Abstract
Free full text

The 9 + 2 Axoneme Anchors Multiple Inner Arm Dyneins and a Network of Kinases and Phosphatases That Control Motility
Cilia and flagella are found on a variety of cell types, ranging from single cell protozoa and sperm to the ciliated epithelia of the respiratory and reproductive tracts. Despite this diversity, most motile cilia and flagella contain a highly ordered structure, the 9 + 2 axoneme (Fig. 1 A), which is composed of >250 distinct, but well conserved polypeptides (Luck 1984). Ciliary motility is generated by the dynein-driven sliding of outer doublet microtubules. Defects in the dynein motors or components that regulate their activity can have profound consequences; in vertebrates, these include infertility, respiratory disease, and defects in the determination of the left–right axis during embryonic development (Afzelius 1999; Supp et al. 2000). The biochemical complexity of the organelle has made it challenging to identify the relevant loci by traditional mapping methods (Blouin et al. 2000). However, significant progress is being made using candidate genes identified in model organisms. For instance, it is much simpler to analyze mutations affecting flagellar motility in Chlamydomonas using biochemical, structural, and molecular approaches (Fig. 1 A; Dutcher 1995; Mitchell 2000), and then identify related genes with similar functions in other species (Neilson et al. 1999; Pennarun et al. 1999).
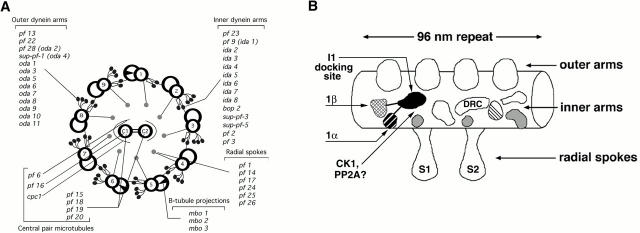
(A) Schematic diagram of the flagellar axoneme in cross-section. The Chlamydomonas mutations that affect the assembly or function of specific structures are also indicated. The inner and outer dynein arms are multisubunit ATPases that generate relative sliding movements between the outer doublet microtubules. The interdoublet sliding is normally constrained by interdoublet linkages and attachment to the basal body, leading to flagellar bending. The radial spokes and central pair microtubules with their associated projections coordinate the dynein-induced sliding to generate a variety of waveforms. (B) The arrangement of the inner dynein arms and other structures on the A-tubule of the outer doublet, as viewed from the B-tubule of the adjacent doublet. The structures repeat along the length of the outer doublet microtubule with a 96-nm periodicity, referred to as the 96-nm repeat. The proximal end (adjacent to the cell body) is to the left. The outer arms (OA) are on the top and the radial spokes (RS) are on the bottom. The I1 dynein is the trilobed structure proximal to the first radial spoke in each repeat. The three p28-associated dyneins are correlated with the three gray lobes. The crescent-shaped structure above the second radial spoke (S2) is associated with the DRC. One of the centrin-associated dyneins is located in the adjacent striped lobe. The postulated locations of CK1 and PP2A are also indicated.
In this review, we discuss recent findings, primarily in Chlamydomonas, that are providing new insights into the regulation of dynein-based motility within the axoneme. The inner arms, which are both necessary and sufficient to generate flagellar bends, determine the size and shape of the waveform; the outer dynein arms add power and increase beat frequency approximately twofold (Brokaw and Kamiya 1987). As the structure and regulation of the outer dynein arms recently have been reviewed (Satir 1998; King 2000), we focus here on components that control inner arm activity through structural interactions and enzymatic phosphorylation. We first review the evidence that each inner arm dynein plays a distinct role in the generation and control of motility, and that the central pair apparatus and radial spokes (CP/RS) interact with the inner arms to control the flagellar waveform. We then discuss new evidence for a network of enzymes closely associated with the CP/RS complex that may locally modulate inner arm activity to regulate motility.
Diversity and Organization of the Inner Dynein Arms
The inner dynein arms are both structurally and functionally diverse. Based on sequence homologies and expression studies, there appear to be 11 inner arm dynein heavy chain (DHC) genes (Gibbons 1995; Porter et al. 1996, Porter et al. 1999), but thus far, only eight inner arm DHCs have been resolved biochemically in flagellar extracts (Kagami and Kamiya 1992). These are organized with various intermediate (IC) and light chains (LC) into seven distinct isoforms, one two-headed isoform (I1) and six single-headed isoforms, whose arrangement is complex, both within the 96-nm repeat and along the length of the axoneme (Fig. 1 B; Porter 1996; Taylor et al. 1999). The motility phenotypes of the dynein mutants and the in vitro motility of isolated inner arm isoforms indicate that the inner arms have distinct but complementary roles in generating motility (Brokaw 1994; Kamiya 1995; Piperno 1995). To work together efficiently and generate the diversity of flagellar waveforms, the multiple inner arm motors must be tightly coordinated with one another and the outer dynein arm (Kamiya 1995; Satir 1998).
The I1 inner arm isoform is most similar to the outer arm and cytoplasmic dyneins (Smith and Sale 1991). It is composed of two DHCs (1α and 1β), three ICs (97, 138, and 140 kD), and three LCs (8, 11, and 14 kD) (Piperno et al. 1990; Porter et al. 1992; Harrison et al. 1998) and forms a trilobed structure proximal to the first radial spoke in each 96-nm repeat (Fig. 1 B) (Goodenough and Heuser 1985; Mastronarde et al. 1992). The isolated I1 dynein binds microtubules in vitro, but it induces only slow microtubule translocation in in vitro gliding assays (Smith and Sale 1991; Kagami and Kamiya 1992). This behavior may be related to its phosphorylation state (see below).
The I1 dynein plays an important role in the control of flagellar motility. Mutations in six genes disrupt the assembly or activity of the I1 dynein, leading to defects in both flagellar waveform and phototaxis (Brokaw and Kamiya 1987; Kamiya et al. 1991; Porter et al. 1992; King and Dutcher 1997; Perrone et al. 1998). Expression of amino-terminal fragments of the DHCs in the appropriate DHC mutant background can partially rescue the motility defects by reassembly of I1 dyneins containing one full-length DHC, one DHC fragment, and the full complement of ICs and LCs (Myster et al. 1997, Myster et al. 1999; Perrone et al. 2000). Attachment of I1 to a specific site on the outer doublet is mediated by the 140-kD IC, a WD-repeat containing protein with homology to other dynein ICs (Smith and Sale 1992a; Perrone et al. 1998; Yang and Sale 1998). However, the docking proteins that form the I1 binding site in the axoneme are unknown.
At least two I1 subunits are thought to regulate I1 activity. Tctex1, a 14-kD LC that is shared with cytoplasmic dynein, interacts with several protein kinases in two-hybrid assays (Bauch et al. 1998; Campbell et al. 1998). Mutant forms of Tctex1 also appear to be involved in the assembly of defective dyneins during spermatogenesis in t-haplotype mice (Harrison et al. 1998). Most pertinent to this discussion, the phosphorylation state of the 138-kD IC (IC138) has been correlated with changes in phototaxis and microtubule sliding velocities (Habermacher and Sale 1997; King and Dutcher 1997; Yang and Sale 2000). EM analysis has localized the two DHC motor domains to two lobes of the I1 structure (Fig. 1 B, Myster et al. 1999; Perrone et al. 2000). The amino-terminal regions of the DHCs and the IC/LC complex must therefore form the third lobe, next to the first radial spoke. The enzymes that modify IC138 and alter I1 activity must also be located close to this site (Fig. 1 B, Perrone et al. 2000; Yang and Sale 2000; Yang et al. 2000).
Although less is known about the organization of the six single-headed inner arm isoforms, biochemical and functional studies indicate that they also contribute to the formation of waveform (Brokaw 1994; Kamiya 1995). Each isoform contains a distinct DHC that can be phosphorylated in vivo (Piperno and Luck 1981), and each has been shown to translocate, and in some cases rotate, microtubules with a distinct velocity (Kagami and Kamiya 1992). At least one isoform, subspecies c, is a processive motor; this behavior may be critical for controlling axonemal oscillation (Shingyoji et al. 1998; Sakakibara et al. 1999).
The six isoforms can be separated into two groups based on their association with specific LCs. Three DHCs are associated with an actin IC and a conserved 28-kD LC (p28) (LeDizet and Piperno 1995a; Kastury et al. 1997). Mutations in p28 disrupt the assembly of the three DHCs, leading to defects in waveform (Kamiya et al. 1991; LeDizet and Piperno 1995b) and the loss of three, single-lobed structures within the 96-nm repeat (Fig. 1 B, Mastronarde et al. 1992). The other three DHCs are associated with an actin IC and a 19-kD LC known as the calcium-binding protein, centrin (LeDizet and Piperno 1995a). Assembly of a centrin-associated DHC isoform is disrupted in ida6 and pf3 (Kato et al. 1993; Gardner et al. 1994). The loss of this isoform alters the flagellar waveform (Kato et al. 1993) and correlates with a defect in a structure located distal to the second radial spoke, in close proximity to the dynein regulatory complex (DRC) (Fig. 1 B; Gardner et al. 1994; C. Perrone, E. O'Toole, and M. Porter, unpublished observations). Further work is needed to determine where the remaining centrin-associated DHCs are located and whether they might be involved in calcium modulation of the flagellar waveform.
The Central Pair Apparatus and Radial Spokes Are Important Regulators of Dynein Activity
Structural and genetic evidence have implicated the central pair and radial spoke (CP/RS) structures as key regulators of dynein activity (reviewed by Smith and Sale 1994; Smith and Lefebvre 1997). The radial spokes are located in close proximity to the inner arms (Fig. 1 B, 2), and in some species, the central pair rotates during the beat cycle (reviewed by Omoto et al. 1999). The central pair microtubules are structurally asymmetric and biochemically distinct; each is associated with a unique set of projections that appear to contact the radial spoke heads (Dutcher et al. 1984; Smith and Lefebvre 1996; Mitchell and Sale 1999). One model is that the central pair projections function like a distributor to provide a local signal to the radial spokes that selectively activates subsets of dynein arms (Omoto et al. 1999). Consistent with this model, mechanically induced changes in the plane of flagellar bending have been correlated with rotation of the central pair apparatus (Shingyoji et al. 1991). Moreover, flagellar mutants lacking CP/RS structures are paralyzed under physiological conditions (Witman et al. 1978).
One function of the CP/RS structures is to regulate the velocity of dynein-driven sliding. CP/RS defective axonemes can be induced to undergo sliding disintegration in vitro, but the rate of microtubule sliding is significantly reduced (Witman et al. 1978; Smith and Sale 1992b). Reconstitution experiments suggested that these changes in sliding velocity are mediated in part by posttranslational modification of the inner arms (Smith and Sale 1992b). This work indicated the presence of a control system that inhibits dynein activity in the absence of signals from the CP/RS complex (see below). A second function of the CP/RS complex is to coordinate inner arm and outer arm activity at physiological levels of ATP (Omoto et al. 1996). CP/RS mutants can be induced to beat by lowering ATP concentrations or by modifying nucleotide or salt conditions in the reactivation media, but flagellar beating under these conditions requires the outer arms (Omoto et al. 1996; Yagi and Kamiya 2000). High levels of ATP are thought to inhibit the outer arms by binding to a regulatory site on an outer arm DHC (Omoto et al. 1996). The CP/RS complex may override ATP inhibition by activating the inner arms in a coordinated fashion.
Additional evidence for a control system that inhibits dynein activity has come from the characterization of bypass suppressor mutations that restore motility to paralyzed CP/RS mutants without restoring the missing structures (Huang et al. 1982). These second-site mutations alter other axonemal components and permit modified motility in the CP/RS mutants (Huang et al. 1982). One group of suppressors (sup-pf-1 and sup-pf-2) alters the activity of the outer arm DHCs (Porter et al. 1994; Rupp et al. 1996). The mechanism of suppression is unknown, but may involve changes in the regulatory sites that otherwise inhibit dynein activity at physiological levels of ATP (Rupp et al. 1996). A second group of suppressors (pf2, pf3, pf9-2, sup-pf-3, sup-pf-4, and sup-pf-5) alters either the inner arms and/or a subset of closely associated polypeptides known as the DRC (Huang et al. 1982; Piperno et al. 1992; Porter et al. 1992). The seven DRC polypeptides are tightly associated with the outer doublets in wild-type axonemes, but they are missing to varying degrees in the DRC mutants. EM analysis has identified a crescent-shaped structure at the base of the second radial spoke that is altered or missing in the most severely defective DRC strains (Fig. 1 B, Gardner et al. 1994). This structure appears to be ideally positioned to mediate local signals, either mechanical, chemical, or both, between the radial spokes, interdoublet linkages, and the inner and outer dynein arms (Mastronarde et al. 1992; Gardner et al. 1994; Woolley 1997).
The cloning of the PF2 gene has recently identified one DRC component as a highly coiled-coil polypeptide that is tightly associated with the outer doublets (Rupp, G., E. O'Toole, and M.E. Porter. 1999. Mol. Biol. Cell. 2128 [Abstr.]). One hypothesis is that the DRC functions as a scaffold for the attachment of regulatory enzymes that modify dynein activity (see below). A second hypothesis is that the DRC interacts with the interdoublet linkages and/or radial spokes to sense tension or strain within the axoneme and provides mechanical feedback to the dynein arms. Interestingly, homologues of PF2 are expressed in several cell types, including tissues not associated with axoneme assembly (G. Rupp and M. Porter, unpublished observations). Further work is needed to characterize additional DRC polypeptides, to determine how they interact with other axoneme components, and to evaluate whether they may regulate dynein activity elsewhere in the cell.
Identifying Kinases and Phosphatases Anchored in the Axoneme
Both pharmacological and biochemical evidence from several species have shown that ciliary and flagellar motility is regulated in part by phosphorylation (reviewed by Tash and Bracho 1994; Walczak and Nelson 1994). For example, cAMP-dependent phosphorylation of axonemal proteins activates sperm motility (e.g., Inaba et al. 1999), increases beat frequency in Paramecium cilia (Hamasaki et al. 1991), and inhibits flagellar motility in Chlamydomonas (Hasegawa et al. 1987). Moreover, several kinases and phosphatases appear to be anchored in the axoneme (Hasegawa et al. 1987; Hamasaki et al. 1989; San Agustin and Witman 1994). The challenge has been to identify the enzymes most directly involved in the control of motility, to determine how each enzyme is anchored in the axoneme, and to identify the relevant targets among the numerous (>80) phosphoproteins within the axoneme (e.g., Piperno et al. 1981; Hamasaki et al. 1991).
One successful strategy for unraveling this complexity has been to combine a pharmacological approach with microtubule sliding assays and isolated axonemes from specific Chlamydomonas flagellar mutants. This approach has revealed that the I1 dynein is regulated by phosphorylation of IC138, and that this phosphorylation state is determined by several kinases and phosphatases whose activities are controlled by the CP/RS complex (Fig. 2 A). For example, treatment of CP/RS mutant axonemes with inhibitors of cAMP-dependent protein kinase (PKA) increased microtubule sliding velocities to wild-type levels (Howard et al. 1994), which suggested that one function of the CP/RS complex is to override the inhibitory action of PKA and dephosphorylate a dynein motor. Subsequent studies using double mutants identified the I1 dynein as the critical target for rescue of microtubule sliding; hyperphosphorylation of IC138 was correlated with inhibition of activity, whereas dephosphorylation of IC138 was correlated with rescue of microtubule sliding (Habermacher and Sale 1997). Rescue of dynein activity also depended on the action of tightly bound axonemal phosphatases, as selective phosphatase inhibitors could block rescue of dynein-driven sliding by the radial spokes (Habermacher and Sale 1996). Controlled phosphorylation of IC138 might simply be used to inhibit flagellar motility. However, hyperphosphorylation of IC138 has also been associated with phototaxis defects in mia mutants, and mutants lacking the I1 complex have defects in both waveform and phototaxis (Brokaw and Kamiya 1987; Porter et al. 1992; King and Dutcher 1997). Thus, it seems likely that locally controlled phosphorylation of IC138 by the CP/RS complex is designed to modulate the flagellar waveform.
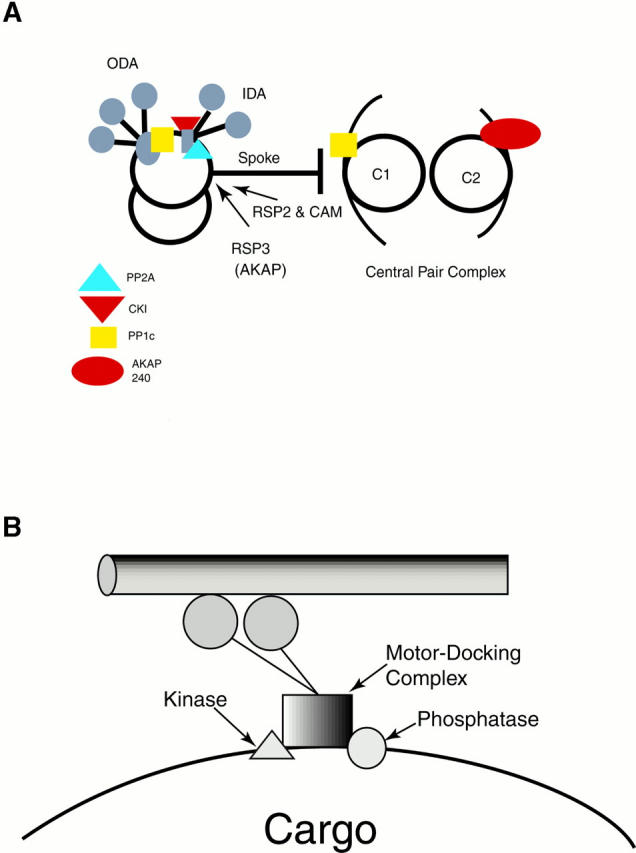
(A) Schematic diagram of the proposed location of axonemal kinases and phosphatases. The proposed locations of several axonemal kinases and phosphatases are also shown in relationship to the outer arms (ODA), the inner arms (IDA), the radial spokes, and the central pair microtubules. These positions are approximate, as they are based on indirect methods. Determining the precise locations will require direct, high resolution structural techniques. (B) General model for motor-regulatory enzyme docking structures. The protein complexes that bind motors to specific cargoes may also be adapted to bind regulatory kinases and phosphatases to the same site (see text).
Several studies have shown that PKA is tightly bound to the axoneme (Hasegawa et al. 1987; Hamasaki et al. 1989; Howard et al. 1994; San Agustin and Witman 1994; San Agustin et al. 1998), but recent work has provided new insight into its possible location(s). The A-kinase anchor proteins (AKAPs) are a diverse group of polypeptides responsible for localizing PKA by binding to its regulatory subunit (reviewed by Edwards and Scott 2000). Based on the analysis of mutant axonemes from Chlamydomonas, AKAP240 is associated with the C2 microtubule of the central pair apparatus, and AKAP97 is radial spoke protein 3 (RSP3) (Fig. 2 A; Roush, A., and W. Sale. 1998. Mol. Biol. Cell. 2306 [Abstr.]; Roush-Gaillard, A., and W. Sale. 2000. Mol. Biol. Cell. 2239 [Abstr.]). RSP3 is located at the base of the radial spoke, where it is responsible for attaching the radial spoke to the outer doublet, near the inner dynein arm (Diener et al. 1993). RSP3 therefore appears to anchor PKA at the base of the radial spoke in a signaling network designed to directly or indirectly control the phosphorylation state of the inner arms.
The identification of RSP3 as an AKAP suggests that part of the signaling circuitry linking the central pair to the dynein arms and the control of motility is built into the radial spoke structure. To test this idea, the molecular organization of the radial spokes must be better understood. Interestingly, the isolation of intact radial spoke structures has further revealed that radial spoke protein 2 (RSP2) is a novel kinase that binds calmodulin (Yang, P., and W. Sale. 2000. Mol. Biol. Cell. 2784 [Abstr.]). RSP2 was previously identified as a stable component of the radial spoke stalk (Fig. 2 A; Piperno et al. 1981). Although the targets of the RSP2 kinase are unknown, its activity might be modified either by mechanical forces within the axoneme or in response to intracellular calcium levels. This hypothesis is consistent with other results indicating that calcium may control flagellar motility by modulating interactions between the central pair and radial spokes (Bannai et al. 2000).
The axoneme also contains other kinases that control motility (Chaudhry et al. 1995). Using pharmacological and biochemical approaches, casein kinase 1 (CK1) recently has been identified in Chlamydomonas axonemes, and like PKA, CK1 inhibits I1 dynein activity (Yang and Sale 2000). CK1 is located on the outer doublet microtubules, and inhibitors of CK1, which increase dynein-driven sliding in CP/RS mutant axonemes, block phosphorylation of IC138. One likely scenario is that CK1 is anchored near the base of the I1 dynein in position to control phosphorylation of IC138 (Fig. 1 B and 2 A).
Activation of the I1 dynein by the CP/RS complex also requires the presence of tightly bound axonemal phosphatases (Habermacher and Sale 1996, Habermacher and Sale 1997), and biochemical studies have recently revealed the locations of these enzymes. The catalytic subunit of protein phosphatase type 1 (PP1c) is primarily associated with the C1 microtubule of the central pair, with a fraction also anchored to the outer doublet microtubules, in close association with the outer arms (Fig. 2 A; Yang et al. 2000; W. Sale and R. Colbran, unpublished results). Further work is needed to determine how PP1c is anchored in the axoneme and to identify the relevant PP1c substrates, but one intriguing implication of its dual location is that it may help to coordinate outer and inner arm activity. The catalytic subunit of protein phosphatase 2A (PP2A) is also anchored on the outer doublet microtubules (Yang et al. 2000). The current hypothesis is that PP2A is located at the base of the I1 complex (Fig. 1 B and 2 A), in position to dephosphorylate IC138 and thereby modify the flagellar waveform through selective activation of the I1 dynein (Yang et al. 2000). Further tests of this model will require an understanding of the relative stoichiometry of PP2A, how PP2A is anchored on the outer doublet, and whether PP2A directly controls the phosphorylation state of IC138.
A General Model for Regulation of Motor Activity
The recent work on the structure and regulation of the I1 dynein suggests a general model for the regulation of motor proteins: the docking complexes that are responsible for binding motors to cargoes may also anchor the kinases and phosphatases required for local control of motor activity (Fig. 2 B). Indeed, recent studies have shown that outer arm activity is regulated in part by components associated with the docking complex that specifies the outer arm binding site (Takada and Kamiya 1997). Similarly, the dynactin complex may play a role in regulating cytoplasmic dynein activity by anchoring enzymes that alter the phosphorylation state of the cytoplasmic dynein LCs (Kumar et al. 2000). Control of the direction of organelle transport may likewise involve kinases and phosphatases anchored on cargo along with dynein and kinesin (Reese and Haimo 2000). Additional work is clearly needed to characterize each of these docking complexes and to determine whether they share any structural or functional homologies.
Summary and Future Directions
Significant progress has been made in characterizing subunits of the I1 dynein, including the localization of functional domains within the I1 structure (Fig. 1 B) (Myster et al. 1999; Perrone et al. 1998; Perrone et al. 2000; Harrison et al. 1998; Yang and Sale 1998). Several enzymes that modify the phosphorylation state of IC138 and alter I1 activity in microtubule sliding assays have also been identified as tightly bound, axonemal polypeptides (Habermacher and Sale 1997; Yang et al. 2000; Yang and Sale 2000). PKA, CK1, and PP2A are likely to be closely associated with the base of the I1 dynein and the radial spokes as part of the signaling pathway linking the central pair apparatus to the dynein arms (Fig. 1 B and 2 A). Future studies using reactivated flagella and site-directed mutagenesis of IC138 are needed to understand how the phosphorylation state of IC138 affects the flagellar waveform.
Less is known about the function or regulation of the other inner arm dyneins, but further analysis of inner arm mutations will eventually provide insights into their contributions to motility. To this end, a new inner arm mutant, ida9, defective in assembly of only the “c” subspecies, has recently been isolated (Fujiwara, A., T. Yagi, M. Hirono, and R. Kamiya personal communication). Continued biophysical studies of the individual inner arm isoforms will also be critical for understanding their mechanochemical properties and how these properties might be altered by phosphorylation (Sakakibara et al. 1999).
The cloning of PF2 (Rupp, G., E. O'Toole, and M.E. Porter. 1999. Mol. Biol. Cell. 2128 [Abstr.]) and the recent isolation of the radial spokes (Yang, P., and W. Sale. 1999. Mol. Biol. Cell. 2244 [Abstr.]) will permit more direct biochemical analyses of both the DRC and the radial spokes. The relationship of these structures to the growing network of axonemal kinases and phosphatases can now be examined. One view is that mechanical interactions between the central pair and the radial spokes are converted into a biochemical signaling pathway that ultimately alters the phosphorylation state of the different dynein isoforms at the level of the 96-nm axoneme repeat. The kinases and phosphatases responsible for these events appear to be bound at discrete sites on the axoneme (Fig. 1 B and 2 A). Additional work is needed to identify the components that anchor the kinases and phosphatases to the axoneme, and to define more precisely their positions relative to the dynein arms. Given the numerous structural and functional homologies between axonemal and cytoplasmic dyneins, these studies will also provide new insights into the basic mechanisms that regulate the activity of the cytoplasmic dynein isoforms.
Acknowledgments
We would like to thank Ritsu Kamiya, Gerald Rupp, Cathy Perrone, Eileen O'Toole, and Anne Roush, and Pinfen Yang for sharing unpublished data. We also thank Tom Hays, Cathy Perrone, Anne Roush, Pinfen Yang, and our reviewers for their thoughtful comments on the manuscript.
Work in our laboratories is supported by grants from the National Institutes of Health General Medical Sciences to M. Porter (GM55667) and W. Sale (GM 51173), and from the March of Dimes Birth Defects Foundation to W. Sale.
Footnotes
Abbreviations used in this paper: AKAP, A-kinase anchoring protein; CK1, casein kinase 1; CP/RS, central pair and radial spoke; DHC, dynein heavy chain; DRC, dynein regulatory complex; IC, intermediate chain; IC138, 138-kD intermediate chain; LC, light chain; p28, 28-kD light chain; PKA, cAMP-dependent protein kinase; PP1c, protein phosphatase type 1 catalytic subunit; PP2A, protein phosphatase type 2A; RSP2, radial spoke protein 2; RSP3, radial spoke protein 3.
References
- Afzelius B.A. Asymmetry of cilia and of mice and men. Int. J. Dev. Biol. 1999;43:283–286. [Abstract] [Google Scholar]
- Bannai H., Yoshimura M., Takahashi K., Shingyoji C. Calcium regulation of microtubule sliding in reactivated sea urchin sperm flagella. J. Cell. Sci. 2000;113:831–839. [Abstract] [Google Scholar]
- Bauch A., Campbell K.S., Reth M. Interaction of the CD5 cytoplasmic domain with the Ca2+/calmodulin-dependent kinase IIδ Eur. J. Immunol. 1998;28:2167–2177. [Abstract] [Google Scholar]
- Blouin J.L., Meeks M., Radhakrishna U., Sainsbury A., Gehring C., Sail G.D., Bartoloni L., Dombi V., O'Rawe A., Walne A. Primary ciliary dyskinesiaa genome-wide linkage analysis reveals extensive locus heterogeneity. Eur. J. Hum. Genet. 2000;8:108–118. [Abstract] [Google Scholar]
- Brokaw C.J. Control of flagellar bendinga new agenda based on dynein diversity. Cell Motil. Cytoskel. 1994;27:150–160. [Abstract] [Google Scholar]
- Brokaw C.J., Kamiya R. Bending patterns of Chlamydomonas flagella. IV. Mutants with defects in inner and outer dynein arms indicate differences in dynein arm function. Cell. Motil. Cytoskel. 1987;8:68–75. [Abstract] [Google Scholar]
- Campbell K.S., Cooper S., Dessing M., Yates S., Buder A. Interaction of p59fyn kinase with the dynein light chain, Tctex1, and colocalization during cytokinesis. J. Immunol. 1998;161:1728–1737. [Abstract] [Google Scholar]
- Chaudhry P.S., Creagh S., Yu N., Brokaw C.J. Multiple protein kinase activities required for activation of sperm flagellar motility. Cell. Motil. Cytoskel. 1995;32:65–79. [Abstract] [Google Scholar]
- Diener D.R., Ang L.H., Rosenbaum J.L. Assembly of flagellar radial spoke proteins in Chlamydomonasidentification of the axoneme binding domain of radial spoke protein 3. J. Cell Biol. 1993;123:183–190. [Europe PMC free article] [Abstract] [Google Scholar]
- Dutcher S.K. Flagellar assembly in two hundred fifty easy-to-follow steps. Trends Genet. 1995;11:398–404. [Abstract] [Google Scholar]
- Dutcher S.K., Huang B., Luck D.J.L. Genetic dissection of the central pair microtubules of the flagella of Chlamydomonas reinhardtii . J. Cell Biol. 1984;98:229–236. [Europe PMC free article] [Abstract] [Google Scholar]
- Edwards A.S., Scott J.D. A-kinase anchoring proteinsprotein kinase A and beyond. Curr. Opin. Cell Biol. 2000;12:217–221. [Abstract] [Google Scholar]
- Gardner L.C., O'Toole E., Perrone C.A., Giddings T., Porter M.E. Components of a “dynein regulatory complex” are located at the junction between the radial spokes and the dynein arms in Chlamydomonas flagella. J. Cell Biol. 1994;127:1311–1325. [Europe PMC free article] [Abstract] [Google Scholar]
- Gibbons I.R. Dynein family of motor proteinspresent status and future questions. Cell Motil. Cytoskeleton. 1995;32:136–144. [Abstract] [Google Scholar]
- Goodenough U.W., Heuser J.E. Substructure of inner dynein arms, radial spokes, and the central pair projection complex. J. Cell Biol. 1985;100:2008–2018. [Europe PMC free article] [Abstract] [Google Scholar]
- Habermacher G., Sale W. Regulation of a flagellar dynein by axonemal type-1 phosphatase in Chlamydomonas . J. Cell Sci. 1996;109:1899–1907. [Abstract] [Google Scholar]
- Habermacher G., Sale W. Regulation of flagellar dynein by phosphorylation of a 138-kD inner arm dynein intermediate chain. J. Cell Biol. 1997;136:167–176. [Europe PMC free article] [Abstract] [Google Scholar]
- Hamasaki T., Murtaugh T.J., Satir B.H., Satir P. In vitro phosphorylation of Paramecium axonemes and permeabilized cells. Cell. Motil. Cytoskel. 1989;12:1–11. [Abstract] [Google Scholar]
- Hamasaki T., Barkalow K., Richmond J., Satir P. cAMP-stimulated phosphorylation of an axonemal polypeptide that copurifies with the 22S dynein arm regulates microtubule translocation velocity and swimming speed in Paramecium . Proc. Natl. Acad. Sci. USA. 1991;88:7918–7922. [Europe PMC free article] [Abstract] [Google Scholar]
- Harrison A., Olds-Clarke P., King S.M. Identification of the t complex-encoded cytoplasmic dynein light chain Tctex1 in inner arm I1 supports the involvement of flagellar dyneins in meiotic drive. J. Cell Biol. 1998;140:1137–1147. [Europe PMC free article] [Abstract] [Google Scholar]
- Hasegawa E., Hayashi H., Asakura S., Kamiya R. Stimulation of in vitro motility of Chlamydomonas axonemes by inhibition of cAMP-dependent phosphorylation. Cell. Motil. Cytoskel. 1987;8:302–311. [Abstract] [Google Scholar]
- Howard D.R., Habermacher G., Glass D.B., Smith E.F., Sale W.S. Regulation of Chlamydomonas flagellar dynein by an axonemal protein kinase. J. Cell Biol. 1994;127:1683–1692. [Europe PMC free article] [Abstract] [Google Scholar]
- Huang B., Ramanis Z., Luck D.J.L. Suppressor mutations in Chlamydomonas reveal a regulatory mechanism for flagellar function. Cell. 1982;28:115–124. [Abstract] [Google Scholar]
- Inaba K., Kagami O., Ogawa K. Tctex2-related outer arm dynein light chain is phosphorylated at activation of sperm motility. Bioch. Biophys. Res. Commun. 1999;256:177–183. [Abstract] [Google Scholar]
- Kagami O., Kamiya R. Translocation and rotation of microtubules caused by multiple species of Chlamydomonas inner-arm dynein. J. Cell Sci. 1992;103:653–664. [Google Scholar]
- Kamiya R. Exploring the function of inner and outer dynein arms with Chlamydomonas mutants. Cell Motil. Cytoskel. 1995;32:98–102. [Abstract] [Google Scholar]
- Kamiya R., Kurimoto E., Muto E. Two types of Chlamydomonas flagellar mutants missing different components of inner-arm dynein. J. Cell Biol. 1991;112:441–447. [Europe PMC free article] [Abstract] [Google Scholar]
- Kastury K., Taylor W.E., Gutierrez M., Ramirez L., Coucke P.J., Van Hauwe P., Van Camp G., Bhasin S. Chromosomal mapping of two members of the human dynein gene family to chromosome regions 7p15 and 11q13 near the deafness loci DFNA 5 and DFNA 11. Genomics. 1997;44:362–364. [Abstract] [Google Scholar]
- Kato T., Kagami O., Yagi T., Kamiya R. Isolation of two species of Chlamydomonas reinhardtii flagellar mutants, ida5 and ida6, that lack a newly identified heavy chain of the inner dynein arm. Cell. Struct. Funct. 1993;18:371–377. [Abstract] [Google Scholar]
- King S.J., Dutcher S.K. Phosphoregulation of an inner dynein arm complex in Chlamydomonas reinhardtii is altered in phototactic mutant strains. J. Cell Biol. 1997;136:177–191. [Europe PMC free article] [Abstract] [Google Scholar]
- King S.M. The dynein microtubule motor. Biochim. Biophys. Acta. 2000;1496:60–75. [Abstract] [Google Scholar]
- Kumar S., Lee I.H., Plamann M. Cytoplasmic dynein ATPase activity is regulated by dynactin-dependent phosphorylation. J. Biol. Chem. 2000;In press [Abstract] [Google Scholar]
- LeDizet M., Piperno G. The light chain p28 associates with a subset of inner dynein arm heavy chains in Chlamydomonas axonemes Mol. Biol. Cell 6 1995. 697 711a [Europe PMC free article] [Abstract] [Google Scholar]
- LeDizet M., Piperno G. ida4-1, ida4-2, and ida4-3 are intron splicing mutations affecting the locus encoding p28, a light chain of Chlamydomonas axonemal inner dynein arms Mol. Biol. Cell 6 1995. 713 723b [Europe PMC free article] [Abstract] [Google Scholar]
- Luck D.J.L. Genetic and biochemical dissection of the eucaryotic flagellum. J. Cell Biol. 1984;98:789–794. [Europe PMC free article] [Abstract] [Google Scholar]
- Mastronarde D.N., O'Toole E.T., McDonald K.L., McIntosh J.R., Porter M.E. Arrangement of inner dynein arms in wild-type and mutant flagella of Chlamydomonas . J. Cell Biol. 1992;118:1145–1162. [Europe PMC free article] [Abstract] [Google Scholar]
- Mitchell D.R. Chlamydomonas flagella. J. Phycol. 2000;36:261–273. [Google Scholar]
- Mitchell D.R., Sale W.S. Characterization of a Chlamydomonas insertional mutant that disrupts flagellar central pair microtubule-associated structures. J. Cell Biol. 1999;144:293–304. [Europe PMC free article] [Abstract] [Google Scholar]
- Myster S.H., Knott J.A., O'Toole E., Porter M.E. The Chlamydomonas Dhc1 gene encodes a dynein heavy chain subunit required for assembly of the I1 inner arm complex. Mol. Biol. Cell. 1997;8:607–620. [Europe PMC free article] [Abstract] [Google Scholar]
- Myster S.H., Knott J.A., Wysocki K.M., O'Toole E., Porter M.E. Domains in the 1α dynein heavy chain required for inner arm assembly and flagellar motility in Chlamydomonas . J. Cell Biol. 1999;146:801–818. [Europe PMC free article] [Abstract] [Google Scholar]
- Neilson L.I., Schneider P.A., Van Deerlin P.G., Kiriakidou M., Driscoll D.A., Pellegrini M.C., Millinder S., Yamamoto K.K., French C.K., Strauss J.F. cDNA cloning and characterization of a human sperm antigen (SPAG6) with homology to the product of the Chlamydomonas PF6 locus. Genomics. 1999;60:272–280. [Abstract] [Google Scholar]
- Omoto C.K., Yagi T., Kurimoto E., Kamiya R. Ability of paralyzed flagella mutants of Chlamydomonas to move. Cell Motil. Cytoskel. 1996;33:88–94. [Abstract] [Google Scholar]
- Omoto C.K., Gibbons I.R., Kamiya R., Shingyoji C., Takahashi K., Witman G.B. Rotation of the central pair microtubules in eukaryotic flagella. Mol. Biol. Cell. 1999;10:1–4. [Europe PMC free article] [Abstract] [Google Scholar]
- Pennarun G., Escudier E., Chapelin C., Bridoux A., Cacheux V., Roger G., Clément A., Goossens M., Amselen S., Duriez B. Loss-of-function mutations in human gene related to Chlamydomonas reinhardtii dynein IC78 result in primary ciliary dyskinesia. Am. J. Hum. Genet. 1999;65:1508–1519. [Europe PMC free article] [Abstract] [Google Scholar]
- Perrone C.A., Yang P., O'Toole E., Sale W.S., Porter M.E. The Chlamydomonas IDA7 locus encodes a 140-kDa dynein intermediate chain required to assemble the I1 inner arm complex. Mol. Biol. Cell. 1998;9:3351–3365. [Europe PMC free article] [Abstract] [Google Scholar]
- Perrone C.A., Myster S.H., Bower R., O'Toole E.T., Porter M.E. Insights into the structural organization of the I1 inner arm dynein from a domain analysis of the 1β dynein heavy chain. Mol. Biol. Cell. 2000;11:2297–2313. [Europe PMC free article] [Abstract] [Google Scholar]
- Piperno G. Regulation of dynein activity within Chlamydomonas flagella. Cell Motil. Cytoskel. 1995;32:103–105. [Abstract] [Google Scholar]
- Piperno G., Luck D.J.L. Inner arm dyneins from flagella of Chlamydomonas reinhardtii . Cell. 1981;27:331–340. [Abstract] [Google Scholar]
- Piperno G., Mead K., Shestak W. The inner dynein arms I2 interact with a “dynein regulatory complex” in Chlamydomonas flagella. J. Cell Biol. 1992;118:1455–1463. [Europe PMC free article] [Abstract] [Google Scholar]
- Piperno G., Huang B., Ramanis Z., Luck D.J.L. Radial spokes of Chlamydomonas flagellapolypeptide composition and phosphorylation of stalk components. J. Cell Biol. 1981;88:73–79. [Europe PMC free article] [Abstract] [Google Scholar]
- Piperno G., Ramanis Z., Smith E.F., Sale W.S. Three distinct inner dynein arms in Chlamydomonas flagellamolecular composition and location in the axoneme. J. Cell Biol. 1990;110:379–389. [Europe PMC free article] [Abstract] [Google Scholar]
- Porter M.E. Axonemal dyneinsassembly, organization, and regulation. Curr. Opin. Cell Biol. 1996;8:10–17. [Abstract] [Google Scholar]
- Porter M.E., Power J., Dutcher S.K. Extragenic suppressors of paralyzed flagellar mutations in Chlamydomonas reinhardtii identify loci that alter the inner dynein arms. J. Cell Biol. 1992;118:1163–1176. [Europe PMC free article] [Abstract] [Google Scholar]
- Porter M.E., Knott J.A., Gardner L.C., Mitchell D.R., Dutcher S.K. Mutations in the SUP-PF-1 locus of Chlamydomonas reinhardtii identify a regulatory domain in the β-dynein heavy chain. J. Cell Biol. 1994;126:1495–1507. [Europe PMC free article] [Abstract] [Google Scholar]
- Porter M.E., Knott J.A., Gardner L.C., Myster S.H., Farlow S.J. The dynein gene family in Chlamydomonas reinhardtii . Genetics. 1996;144:569–585. [Europe PMC free article] [Abstract] [Google Scholar]
- Porter M.E., Bower R., Knott J.A., Byrd P., Dentler W. Cytoplasmic dynein heavy chain 1b is required for flagellar assembly in Chlamydomonas . Mol. Biol. Cell. 1999;10:693–712. [Europe PMC free article] [Abstract] [Google Scholar]
- Reese E.L., Haimo L.T. Dynein, dynactin, and kinesin II's interaction with microtubules is regulated during bidirectional organelle transport. J. Cell Biol. 2000;151:155–165. [Europe PMC free article] [Abstract] [Google Scholar] Retracted
- Rupp G., O'Toole E., Gardner L.C., Mitchell B.F., Porter M.E. The sup-pf-2 mutations of Chlamydomonas alter the activity of the outer dynein arms by modification of the γ-dynein heavy chain. J. Cell Biol. 1996;135:1853–1865. [Europe PMC free article] [Abstract] [Google Scholar]
- Sakakibara H., Kojima H., Sakai Y., Katayama E., Oiwa K. Inner-arm dynein c of Chamydomonas flagella is a single-headed processive motor. Nature. 1999;400:586–590. [Abstract] [Google Scholar]
- San Agustin J.T., Witman G.B. Role of cAMP in the reactivation of demembranated ram spermatozoa. Cell Motil. Cytoskel. 1994;27:206–218. [Abstract] [Google Scholar]
- San Agustin J.T., Leszyk J.D., Nuwaysir L.M., Witman G.B. The catalytic subunit of the cAMP-dependent protein kinase of ovine sperm flagella has a unique amino-terminal sequence. J. Biol. Chem. 1998;273:24874–24883. [Abstract] [Google Scholar]
- Satir P. Mechanisms of ciliary motilityan update. Eur. J. Protistol. 1998;34:267–272. [Google Scholar]
- Shingyoji C., Takahashi J., Gibbons I.R. Rotating the plane of imposed vibration can rotate the plane of flagellar beating in sea-urchin sperm without twisting the axoneme. J. Cell. Sci. 1991;98:175–181. [Abstract] [Google Scholar]
- Shingyoji C., Higuchi H., Yoshimura M., Katayama E., Yanagida T. Dynein arms are oscillating force generators. Nature. 1998;393:711–714. [Abstract] [Google Scholar]
- Smith E.F., Lefebvre P.A. PF16 encodes a protein with armadillo repeats and localizes to a single microtubule of the central apparatus in Chlamydomonas flagella. J. Cell Biol. 1996;132:359–370. [Europe PMC free article] [Abstract] [Google Scholar]
- Smith E.F., Lefebvre P.A. The role of central apparatus components in flagellar motility and microtubule assembly. Cell. Motil. Cytoskel. 1997;38:1–8. [Abstract] [Google Scholar]
- Smith E.F., Sale W.S. Microtubule binding and translocation by inner dynein arm subtype I1. Cell. Motil. Cytoskel. 1991;18:258–268. [Abstract] [Google Scholar]
- Smith E.F., Sale W.S. Structural and functional reconstitution of inner dynein arms in Chlamydomonas flagellar axonemes J. Cell Biol. 117 1992. 573 581a [Europe PMC free article] [Abstract] [Google Scholar]
- Smith E.F., Sale W.S. Regulation of dynein-driven microtubule sliding by the radial spokes in flagella Science 257 1992. 1557 1559b [Abstract] [Google Scholar]
- Smith E.F., Sale W.S. Mechanisms of flagellar movementfunctional interactions between dynein arms and the radial spoke-central apparatus complex. In: Hyams J.S., Lloyd C.W., editors. Microtubules. John Wiley & Sons, Inc.; New York: 1994. pp. 381–392. [Google Scholar]
- Supp D.M., Potter S.S., Brueckner M. Molecular motorsthe driving force behind mammalian left–right development. Trends Cell Biol. 2000;10:41–45. [Abstract] [Google Scholar]
- Takada S., Kamiya R. Beat frequency difference between the two flagella of Chlamydomonas depends on the attachment site of outer dynein arms on the outer-doublet microtubules. Cell. Motil. Cytoskel. 1997;36:68–75. [Abstract] [Google Scholar]
- Tash J.S., Bracho G.E. Regulation of sperm motilityemerging evidence for a major role for protein phosphatases. J. Androl. 1994;15:505–509. [Abstract] [Google Scholar]
- Taylor H.C., Satir P., Holwill M.E.J. Assessment of inner dynein arm structure and possible function in ciliary axonemes. Cell. Motil. Cytoskel. 1999;43:167–177. [Abstract] [Google Scholar]
- Walczak C.E., Nelson D.L. Regulation of dynein-driven motility in cilia and flagella. Cell Motil. Cytoskel. 1994;27:101–107. [Abstract] [Google Scholar]
- Witman G.B., Plummer J., Sander G. Chlamydomonas flagellar mutants lacking radial spokes and central tubules. J. Cell Biol. 1978;76:729–747. [Europe PMC free article] [Abstract] [Google Scholar]
- Woolley D.M. Studies on the eel sperm flagellum. I. The structure of the inner dynein arm complex. J. Cell Sci. 1997;110:85–94. [Abstract] [Google Scholar]
- Yagi T., Kamiya R. Vigorous beating of Chlamydomonas axonemes lacking central pair/radial spoke structures in the presence of salts and organic compounds. Cell. Motil. Cytoskel. 2000;46:190–199. [Abstract] [Google Scholar]
- Yang P., Sale W. The Mr 140,000 intermediate chain of Chlamydomonas flagellar inner arm dynein is a WD-repeat protein implicated in dynein arm anchoring. Mol. Biol. Cell. 1998;9:3335–3349. [Europe PMC free article] [Abstract] [Google Scholar]
- Yang P., Sale W.S. Casein kinase I is anchored on axonemal doublet microtubules and regulates flagellar dynein phosphorylation and activity. J. Biol. Chem. 2000;275:18905–18912. [Abstract] [Google Scholar]
- Yang P., Fox L., Colbran R.J., Sale W.S. Protein phosphatases PP1 and PP2A are located in distinct positions in the Chlamydomonas flagellar axoneme. J. Cell Sci. 2000;113:91–102. [Abstract] [Google Scholar]
Articles from The Journal of Cell Biology are provided here courtesy of The Rockefeller University Press
Full text links
Read article at publisher's site: https://doi.org/10.1083/jcb.151.5.f37
Read article for free, from open access legal sources, via Unpaywall:
https://rupress.org/jcb/article-pdf/151/5/F37/1294757/0007057.pdf
Citations & impact
Impact metrics
Article citations
Protofilament-specific nanopatterns of tubulin post-translational modifications regulate the mechanics of ciliary beating.
Curr Biol, 34(19):4464-4475.e9, 12 Sep 2024
Cited by: 1 article | PMID: 39270640 | PMCID: PMC11466076
Biallelic Variants in MNS1 Are Associated with Laterality Defects and Respiratory Involvement.
Cells, 13(12):1017, 11 Jun 2024
Cited by: 0 articles | PMID: 38920647 | PMCID: PMC11202006
Effect of α-tubulin acetylation on the doublet microtubule structure.
Elife, 12:RP92219, 10 Apr 2024
Cited by: 0 articles | PMID: 38598282 | PMCID: PMC11006419
Light-controlled soft bio-microrobot.
Light Sci Appl, 13(1):55, 26 Feb 2024
Cited by: 4 articles | PMID: 38403642 | PMCID: PMC10894875
Primary cilia: a novel research approach to overcome anticancer drug resistance.
Front Mol Biosci, 10:1270639, 02 Oct 2023
Cited by: 2 articles | PMID: 37900915 | PMCID: PMC10602908
Review Free full text in Europe PMC
Go to all (220) article citations
Other citations
Wikipedia
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The regulation of dynein-driven microtubule sliding in Chlamydomonas flagella by axonemal kinases and phosphatases.
Methods Cell Biol, 92:133-151, 21 Nov 2009
Cited by: 9 articles | PMID: 20409803
Regulation of ciliary motility: conserved protein kinases and phosphatases are targeted and anchored in the ciliary axoneme.
Arch Biochem Biophys, 510(2):93-100, 14 Apr 2011
Cited by: 42 articles | PMID: 21513695 | PMCID: PMC3114296
Review Free full text in Europe PMC
Casein kinase I is anchored on axonemal doublet microtubules and regulates flagellar dynein phosphorylation and activity.
J Biol Chem, 275(25):18905-18912, 01 Jun 2000
Cited by: 65 articles | PMID: 10858448
Functional binding of inner-arm dyneins with demembranated flagella of Chlamydomonas mutants.
Cell Motil Cytoskeleton, 63(5):258-265, 01 May 2006
Cited by: 6 articles | PMID: 16518818
Funding
Funders who supported this work.
NIGMS NIH HHS (6)
Grant ID: GM 51173
Grant ID: R37 GM055667
Grant ID: R01 GM051173
Grant ID: R01 GM055667
Grant ID: R37 GM051173
Grant ID: GM55667





