independently reviewed the data collection forms to double-check the collected data.
Statistical Analysis
Descriptive data are presented as frequencies (percentages) for discrete variables and as means (standard deviations [SDs]) or medians (interquartile ranges [IQRs]) for continuous variables. Comparisons were performed using Student’s t test or Mann-Whitney U test for continuous variables as appropriate, and the χ2 test for categorical variables. The statistical significance level was set at 0.05 (two-tailed). All analyses were conducted with MedCalc and SPSS version 23.0 statistical software.
Results
Characteristics of the Study Patients
In Wuhan city, a total of 48 hospitals (35 in urban districts and 13 in suburban areas) were designated as “COVID-19 hospitals” by the local government. Pulmonary Hospital and Jinyintan Hospital are the two special hospitals for infectious diseases in Wuhan. It was local policy to transfer critically ill patients with COVID pneumonia from general hospitals, including Tianyou Hospital and Central Hospital, to the special hospitals for treatment. During the study period, 1,017 patients with confirmed COVID-19 pneumonia were admitted to our three study hospitals. As of February 24, 2020, the numbers (percentages) of dead patients in Pulmonary Hospital, Tianyou Hospital, and Central Hospital were 45 (16.5%), 29 (9.6%), and 40 (9.0%), respectively. In Central Hospital, 40 nonsurvivors were distributed among different wards. Five of these patients died within a few hours after they were admitted to the hospital and thus the required data could not be obtained; therefore, only 35 cases were included in the present study. In total, the study population of this multicenter observational study consisted of 109 decedents.
Throat-swab specimens from the 109 decedents showed positive SARS-CoV-2 test results. Pulmonary involvement was seen on computed tomography of all of the patients, and the most frequent computed tomography findings were bilateral extensive ground-glass opacification and/or consolidation.
The mean age of the patients with COVID-19 pneumonia was 70.7 years (SD = 10.9 yr; range 43–99 yr), and 35 patients (32.1%) were female (Table 1). Three patients (2.8%) were <50 years old, and 76.1% were ≥65 years old. Eight-five patients (78.0%) suffered from one or more underlying comorbidities, with the three most common being hypertension, cardiovascular or cerebrovascular disease, and diabetes (Table 1).
Table 1.
Demographics and clinical presentation of 109 decedents with COVID-19 pneumonia
| Characteristic | Total (N = 109) | ICU (n = 51) | Non-ICU (n = 58) | P Value |
|---|---|---|---|---|
| Age, yr | ||||
| Mean ± SD, yr | 70.7 ± 10.9 | 68.4 ± 9.7 | 72.7 ± 11.6 | 0.038 |
| Subgroup, n (%) | — | — | — | 0.005 |
| ≤49 yr | 3 (2.8) | 3 (5.9) | 0 (0) | — |
| 50–64 yr | 23 (21.1) | 10 (19.6) | 13 (22.4) | — |
| 65–74 yr | 45 (41.3) | 27 (52.9) | 18 (31.0) | — |
| ≥75 yr | 38 (34.8) | 11 (21.6) | 27 (46.6) | — |
| Female, n (%) | 35 (32.1) | 15 (29.4) | 20 (34.5) | 0.572 |
| Underlying diseases, n (%) | ||||
| Hypertension | 65 (59.6) | 29 (56.9) | 36 (62.1) | 0.580 |
| Cardiovascular or cerebrovascular diseases | 37 (33.9) | 15 (29.4) | 22 (37.9) | 0.349 |
| Diabetes | 34 (31.2) | 18 (35.3) | 16 (27.6) | 0.386 |
| Chronic respiratory disease | 17 (15.6) | 4 (7.8) | 13 (22.4) | 0.036 |
| Chronic digestive disorders | 16 (14.7) | 6 (11.8) | 10 (17.2) | 0.420 |
| Chronic renal insufficiency | 8 (7.3) | 1 (2.0) | 7 (12.1) | 0.099 |
| Peripheral vascular disease | 8 (7.3) | 2 (3.9) | 6 (10.3) | 0.360 |
| Malignancy | 8 (7.3) | 2 (3.9) | 6 (10.3) | 0.360 |
| Chronic hepatic insufficiency | 2 (1.8) | 0 (0) | 2 (3.4) | 0.497 |
| Autoimmune diseases | 1 (0.9) | 1 (2.0) | 0 (0) | 0.468 |
| Symptoms at onset of illness, n (%) | ||||
| Fever | 99 (90.8) | 49 (96.1) | 50 (86.2) | 0.147 |
| Cough | 77 (70.6) | 36 (70.6) | 41 (70.7) | 0.991 |
| Dyspnea | 75 (68.8) | 38 (74.5) | 37 (63.8) | 0.228 |
| Fatigue | 58 (53.2) | 22 (43.1) | 36 (62.1) | 0.048 |
| Sputum production | 47 (43.1) | 21 (41.2) | 26 (44.8) | 0.701 |
| Diarrhea | 29 (26.6) | 15 (29.4) | 14 (24.1) | 0.534 |
| Myalgia | 19 (17.4) | 12 (23.5) | 7 (12.1) | 0.116 |
| Headache | 8 (7.3) | 4 (7.8) | 4 (6.9) | 1.000 |
| Hemoptysis | 8 (7.3) | 6 (11.8) | 2 (3.4) | 0.196 |
| Vital signs on admission | ||||
| Temperature | ||||
| Median (IQR), °C | 36.6 (36.4–37.2) | 36.6 (36.5–37.3) | 36.6 (36.4–37.2) | 0.367 |
| Subgroup, n (%) | — | — | — | 0.184 |
| <37.3°C | 83 (76.1) | 38 (74.5) | 45 (77.6) | — |
| 37.3–38.0°C | 17 (15.6) | 7 (13.7) | 10 (17.2) | — |
| 38. 1–39.0°C | 6 (5.5) | 3 (5.9) | 3 (5.2) | — |
| >39.0°C | 3 (2.8) | 3 (5.9) | 0 (0) | — |
| Systolic blood pressure, mm Hg | 132 ± 22 | 131 ± 23 | 133 ± 22 | 0.713 |
| Diastolic blood pressure, mm Hg | 75 ± 12 | 73 ± 11 | 76 ± 13 | 0.326 |
| Respiratory rate | ||||
| Median (IQR), breath/min | 20 (20–26) | 21 (20–28) | 20 (20–25) | 0.387 |
| >24 breaths/min, n (%) | 32 (29.4) | 17 (33.3) | 15 (25.9) | 0.393 |
| Heart rate | ||||
| Mean ± SD, breath/min | 88.5 ± 18.5 | 92.3 ± 19.6 | 85.2 ± 16.9 | 0.043 |
| >100 beats/min, n (%) | 29 (26.6) | 17 (33.3) | 12 (20.7) | 0.136 |
| APACHE II score | 22.0 (16.0–34.8) | 26.5 (17.0–35.3) | 21.0 (14.8–33.5) | 0.163 |
| SOFA score | 3.0 (2.0–6.0) | 3.0 (2.0–7.0) | 3.0 (2.0–6.0) | 0.880 |
Definition of abbreviations: APACHE II = Acute Physiology and Chronic Health Evaluation II; COVID-19 = coronavirus disease; ICU = intensive care unit; IQR = interquartile range; SD = standard deviation; SOFA = Sequential Organ Failure Assessment.
Comparisons were determined by Student’s t test, Mann-Whitney U test, or χ2 test as appropriate.
At the onset of illness, fever, fatigue, and dry cough were the three most common symptoms reported in the overall population (2, 8). We noted that dyspnea took the place of fatigue, becoming one of the top three symptoms of decedents, followed by fatigue, sputum production, and diarrhea (Table 1). On admission to the hospital, all of the patients were critically ill, and the most frequent and most significant symptom was rapid, progressive dyspnea. As shown in Table 1, the medians of Acute Physiology and Chronic Health Evaluation (APACHE) II scores and SOFA scores were 22.0 and 3.0, respectively, indicating that more severe conditions, including multiple organ injury, were common.
The numbers of ICU beds provided for patients with COVID-19 were 21, 10, and 12 in Pulmonary Hospital, Tianyou Hospital, and Central Hospital, respectively. All 109 hospitalized patients needed ICU admission; however, because of very limited availability, only 51 (46.8%) could be admitted. As a matter of fact, during this study period, only after someone in the ICU had died could the next patient be admitted to occupy the ICU bed. When we compared the demographic and clinical characteristics of patients who were admitted to the ICU with those of the patients who were not, we noted that most characteristics, including APACHE II and SOFA scores, were similar, except that younger age, less underlying chronic respiratory disease, less fatigue symptoms, and faster heart rates were seen in the ICU group (Table 1).
Laboratory Findings
The white blood cell numbers in 55.0% of the decedents with COVID pneumonia were not outside the normal range: 31.2% were above the upper boundary of normal range, and approximately half had increased neutrophilic granulocytosis (Table 2). Lymphopenia is always a leading feature of viral pneumonia. Similar to findings from the overall population of patients with COVID-19 pneumonia throughout China (9), a majority (82.6%) of decedents in our study demonstrated a remarkable lymphopenia; only one (0.9%) had lymphocyte numbers above the upper boundary of normal range. In addition, anemia and thrombocytopenia were also common (Table 2). As shown in Figure E1 in the online supplement, leukocytosis, neutrophilic granulocytosis, lymphopenia, and anemia occurred right after hospitalization and lasted for at least 2 weeks or until death.
Table 2.
Blood analysis in 109 decedents with COVID-19 pneumonia
| Parameter | Total (N = 109) | ICU (n = 51) | Non-ICU (n = 58) | P Value |
|---|---|---|---|---|
| White blood cells | ||||
| Mean ± SD, ×109/L | 8.7 ± 4.8 | 9.6 ± 5.5 | 7.9 ± 4.1 | 0.067 |
| Subgroup, n (%) | — | — | — | 0.897 |
| <4 × 109/L | 15 (13.8) | 7 (13.7) | 8 (13.8) | — |
| 4–10 × 109/L | 60 (55.0) | 27 (52.9) | 33 (56.9) | — |
| >10 × 109/L | 34 (31.2) | 17 (33.3) | 17 (29.3) | — |
| Neutrophils | ||||
| Mean ± SD,×109/L | 7.5 ± 4.6 | 8.3 ± 5.2 | 6.9 ± 4.1 | 0.101 |
| Subgroup, n (%) | — | — | — | 0.033 |
| <1.8 × 109/L | 4 (3.7) | 0 (0) | 4 (6.9) | — |
| 1.8–6.3 × 109/L | 50 (45.9) | 21 (41.2) | 29 (50.0) | — |
| >6.3 × 109/L | 55 (50.5) | 30 (58.8) | 25 (43.1) | — |
| Lymphocytes | ||||
| Median (IQR), ×109/L | 0.6 (0.4–0.9) | 0.6 (0.4–0.9) | 0.6 (0.5–0.8) | 0.817 |
| Subgroup, n (%) | — | — | — | 0.696 |
| <1.1 × 109/L | 90 (82.6) | 41 (80.4) | 49 (84.5) | — |
| 1.1–3.2 × 109/L | 18 (16.5) | 9 (17.6) | 9 (15.5) | — |
| >3.2 × 109/L | 1 (0.9) | 1 (2.0) | 0 (0) | — |
| Red blood cells | ||||
| Mean ± SD, ×1012/L | 4.2 ± 0.7 | 4.3 ± 0.6 | 4.0 ± 0.8 | 0.022 |
| Subgroup, n (%) | — | — | — | 0.051 |
| <4.3 × 1012/L | 62 (56.9) | 24 (47.1) | 38 (65.5) | — |
| 4.3–5.8 × 1012/L | 46 (42.2) | 27 (52.9) | 19 (32.8) | — |
| >5.8 × 1012/L | 1 (0.9) | 0 (0) | 1 (1.7) | — |
| Hemoglobulin | ||||
| Mean ± SD, g/L | 125.8 ± 21.2 | 131.2 ± 17.3 | 121.0 ± 23.2 | 0.010 |
| <110 g/L, n (%) | 19 (17.4) | 4 (7.8) | 15 (25.9) | 0.013 |
| Platelets | ||||
| Mean ± SD, × 109/L | 169.4 ± 78.4 | 169.2 ± 72.5 | 169.5 ± 83.9 | 0.983 |
| <100 × 109/L, n (%) | 16 (14.7) | 7 (13.7) | 9 (15.5) | 0.792 |
| C-reactive protein | ||||
| Mean ± SD, mg/L | 85.7 ± 57.3 | 87.1 ± 63.0 | 84.5 ± 52.3 | 0.815 |
| ≥10 mg/L, n (%) | 104 (95.4) | 48 (94.1) | 56 (96.6) | 0.883 |
| Procalcitonin | ||||
| Median (IQR), μg/L | 0.1 (0.1–0.3) | 0.1 (0.1–0.4) | 0.1 (0.0–0.3) | 0.395 |
| ≥0.5 μg/L, n (%) | 20 (18.3) | 10 (19.6) | 10 (17.2) | 0.750 |
| PaO2 | ||||
| Mean ± SD, mm Hg | 62.3 ± 25.4 | 63.6 ± 27.4 | 61.1 ± 23.6 | 0.617 |
| <80 mm Hg, n (%) | 91 (83.5) | 42 (82.4) | 49 (84.5) | 0.765 |
| SaO2 | ||||
| Median (IQR),% | 90.0 (80.5–94.0) | 90.0 (80.0–95.0) | 90.0 (81.5–93.3) | 0.803 |
| <95%, n (%) | 84 (77.1) | 38 (74.5) | 46 (79.3) | 0.552 |
| PaO2/FiO2 | ||||
| Median (IQR), mm Hg | 124 (90–186) | 121 (90–158) | 133 (90–230) | 0.143 |
| <200 mm Hg, n (%) | 84 (77.1) | 43 (84.3) | 41 (70.7) | 0.091 |
| Cardiac troponin I | ||||
| Median (IQR), μg/L | 0.0 (0.0–0.1) | 0.0 (0.0–0.1) | 0.0 (0.0–0.1) | 0.543 |
| ≥0.05 μg/L, n (%) | 52 (47.7) | 25 (49.0) | 27 (46.6) | 0.797 |
| Creatine kinase MB | ||||
| Median (IQR), μg/L | 3.0 (1.7–9.5) | 2.7 (1.4–7.0) | 3.9 (1.7–10.7) | 0.182 |
| >5 μg/L, n (%) | 39 (35.8) | 15 (29.4) | 24 (41.4) | 0.193 |
| Myoglobin | ||||
| Median (IQR), μg/L | 68.3 (36.6–141.1) | 71.4 (37.3–153.9) | 68.1 (35.5–133.7) | 0.491 |
| >100 μg/L, n (%) | 42 (38.5) | 23 (45.1) | 19 (32.8) | 0.187 |
| NT-pro-brain natriuretic peptide* | ||||
| Median (IQR), ng/L | 582.0 (307.0–1,097.5) | 480.0 (164.0–1,046.5) | 722.0 (498.5–1,335.0) | 0.134 |
| >300 ng/L, n (%) | 34/45 (75.6) | 18/28 (64.3) | 16/17 (94.1) | 0.057 |
| Albumin | ||||
| Median (IQR), g/L | 34.2 (31.6–37.0) | 33.2 (30.7–35.2) | 35.6 (32.0–38.9) | 0.011 |
| <40 g/L, n (%) | 92 (84.4) | 47 (92.2) | 45 (77.6) | 0.036 |
| Alanine aminotransferase | ||||
| Median (IQR), U/L | 24.0 (19.0–39.0) | 27.0 (21.0–45.0) | 21.6 (16.8–36.5) | 0.103 |
| >50 U/L, n (%) | 18 (16.5) | 10 (19.6) | 8 (13.8) | 0.415 |
| Aspartate aminotransferase | ||||
| Median (IQR), U/L | 37.0 (25.7–50.0) | 40.0 (27.0–56.0) | 32.0 (24.8–47.0) | 0.216 |
| >40 U/L, n (%) | 50 (45.9) | 25 (49.0) | 25 (43.1) | 0.536 |
| Urea nitrogen | ||||
| Median (IQR), mmol/L | 7.3 (5.7–10.1) | 7.3 (5.4–8.7) | 7.7 (5.7–12.4) | 0.101 |
| >8 mmol/L, n (%) | 43 (39.4) | 16 (31.4) | 27 (46.6) | 0.106 |
| Creatinine | ||||
| Median (IQR), μmol/L | 76.0 (62.3–96.0) | 71.0 (58.0–86.0) | 85.8 (70.0–110.3) | 0.002 |
| >133 μmol/L, n (%) | 12 (11.0) | 2 (3.9) | 10 (17.2) | 0.027 |
| D-dimer, mg/L | ||||
| Median (IQR), mg/L | 1.4 (0.6–4.8) | 2.3 (0.9–7.5) | 1.0 (0.4–4.2) | 0.011 |
| >0.5 mg/L, n (%) | 85 (78.0) | 44 (86.3) | 41 (70.7) | 0.050 |
| Prothrombin time | ||||
| Median (IQR), s | 12.9 (11.6–15.4) | 13.6 (12.1–16.0) | 12.3 (11.3–14.4) | 0.007 |
| Subgroup, n (%) | — | — | — | 0.023 |
| <11.0 s | 13 (11.9) | 3 (5.9) | 10 (17.2) | — |
| 11.0–13.0 s | 44 (40.4) | 17 (33.3) | 27 (46.6) | — |
| >13.0 s | 52 (47.7) | 31 (60.8) | 21 (36.2) | — |
| Activated partial thromboplastin time | ||||
| Median (IQR), s | 34.3 ± 10.7 | 35.0 ± 11.9 | 33.8 ± 9.6 | 0.546 |
| Subgroup, n (%) | — | — | — | 0.545 |
| <21.0 s | 7 (6.4) | 2 (3.9) | 5 (8.6) | — |
| 21.0–34.0 s | 54 (49.5) | 27 (52.9) | 27 (46.6) | — |
| >34.0 s | 48 (44.1) | 22 (43.2) | 26 (44.8) | — |
| Potassium | ||||
| Mean ± SD, mmol/L | 4.1 ± 0.6 | 4.0 ± 0.7 | 4.1 ± 0.6 | 0.650 |
| Subgroup, n (%) | — | — | — | 0.700 |
| <3.5 mmol/L | 16 (14.7) | 9 (17.7) | 7 (12.1) | — |
| 3.5–5.5 mmol/L | 89 (81.7) | 40 (78.4) | 49 (84.5) | — |
| >5.5 mmol/L | 4 (3.7) | 2 (3.9) | 2 (3.4) | — |
| Sodium | ||||
| Mean ± SD, mmol/L | 138.8 ± 5.3 | 139.2 ± 4.8 | 138.5 ± 5.7 | 0.508 |
| Subgroup, n (%) | — | — | — | 0.753 |
| <135 mmol/L | 24 (22.0) | 10 (19.6) | 14 (24.1) | — |
| 135–145 mmol/L | 73 (67.0) | 36 (70.6) | 37 (63.8) | — |
| >145 mmol/L | 12 (11.0) | 5 (9.8) | 7 (12.1) | — |
Definition of abbreviations: COVID-19 = coronavirus disease; FiO2 = fraction of inspired oxygen; ICU = intensive care unit; IQR = interquartile range; PaO2 = arterial partial pressure of oxygen; SaO2 = arterial oxygen saturation; SD = standard deviation.
Comparisons were determined by Student’s t test, Mann-Whitney U test, or χ2 test as appropriate.
Data from 45 patients in Wuhan Pulmonary Hospital.
All of the patients were severely hypoxemic, as indicated by a remarkable decrease in arterial oxygen pressure, arterial oxygen saturation, and the ratio of arterial oxygen pressure/fraction of inspired oxygen at hospital admission (Table 2 and Figure E2). They suffered more frequent and more severe heart injuries, as an elevation in all laboratory parameters reflecting heart injury, including cardiac troponin I, creatine kinase MB, myoglobin, and NT-pro-brain natriuretic peptide, was seen in a high proportion of the decedents (Table 2). Furthermore, they exhibited such high concentrations above the upper boundaries of normal ranges for at least the first 2 weeks (Figure E3). In some patients, the concentrations of alanine aminotransferase, aspartate aminotransferase, urea nitrogen, and creatinine were increased, especially in the late stage of illness (Table 2 and Figure E4), indicating that they were also susceptible to hepatic or renal insufficiency. Multiple organ failure was further manifested by coagulation disorders, as D-dimer concentrations were elevated, and prothrombin time and activated partial thromboplastin time were prolonged, and these abnormalities were long lasting (Table 2 and Figure E5).
A comparison of laboratory findings from the ICU group and non-ICU group demonstrated some statistical differences in anemia, hypoproteinemia, creatinine, D-dimer, and prothrombin time (Table 2); however, these differences did not show any clinical significance.
Treatment
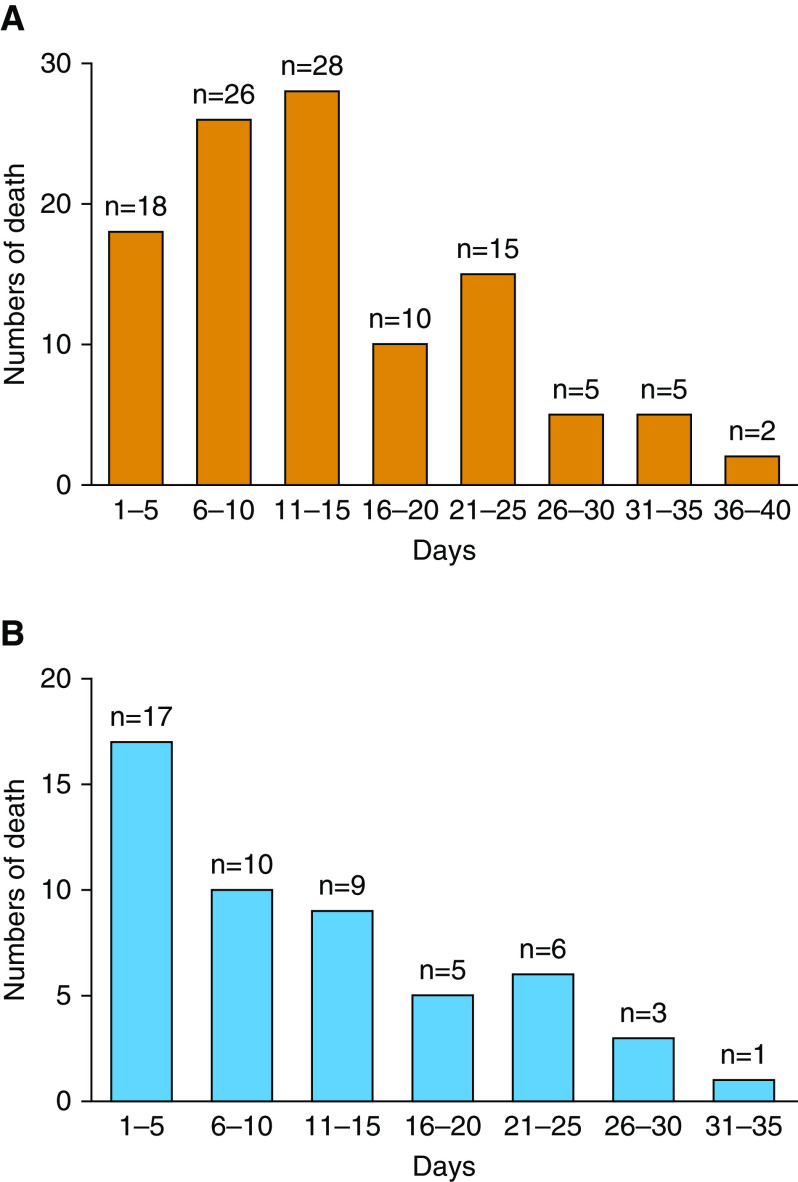
The mean duration that patients with COVID-19 pneumonia had to wait for a hospital bed was 9.7 days (SD = 5.3 d). One day (range, 0–4 d) after hospitalization, 51 patients had an opportunity to be transferred from a non-ICU setting to an ICU. After they were admitted to the ICU, these patients stayed there for 11.8 days (SD = 8.8 d) before they died. Overall, the mean time from onset of symptoms to death was 22.3 days (SD = 9.2 d) (Table 3). As shown in Figure 1, 66.1% of the patients (72/109) died in the first 15 days after hospitalization, and 70.6% (36/51) died in the first 15 days after ICU admission. As expected, patients in the ICU group lived longer than those in the non-ICU group (Table 3). Given that the surge in patients requiring ICU admission was overwhelming, and that there were no specific decision rules, risk scores, or criteria for deciding who should receive ICU admission in China, our physicians made the decision at their sole discretion.
Table 3.
Treatments in 109 decedents with COVID-19 pneumonia
| Treatment | Total (N = 109) | ICU (n = 51) | Non-ICU (n = 58) | P Value |
|---|---|---|---|---|
| Onset of symptom to, mean ± SD, d | ||||
| Hospitalization* | 9.7 ± 5.3 | 10.5 ± 4.9 | 9.0 ± 5.5 | 0.128 |
| ICU admission | 14.0 ± 7.6 | 14.0 ± 7.6 | — | — |
| Death | 22.3 ± 9.2 | 24.8 ± 9.4 | 20.1 ± 8.5 | 0.007 |
| Period of hospitalization, mean ± SD, d | 14.1 ± 8.8 | 15.9 ± 8.8 | 12.5 ± 8.6 | 0.044 |
| ICU admission | ||||
| n (%) | 51 (46.8) | 51 (100) | — | — |
| Period, d | 11.8 ± 8.8 | 11.8 ± 8.8 | — | — |
| Period of hospitalization to ICU, d | 1.0 (0–4.0) | 1.0 (0–4.0) | — | — |
| Antibiotics, n (%) | 109 (100) | 51 (100) | 58 (100) | — |
| Antiviral drugs, n (%) | 103 (94.5) | 49 (96.1) | 54 (93.1) | 0.796 |
| Antifungal drugs, n (%) | 20 (18.3) | 20 (39.2) | 0 (0) | <0.001 |
| Glucocorticoids, n (%) | 83 (76.1) | 34 (66.7) | 49 (84.5) | 0.029 |
| Intravenous immune globulin, n (%) | 66 (60.6) | 41 (80.4) | 25 (43.1) | <0.001 |
| Anticoagulant therapy, n (%) | 42 (38.5) | 26 (51.0) | 16 (27.6) | 0.012 |
| Oxygen therapy | ||||
| High-flow nasal cannula oxygen therapy, n (%) | 43 (39.4) | 21 (41.2) | 22 (37.9) | 0.729 |
| Mechanical ventilation, n (%) | ||||
| Noninvasive | 64 (58.7) | 39 (76.5) | 25 (43.1) | <0.001 |
| Invasive | 33 (30.3) | 33 (64.7) | 0 (0) | <0.001 |
| Continuous renal-replacement therapy, n (%) | 12 (11.0) | 10 (19.6) | 2 (3.4) | 0.007 |
| Extracorporeal membrane oxygenation, n (%) | 7 (6.4) | 7 (13.7) | 0 (0) | 0.012 |
Definition of abbreviations: COVID-19 = coronavirus disease; ICU = intensive care unit; SD = standard deviation.
Comparisons were determined by Student’s t test or χ2 test as appropriate.
Data were obtained from 107 patients, as 2 patients were hospitalized because of other diseases; n = 50 in the ICU group and n = 57 in the non-ICU group.
Figure 1.
(A and B) Distribution of time to death after hospitalization (A, N = 109) and after admission to an intensive care unit (B, n = 51) among decedents with coronavirus disease (COVID-19) pneumonia.
As shown in Table 3, all patients (100%) were administered antibiotics to prevent or treat coexisting or secondary bacterial infection, and almost all patients in the ICU group (96.1%) and non-ICU group (93.1%) were administered antiviral drugs (oseltamivir or peramivir) to treat possible influenza. Twenty of the patients (39.2%) in the ICU group, but none in the non-ICU group, were given antifungal drugs. However, actual lung bacterial or fungal infections were documented in only 42 patients (38.5%).
In the hope of alleviating a severe inflammatory response, glucocorticoids were given to 83 patients (76.1%), and to enhance immunocompetence, intravenous immune globulin was given to 66 patients (60.6%) (Table 3). As indicated by an elevation of D-dimer and extension of prothrombin time and/or activated partial thromboplastin time, anticoagulant therapy was given to 42 patients (38.5%). In addition, immune globulin and anticoagulant therapy was given to more patients in the ICU group than in the non-ICU group (Table 3).
In response to rapidly progressive hypoxemia, oxygen therapy was provided for all patients, including high-flow nasal cannula oxygen therapy (39.4%), noninvasive mechanical ventilation (58.7%), and invasive ventilation (30.3%). In addition, 12 patients (11.0%) received continuous renal-replacement therapy, and 7 (6.4%) received extracorporeal membrane oxygenation (Table 3). In our settings, invasive ventilation and extracorporeal membrane oxygenation could only be performed in the ICU. All 51 patients who were admitted to the ICU needed invasive mechanical ventilation; unfortunately, owing to a lack of ventilators, only 33 (64.7%) received it.
Discussion
This multicenter observational study of 109 decedents indicated that mortality due to COVID-19 pneumonia was concentrated in people above the age of 65 years, especially those with major comorbidities, and that 51 patients who were admitted to an ICU lived longer than 58 patients who were not. Healthcare systems must address the difficult issue of allocating ICU resources when facing overwhelming numbers of critically ill patients. Our three designated COVID-19 hospitals had a total of 1,379 beds; however, only 43 ICU beds were available for patients with severe COVID-19 pneumonia. All 51 patients who were admitted to the ICU died there; because of the rapidly progressive nature of this disease, none had a chance to be transferred to a general ward. Given that the number of critically ill patients with COVID-19 pneumonia far exceeded the number of ICU beds, and that no specific decision rules, risk scores, or criteria were available to determine who should receive ICU admission, our physicians made the decision at their sole discretion. Usually, during the study period, only after someone in an ICU died could the next patient be transferred into that ICU. Occasionally (if at all), the physicians tended to allocate ICU care to the younger patients, as they seemed to have a greater chance of surviving.
It is not surprising that there were approximately twice as many men as women among the nonsurvivors with COVID-19 pneumonia, as in most infectious diseases and related conditions, such as sepsis and septic shock, men always account for a larger proportion of cases and have a higher mortality (10, 11). In a single-center retrospective study, 32 nonsurvivors were of older age and more likely to have a preexisting chronic medical illness (12). In the present study, we noted that only 3 (2.8%) of the decedents were younger than 50 years, and 83 (76.1%) were above the age of 65 years. We also noted that most patients suffered from underlying comorbidities, with the most common ones being hypertension, cardiovascular or cerebrovascular diseases, diabetes, and chronic digestive disorders. In contrast, severe disease and mortality from 2009 influenza A (H1N1) infection is concentrated in relatively healthy adolescents and adults between the ages of 10 and 60 years without major comorbidities (13–15).
A pathological study of a patient who died from COVID-19 pneumonia revealed bilateral diffuse alveolar damage with cellular fibromyxoid exudates, interstitial lymphocyte infiltrates, and multinucleated syncytial cells in the intraalveolar spaces (16). Consistent with these extensive pathological abnormalities, widespread bilateral ground-glass opacification and/or consolidation on pulmonary computed tomography were present in all decedents with COVID-19 pneumonia, accounting for the fact that dyspnea was a remarkable symptom. Unfortunately, because these patients had very severe disease, none of them had the chance to undergo a second computed tomography scan. These patients experienced symptoms for a median of ∼10 days before they were hospitalized, but rapidly worsened and required care in the ICU within a short time. Some patients with severe disease died right after they arrived at the hospital, and some died in general wards before they were admitted to the ICU at Tianyou Hospital or Central Hospital, or were transferred to Pulmonary Hospital or Wuhan Jinyintan Hospital for critical care. Although 45 patients in Pulmonary Hospital had an opportunity to receive advanced ventilatory support and rescue therapies for profound hypoxemic respiratory failure, including noninvasive ventilation, invasive ventilation, high-frequency oscillatory ventilation, and extracorporeal membrane oxygenation, they did not survive owing to rapidly progressive multiple organ failure, especially respiratory failure and heart failure.
No antiviral drug, antibiotic, antifungal drug, corticosteroid treatment, or immune globulin is routinely recommended for treatment of COVID-19 pneumonia (7); however, a combination of two or more of these drugs was given to all patients included in the study. Antiviral drugs were prescribed because a diagnosis of influenza had been suspected or could not be excluded before the SARS-CoV-2 test result was available. Empirical broad-spectrum antibacterial agents and/or antifungal regimens were initiated because of a suspicion of community-acquired bacterial pneumonia and/or secondary bacterial and fungal infection. However, actual lung bacterial or fungal infections were documented in only 42 patients (38.5%) in the late stage of disease. It was believed that corticosteroids might be able to alleviate the inflammatory response in the patients’ lungs, and therefore were given to most patients.
As mentioned above, patients who were admitted to an ICU lived longer than those who were not, because the ICU group received more active therapies, including mechanical ventilation, continuous renal-replacement therapy, and extracorporeal membrane oxygenation. Unfortunately, all 51 patients who were admitted to an ICU eventually died there; because of the rapidly progressive nature of this disease, none had a chance to be transferred to a general ward. It is possible that some of these patients could have survived if they had received earlier ICU care, especially earlier incubation and invasive ventilation.
This study has a major strength. It represents the largest and most detailed series of nonsurvivors of severe COVID-19 pneumonia yet described. These observations of typical clinical features, laboratory findings, and responses to therapy should aid in the recognition and clinical management of such infections, especially with regard to ICU resource allocation. This study also has major limitations. One is that we did not provide comparisons of nonsurvivors and survivors in this observational study. Another major limitation is that only decedents from three designated COVID-19 hospitals were included in the present study. Actually, there were 35 such hospitals in the urban districts of Wuhan.
In conclusion, our analysis of a multicenter cohort of decedents with COVID-19 pneumonia reveals that deaths were concentrated in an older patient group, especially those with one or more underlying diseases. Because multiple organ failure, especially respiratory failure and heart failure, occurred rapidly after hospital admission, ICU care should be provided as soon as possible for patients with severe COVID-19 pneumonia. A social distancing policy should be proposed to slow the rate of cases and prevent healthcare systems from being overwhelmed by patients for whom they cannot provide ICU care.
Supplementary Material
Footnotes
Supported by Beijing Municipal Administration of Hospitals’ Mission Plan, China (SML20150301), and the 1351 Talents Program of Beijing Chao-Yang Hospital, China (WXZXZ-2017-01).
Author Contributions: H.-Z.S. conceived the idea, designed and supervised the study, drafted the manuscript, had full access to all of the data, and takes responsibility for the integrity of the data. R.-H.D., L.-M.L., W.Y., L.-L.G., M.-L.Y., Y.-L.L., Y.H., and X.-Y.L. collected data. W.W., B.S., and P.P. analyzed data and performed statistical analyses. All authors reviewed and approved the final version of the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Johns Hopkins University CSSE. Wuhan coronavirus (2019-ncov) global cases[accessed 2020 Mar 30]. Available from: https://gisanddata.Maps.Arcgis.Com/apps/opsdashboard/index.Html#/bda7594740fd40299423467b48e9ecf6
- 2.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. doi: 10.1001/jamainternmed.2020.0994. [online ahead of print] 13 Mar 2020; DOI: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: interim guidance; 2020[accessed 2020 Feb 26]. Available from: https://www.Who.Int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected
- 8.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. doi: 10.1001/jama.2020.1585. [online ahead of print] 7 Feb 2020; DOI: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. [online ahead of print] 28 Feb 2020; DOI: 10.1056/NEJMoa2002032. [Google Scholar]
- 10.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 11.Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 12.Yang XB, Yu Y, Xu JQ, Shu HQ, Xia JA, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:P475–P481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fowler RA, Lapinsky SE, Hallett D, Detsky AS, Sibbald WJ, Slutsky AS, et al. Toronto SARS Critical Care Group. Critically ill patients with severe acute respiratory syndrome. JAMA. 2003;290:367–373. doi: 10.1001/jama.290.3.367. [DOI] [PubMed] [Google Scholar]
- 14.Kumar A, Zarychanski R, Pinto R, Cook DJ, Marshall J, Lacroix J, et al. Canadian Critical Care Trials Group H1N1 Collaborative. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA. 2009;302:1872–1879. doi: 10.1001/jama.2009.1496. [DOI] [PubMed] [Google Scholar]
- 15.Domínguez-Cherit G, Lapinsky SE, Macias AE, Pinto R, Espinosa-Perez L, de la Torre A, et al. Critically ill patients with 2009 influenza A(H1N1) in Mexico. JAMA. 2009;302:1880–1887. doi: 10.1001/jama.2009.1536. [DOI] [PubMed] [Google Scholar]
- 16.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.