Abstract
Free full text

Structural organization of the endoplasmic reticulum
Abstract
The endoplasmic reticulum (ER) is a continuous membrane system but consists of various domains that perform different functions. Structurally distinct domains of this organelle include the nuclear envelope (NE), the rough and smooth ER, and the regions that contact other organelles. The establishment of these domains and the targeting of proteins to them are understood to varying degrees. Despite its complexity, the ER is a dynamic structure. In mitosis it must be divided between daughter cells and domains must be re-established, and even in interphase it is constantly rearranged as tubules extend along the cytoskeleton. Throughout these rearrangements the ER maintains its basic structure. How this is accomplished remains mysterious, but some insight has been gained from in vitro systems.
Introduction
The endoplasmic reticulum (ER) has many different functions. These include the translocation of proteins (such as secretory proteins) across the ER membrane; the integration of proteins into the membrane; the folding and modification of proteins in the ER lumen; the synthesis of phospholipids and steroids on the cytosolic side of the ER membrane; and the storage of calcium ions in the ER lumen and their regulated release into the cytosol (reviewed in Matlack et al., 1998; Meldolesim and Pozzan, 1998; Ma and Hendershot, 2001; McMaster, 2001). These functions have been studied extensively. Here, we concentrate on structural and other aspects of the ER that are less well understood.
ER shape
At the light microscopy level, when stained by fluorescent dyes, or with antibodies, when marked with GFP-tagged proteins, the interphase ER can be divided into nuclear and peripheral ER (Figure 1A and andC).C). The nuclear ER, or nuclear envelope (NE), consists of two sheets of membranes with a lumen (Figure 2). The NE surrounds the nucleus, with the inner and outer membranes connecting only at the nuclear pores. It is underlaid by a network of lamins. The peripheral ER is a network of interconnected tubules that extends throughout the cell cytoplasm (Figure 1A; Terasaki and Jaffe, 1991). In some cell types, such as sea urchin eggs, flat sheets are also abundant (Terasaki and Jaffe, 1991). The lumenal space of the peripheral ER is continuous with that of the nuclear envelope and together they can comprise >10% of the total cell volume. In Saccharomyces cerevisiae, the peripheral, tubular ER network is located exclusively underneath the plasma membrane, and about a dozen large tubules connect it to the membrane sheets of the NE (Koning et al., 1993; Prinz et al., 2000; Figure 1C).

Ultrastructure of the RER, SER and NE. (A) Localization of a GFP–ER fusion protein (GFP-Sec61β) expressed in a COS cell and visualized by epifluorescence microscopy. The fine ER network is particularly clear in the thin edges of the cell periphery; three-way junctions and polygonal reticulum are easily visualized. (B) An electron micrograph of a liver cell shows RER (rough reticulum) and patches of SER (smooth reticulum) (picture taken from Fawcett, 1966, with copyright permission from the publisher, W.B. Saunders Co.). (C) Localization of GFP–ER fusion protein (GFPsec63) expressed in yeast and visualized by fluorescence microscopy outlines the structure of the ER (the upper picture focuses on the middle of the cell, the lower on the top of the cell; taken from Prinz et al., 2001, with copyright permission of the Rockefeller University Press). Several tubules (arrow in top panel) connect the outer NE (top panel) to the peripheral ER (bottom panel).
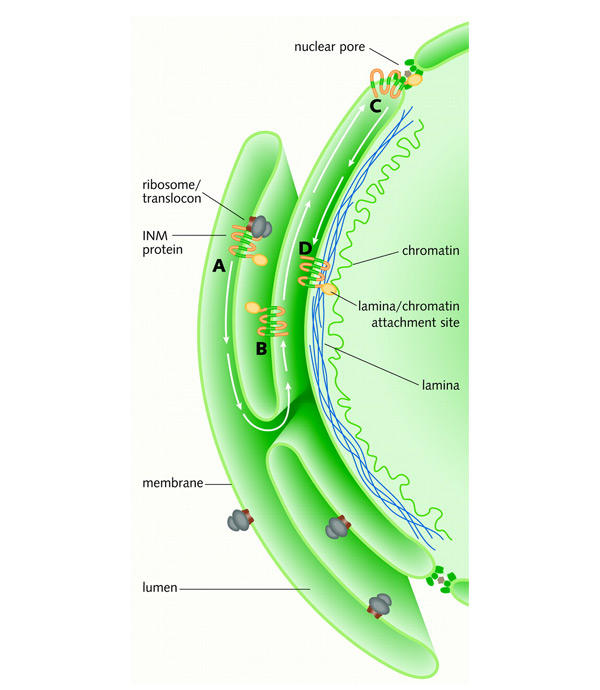
Targeting and retention of an inner nuclear membrane (INM) protein. (A) The newly translated protein is translocated into the ER membrane. (B) It diffuses through the peripheral ER. (C) It then diffuses through the nuclear pore membrane to the inner nuclear envelope. (D) Retention of the INM protein as a result of its binding to the nuclear lamina (green) and chromatin (yellow).
The ultrastructure of the ER has been visualized by electron microscopy in a number of cell types. The most obvious difference seen is between rough, i.e. ribosome-studded, and smooth regions of the ER (RER and SER, respectively; Figure 1B). The RER often has a tubular appearance, whereas the SER is often more dilated and convoluted (for a review, see Baumann and Walz, 2001). The relative abundance of RER and SER found among different cell types correlates with their functions. For example, cells that secrete a large percentage of their synthesized proteins contain mostly RER.
The ER is a single compartment
Several approaches have provided evidence that the ER is a single membrane system with a continuous intralumenal space. In one experiment, a fluorescent dye that cannot exchange between discontinuous membranes was injected into cells in an oil droplet. The dye diffused throughout the cell in a membrane network that, based on morphological criteria, was the ER. This was observed in a number of different cell types including sea urchin eggs (Terasaki and Jaffe, 1991), starfish oocytes (Jaffe and Terasaki, 1994) and Purkinje neurons (Terasaki et al., 1994). Because the dye spread in fixed as well as live cells it must be diffusing through a continuous network rather than being transported by active trafficking (Terasaki et al., 1994). In another type of experiment, GFP-tagged proteins were targeted either to the lumen or membrane of the ER, and then one region of the cell was repeatedly bleached (fluorescence loss in photobleaching, FLIP). All fluorescence was rapidly lost from the entire cell (Cole et al., 1996; Subramanian and Meyer, 1997; Dayel and Verkman, 1999; Terasaki, 2000).
Creating domains in the ER—the nuclear envelope
How can different ER domains, such as the NE or the RER, be generated in a continuous membrane system? For the NE, domain establishment is attributed to a set of proteins that is concentrated within the inner membrane. In mammalian cells, these include the lamin B receptor (LBR), lamin-associated proteins 1 and 2 (LAP1 and 2), emerin, MAN1, nurim (for review, see Holmer and Worman, 2001), LUMA and a murine protein related to UNC-84 (Dreger et al., 2001). The property that unites all of these proteins is direct or indirect attachment to nuclear structures, like the lamina or chromatin, as indicated by either resistance to detergent extraction or slow fluorescence recovery after photobleaching (FRAP) (Ellenberg et al., 1997; Rolls et al., 1999; Dreger et al., 2001). The only sequence or structural features these proteins share is an N-terminal nucleoplasmic domain of >200 amino acids found in LBR, LAP1 and 2, emerin and MAN1, and a short stretch of sequence similarity within this domain in LAP2, emerin and MAN1 (LEM domain).
Inner NE proteins are synthesized on the RER. The classical nuclear localization sequences (NLSs) that target soluble nuclear proteins to the nucleus are unable to target membrane proteins to the NE (Soullam and Worman, 1995). Instead, these proteins appear to travel through the continuous outer NE and enter into the inner NE membrane by diffusion through the pore membrane (Figure 2). It is believed that once the proteins arrive in the inner membrane, they are retained by their association with lamins and/or chromatin (Soullam and Worman, 1995). In support of this hypothesis, the N-terminal region of LBR that is responsible for its accumulation in the inner membrane contains lamin- and chromatin-binding determinants (Ye and Worman, 1994). The inner NE membrane proteins that bind directly to lamins or chromatin are likely to be responsible for establishing and maintaining NE structure. This is supported by their involvement in NE assembly in vitro (for review, see Gant and Wilson, 1997; Holmer and Worman, 2001).
Rough and smooth ER
The morphological differences between RER and SER allow these two regions of the ER to be distinguished visually; for example, the SER is often more convoluted than RER, and the RER tends to be more granular in texture. These differences in appearance may be directly related to the presence of bound ribosomes on the RER as there is some evidence that this affects ER structure (Prinz et al., 2000). Ultimately, however, the distinction between the two must be explained by differences in membrane protein composition. Most membrane proteins are shared between RER and SER (general ER proteins), but several proteins involved in translocation or processing of newly synthesized proteins are enriched in RER, as shown by the fractionation of liver cells (Kreibich et al., 1978; Amar-Cortesec et al., 1989; Vogel et al., 1990).
Since protein translocation is essential for all eukaryotic cells, they all have RER. One type of SER that is also found in all cells is the transitional ER (Palade, 1975). It is involved in packaging proteins for transport from the ER to the Golgi and is enriched in proteins required for this process (Hobman et al., 1998). However, SER is abundant only in certain cell types, such as in steroidsynthesizing cells, liver cells, neurons and muscle cells. The primary activities of the SER are very different in each of these cell types (reviewed in Hopkins, 1978). In liver, the SER is important for detoxification of hydrophobic substances. In steroid producing cells, it is the site of many of the synthesis steps. In muscle, it is called sarcoplasmic reticulum (SR) and is primarily involved in calcium release and uptake for muscle contraction and in neurons, although less well established, it is also probably required for calcium handling. Thus, the SER acts as an overflow site to house upregulated enzymes, and as these enzymes vary, it is also a cell typespecific suborganelle. Why are bound ribosomes concentrated in the RER and excluded from SER, rather than being found at lower levels throughout the ER? One proposed explanation is that the functions associated with bound ribosomes (translocation and modification of newly synthesized proteins) are more efficient if the proteins performing them are concentrated in one part of the membrane (Bergmann and Fusco, 1990).
It is not known how the RER and SER maintain distinct protein compositions. Perhaps, like inner NE proteins, RER membrane proteins are localized by tight binding to a fixed substrate. In this case, the best candidate substrate would be ribosomes as they are essentially fixed and, at least in one system, co-localize with RER membrane proteins (Rolls et al., 2002). The parallel between RER and NE protein targeting is not, however, complete, as FRAP experiments show that localized RER membrane proteins are not immobilized like NE membrane proteins (Rolls et al., 2002). Other mechanisms, for example, active retrieval from the SER, may play a role in the concentration of RER membrane proteins.
The ER contacts other organelles
The ER is closely associated with essentially all other organelles in the cell (for a review, see: Staehelin, 1997). These include the plasma membrane, Golgi, vacuoles, mitochondria, peroxisomes, late endosomes and lysosomes. The contact sites may establish separate ER domains.
In skeletal muscle, the SR abuts either the plasma membrane or the T-tubules, specialized extensions of the plasma membrane that invaginate into the muscle cell, thereby forming junctional membranes. Several proteins including ryanodine receptors (RyRs), which are ER calcium release channels, localize to these structures (Franzini-Armstrong and Jorgensen, 1994). The junctophilin (JP) family members contribute to the formation of these structures; transfection of cells with at least one of the junctophilin proteins (JP-1) establishes regions of proximity between the plasma membrane and SR (Takeshima et al., 2000), and its elimination in mice disrupts the junctional membrane structure (Ito et al., 2001). The JP proteins are proposed to reside in the SR membrane and to bind an unidentified plasma membrane component to establish the contact (Takeshima et al., 2000). It is not clear how the other membrane proteins, for example the RyRs, are targeted to junctional membranes once these structures are established.
The most complete story of how contact between the ER and another organelle is established is that of the nucleus–vacuole junction in yeast. It has been observed by electron microscopy that the nuclei and vacuoles of yeast are often in close contact (Pan et al., 2000) and two proteins that mediate this contact have been identified. Nvj1p localizes to regions of the NE adjacent to vacuoles and binds to the vacuolar membrane protein Vac8p. The absence of either of these proteins reduces the number of junctions (Pan et al., 2000). At least one other protein, Osh1p, also localizes to this domain (Levine and Munro, 2001).
Peroxisomes (Zaar et al., 1987) and mitochondria are also in close contact with the ER. A portion of the ER can be isolated with mitochondria and is enriched in some enzymes involved in lipid synthesis, such as phosphatidylserine (PS) synthase (Voelker, 2000). Some of the PS synthesized in the ER is transferred to mitochondria where it is decarboxylated to phosphatidylethanolamine (PE) and then transported back to the ER. Peroxisomes and ER are similarly intertwined by the synthesis of complex lipids, which requires both ER and peroxisomal enzymes.
Microscopy has linked the ER to two other classes of organelle. Electron microscopy tomography shows that the contact between an ER domain and the trans-Golgi is as intimate as that between the stacks of the Golgi itself (Ladinsky et al., 1999). In addition, one class of late endosomes/lysosomes has been observed to move in coordination with ER tubules, and to perhaps be deformed by the force of this interaction (Ko et al., 2001).
Why is the ER close to other organelles in the cell? Most likely, the major reason is that all organelles need lipids that are made in the ER; close contact may allow their direct transfer to these other membranes. Some organelles, like mitochondria, have no connection with the ER via vesicular trafficking, and therefore direct transfer of lipids seems to be the only way they could receive lipids from the ER. Another reason for the close proximity of the ER with other organelles is calcium signaling. The connection between ER and plasma membrane in muscle cells facilitates calcium release upon membrane depolarization, and the proximity between ER and mitochondria may also contribute to calcium signaling and regulation (see, for example, Rizzuto et al., 1998).
Propagation of the ER during cell division
All components of the cell are dramatically rearranged during cell division. In the face of this turbulence, does the ER maintain its structure as a single tubular network, and do its domains remain distinct? Accumulating evidence suggests that the ER network does not disassemble into vesicles during the cell cycle, but that it is divided between daughter cells by cytokinesis. The strongest support for maintenance of ER continuity comes from FLIP and FRAP experiments demonstrating that ER markers retain interphase patterns of motility during mitosis (Ellenberg et al., 1997). In addition, both light and electron microscopy show that ER networks can be visualized during cell division (Terasaki et al., 1986; Koch and Booth, 1988; Ellenberg et al., 1997; Yang et al., 1997; Terasaki, 2000). Are domainspecific proteins still concentrated in subregions of the mitotic network? Of the domain-specific ER proteins, the inner NE membrane proteins are the only ones whose fates during mitosis have been examined. The NE disassembles during mitosis in most eukaryotic cells: the scaffolds to which NE membrane proteins are bound in interphase are reorganized, the lamina is disassembled and the chromatin is condensed. In addition, phosphorylation of many NE proteins reduces their affinity for these partners (Bailer et al., 1991; Foisner and Gerace, 1993) and imaging of these proteins suggests that once freed, they diffuse throughout the ER network (reviewed in Collas and Courvalin, 2000). However, biochemical fractionation of mitotic or meiotic cells has shown that vesicles are enriched in NE proteins, particularly in egg cells (reviewed in Gant and Wilson, 1997). It is not clear whether this result reflects a portion of the ER that maintains a distinct composition because it is not part of the bulk ER network, or whether domains are somehow retained in the absence of scaffolds like the lamina.
The ER is dynamic and connected to the cytoskeleton
In interphase cells, the peripheral ER is a dynamic network consisting of cisternal sheets, linear tubules, polygonal reticulum and three-way junctions (Figure 1; Lee and Chen, 1988; Allan and Vale, 1991; Dreier and Rapoport, 2000). Several basic movements contribute to its dynamics: elongation and retraction of tubules, tubule branching, sliding of tubule junctions and the disappearance of polygons (Lee and Chen, 1988). These movements are constantly rearranging the ER network while maintaining its characteristic structure.
The dynamics of the ER network depend on the cytoskeleton. In mammalian tissue culture cells, goldfish scale cells, and Xenopus and sea urchin embryos the ER tubules often co-align with microtubules (Terasaki et al., 1986). Microtubule-based ER dynamics were studied with time-lapse microscopy and appear to be based on three different mechanisms. First, new ER tubules can be pulled out of existing tubules by motor proteins migrating along microtubules. Secondly, new tubules may be dragged along by the tips of polymerizing microtubules. Finally, ER tubules may associate with the sides of microtubules, via motor proteins, as they slide along other microtubules. Each of these mechanisms can lead to tubule extension and, when tubules intersect, they fuse and create three-way junctions (Allan and Vale, 1991; Waterman-storer and Salmon, 1998). In yeast and plants, the actin cytoskeleton, rather than the microtubule network, is required for ER dynamics (Liebe and Menzel, 1995; Prinz et al., 2000).
The cytoskeleton contributes to ER dynamics, but it is not necessary for the maintenance of the existing ER network. Although depolymerization of microtubules by nocodazole in mammalian tissue culture cells inhibits new tubule growth and causes some retraction of ER tubules from the cell periphery, the basic tubular-cisternal structure of the ER remains intact (Terasaki et al., 1986). Similarly, actin depolymerization in yeast blocks ER movements but does not disrupt its structure (Prinz et al., 2000).
Formation of ER tubules
The cytoskeleton is also not necessary for the formation of a tubular network in vitro. In Xenopus egg extracts, ER networks can form de novo and this process is not affected by the addition of inhibitors of microtubule polymerization, by the depletion of tubulin from the extract or by inhibitors of actin polymerization (Dreier and Rapoport, 2000).
If the ER network is not formed along a cytoskeleton, how is it generated? The answer is not known, but some properties of ER formation have been elucidated using in vitro systems. ER network formation in extracts requires ATP and GTP, and is NEM (N-ethyl maleimide)-sensitive (Dabora and Sheetz, 1988; Allan and Vale, 1991; Dreier and Rapoport, 2000). In a Xenopus in vitro system, incubation of membranes in the absence of cytosol leads to the formation of large vesicles that cannot subsequently be converted into networks by the addition of cytosol (Dreier and Rapoport, 2000), and it is thought that cytosolic factors convert a basic fusion reaction into a regulated process that produces tubular networks.
Inhibition of network formation by GTPγS and NEM (Allan and Vale, 1991; Dreier and Rapoport, 2000), suggests that a Rab protein and/or a factor similar to the NEMsensitive fusion protein (NSF) may be involved (for a review, see Gotte and Mollard, 1998). There is some evidence that a homolog of NSF, p97, and its co-factor p47, contribute to efficient ER network formation in Xenopus egg extracts (Hetzer et al., 2001), and the yeast homolog of p97, Cdc48, has been shown to be involved in homotypic ER fusion (Latterich et al., 1995). A role for p97/p47 in the in vitro formation of the transitional ER has also been suggested (Roy et al., 2000). Surprisingly, however, a mutant of Cdc48 does not affect ER structure in yeast (Prinz et al., 2000).
Possible mechanisms behind tubulation
Membrane tubules are a structural feature of both the ER and the Golgi complex (Lee et al., 1989; Banta et al., 1995; Dreier and Rapoport, 2000). Both types of tubule have similar diameters (50–100 nm), whether formed in vitro or in vivo, and in the case of the ER, tubule diameter is conserved from yeast to mammalian cells (Dabora and Sheetz, 1988; Lee and Chen, 1988; Dreier and Rapoport, 2000; Prinz et al., 2000), suggesting that their formation is a regulated and fundamental process.
The mechanism behind tubulation is mysterious. The simplest model is that tubules form along a cytoskeletal scaffold, although the in vitro experiments do not support a role for the cytoskeleton (Dreier and Rapoport, 2000). Perhaps a different scaffold within or around the membrane is used. Regulation of lumenal volume could also contribute to tubule shape. If lumenal volume is restricted during vesicle fusion, the shape that the fused membrane could adopt would also be restricted. For example, a sphere has a different ratio of surface area to volume than a tubule. The most obvious way to control lumenal volume would be with an ion pump to maintain an ion gradient, resulting in expulsion of solution from the lumen; however, no such mechanism has been identified. Tubules also have a unique curvature of the lipid bilayer. The regulation of this curvature, by flippases or by the modification of lipids on one side of the membrane, could be another mechanism by which tubulation could be promoted. For example, modifications that change the ratio of coneshaped to inverted cone-shaped phospholipids in one leaflet of the membrane bilayer relative to the other leaflet will alter membrane curvature (for a review, see Sprong et al., 2001). Studies on Golgi tubule formation indicate a potential role for a lipid modification reaction in tubule formation; several inhibitors of phospholipase A2 inhibit Golgi tubule formation in vitro (de Figueirido et al., 1999).
Perspectives
Exciting progress has been made in understanding ER structure and function, but some of the most interesting questions remain to be answered. How are proteins concentrated in the RER? What are the molecules that link the ER to other organelles? How are lipids transported between the ER and other organelles? How are membrane tubules generated and maintained? Some of these problems can probably be addressed by genetic experiments and with in vitro assays that reproduce these complex cellular events. In addition, visual methods employing fluorescent protein fusions will help to understand the dynamics of ER components. We are clearly at the beginning of a new era in understanding how biological membrane structures are generated and maintained.
Acknowledgments
We thank W. Prinz, K. Matlack, S. Munro and M. Terasaki for critical reading of the manuscript and helpful comments. G.K.V. is supported by a fellowship from the Jane Coffin Childs Memorial Fund for Medical Research. M.M.R. is supported by the Howard Hughes Medical Institute. T.A.R. is a Howard Hughes Medical Institute Investigator.
References
- Allan V.J. and Vale R.D. (1991) Cell cycle control of microtubule-based membrane transport and tubule formation in vitro. J. Cell Biol., 113, 346–359. [Europe PMC free article] [Abstract] [Google Scholar]
- Amar-Cortesec A., Dublet B. and Beaufay H. (1989) Translocation and proteolytic processing of nascent secretory polypeptide chains: two functions associated with the ribosomal domain of the endoplasmic reticulum. Mol. Biol. Cell, 65, 99–108. [Abstract] [Google Scholar]
- Bailer S.M., Eppenberger H.M., Griffiths G. and Nigg E.A. (1991) Characterization of a 54-kD protein of the inner nuclear membrane: evidence for cell cycle-dependent interaction with the nuclear lamina. J. Cell Biol., 114, 389–400. [Europe PMC free article] [Abstract] [Google Scholar]
- Banta M., Polizotto R.S., Wood S.A., de Figueiredo P. and Brown W.J. (1995) Characterization of a cytosolic activity that induces the formation of Golgi membrane tubules in a cell-free reconstitution system. Biochemistry, 34, 13359–13366. [Abstract] [Google Scholar]
- Baumann O. and Walz B. (2001) Endoplasmic reticulum of animal cells and its organization into structural and functional domains. Int. Rev. Cytol., 205, 149–214. [Abstract] [Google Scholar]
- Bergmann J.E. and Fusco P.J. (1990) The G protein of vesicular stomatitis virus has free access into and egress from the smooth endoplasmic reticulum of UT-1 cells. J. Cell Biol., 110, 625–635. [Europe PMC free article] [Abstract] [Google Scholar]
- Cole N.B., Smith C.L., Sciaky N., Terasaki M., Edidin M. and Lippincottschwartz J. (1996) Diffusional mobility of Golgi proteins in membranes of living cells. Science, 273, 797–801. [Abstract] [Google Scholar]
- Collas I. and Courvalin J.C. (2000) Sorting nuclear membrane proteins at mitosis. Trends Cell Biol., 10, 5–8. [Abstract] [Google Scholar]
- Dabora S.L. and Sheetz M.P. (1988) The microtubule-dependent formation of a tubulovesicular network with characteristics of the ER from cultured cell extracts. Cell, 54, 27–35. [Abstract] [Google Scholar]
- Dayel M.J. and Verkman A.S. (1999) Diffusion of green fluorescent protein in the aqueous-phase lumen of the endoplasmic reticulum. Biophys. J., 76, 2843–2851. [Europe PMC free article] [Abstract] [Google Scholar]
- de Figueirido P., Polizotto R.S., Drecktrah D. and Brown W.J. (1999) Membrane tubule-mediated reassembly and maintenance of the Golgi complex is disrupted by phospholipase A2 antagonists. Mol. Biol. Cell, 10, 1763–1782. [Europe PMC free article] [Abstract] [Google Scholar]
- Dreger M., Bengtsson L., Schoneberg T., Otto H. and Hucho F. (2001) Nuclear envelope proteomics: novel integral membrane proteins of the inner nuclear membrane. Proc. Natl Acad. Sci. USA, 98, 11943–11948. [Europe PMC free article] [Abstract] [Google Scholar]
- Dreier L. and Rapoport T.A. (2000) In vitro formation of the endoplasmic reticulum occurs independently of microtubules by a controlled fusion reaction. J. Cell Biol., 148, 883–898. [Europe PMC free article] [Abstract] [Google Scholar]
- Ellenberg J., Siggia E.D., Moreira J.E., Smith C.L., Presley J.F., Worman H.J. and Lippincottschwartz J. (1997) Nuclear membrane dynamics and reassembly in living cells: targeting of inner nuclear membrane protein in interphase and mitosis. J. Cell Biol., 138, 1193–1206. [Europe PMC free article] [Abstract] [Google Scholar]
- Fawcett D.W. (1966) An Atlas of Fine Structure: The Cell, its Organelles and Inclusions. W.B. Saunders Co., Philadelphia, PA. [Google Scholar]
- Foisner R. and Gerace L. (1993) Integral membrane proteins of the nuclear envelope interact with lamins and chromosomes, and binding is modulated by mitotic phosphorylation. Cell, 73, 1267–1279. [Abstract] [Google Scholar]
- Franzini-Armstrong C. and Jorgensen A.O. (1994) Structure and development of E–C coupling units in skeletal muscle. Annu. Rev. Physiol., 56, 509–534. [Abstract] [Google Scholar]
- Gant T.M. and Wilson K.L. (1997) Nuclear assembly. Annu. Rev. Cell Dev. Biol., 13, 695–699. [Abstract] [Google Scholar]
- Gotte M. and Mollard G.F. (1998) A new beat for the SNARE drum. Trends Cell Biol., 8, 215–218. [Abstract] [Google Scholar]
- Hetzer M., Meyer H.H., Walther T.C., Bilbao-Cortes D., Warren G. and Mattaj I.W. (2001) Distinct AAA-ATPase p97 complexes function in discrete steps of nuclear assembly. Nat. Cell Biol., 3, 1086–1091. [Abstract] [Google Scholar]
- Hobman T.C., Zhao B., Chan H. and Farquhar M.G. (1998) Immuno-isolation and characterization of a subdomain of the endoplasmic reticulum that concentrates proteins involved in COPII vesicle biogenesis. Mol. Biol. Cell, 9, 1265–1278. [Europe PMC free article] [Abstract] [Google Scholar]
- Holmer L. and Worman H.J. (2001) Inner nuclear membrane proteins: functions and targeting. Cell. Mol. Life Sci., 58, 1741–1747. [Abstract] [Google Scholar]
- Hopkins C.R. (1978) Structure and Function of Cells. W.B. Saunders Co., Philadelphia, PA. [Google Scholar]
- Ito K., Komazaki S., Sasamoto K., Yoshida M., Nishi M., Kitamura K. and Takeshima H. (2001) Deficiency of triad junction and contraction in mutant skeletal muscle lacking junctophilin type 1. J. Cell Biol., 154, 1059–1067. [Europe PMC free article] [Abstract] [Google Scholar]
- Jaffe L.A. and Terasaki M. (1994) Structural changes in the endoplasmic reticulum of starfish oocytes during meiotic maturation and fertilization. Dev. Biol., 164, 579–587. [Abstract] [Google Scholar]
- Ko D.C., Gordon M.D., Jin J.Y. and Scott M.P. (2001) Dynamic movements of organelles containing Niemann-Pick C1 protein: NPC1 involvement in late endocytic events. Mol. Biol. Cell, 12, 601–614. [Europe PMC free article] [Abstract] [Google Scholar]
- Koch G.L.E. and Booth C. (1988) Dissociation and re-assembly of the endoplasmic reticulum in live cells. J. Cell Sci., 91, 511–522. [Abstract] [Google Scholar]
- Koning A.J., Lum P.Y., Williams J.M. and Wright R. (1993) DiOC6 staining reveals organelle structure and dynamics in living yeast cells. Cell Motil. Cytoskel., 25, 111–128. [Abstract] [Google Scholar]
- Kreibich G., Ulrich B.L. and Sabatini D.D. (1978) Proteins of rough microsomal membranes related to ribosome binding: 1. Identification of ribophorins I and II, membrane proteins characteristic of rough microsomes. J. Cell Biol., 77, 464–487. [Europe PMC free article] [Abstract] [Google Scholar]
- Ladinsky M.S., Mastronarde D.N., McIntosh J.R., Howell K.E. and Staehelin L.A. (1999) Golgi structure in three dimensions: functional insights from the normal rat kidney cell. J. Cell Biol., 144, 1135–1149. [Europe PMC free article] [Abstract] [Google Scholar]
- Latterich M., Frolich K.U. and Schekman R. (1995) Membrane fusion and the cell cycle; Cdc48p participates in the fusion of ER membranes. Cell, 82, 885–893. [Abstract] [Google Scholar]
- Lee C. and Chen L.B. (1988) Dynamic behavior of endoplasmic reticulum in living cells. Cell, 54, 37–46. [Abstract] [Google Scholar]
- Lee C., Ferguson M. and Chen L.B. (1989) Construction of the endoplasmic reticulum. J. Cell Biol., 109, 2045–2055. [Europe PMC free article] [Abstract] [Google Scholar]
- Levine T.P. and Munro S. (2001) Dual targeting of Osh1p, a yeast homologue of oxysterol-binding protein, to both the Golgi and the nucleus–vacuole junction. Mol. Biol. Cell, 12, 1633–1644. [Europe PMC free article] [Abstract] [Google Scholar]
- Liebe S. and Menzel D. (1995) Actomyosin-based motility of endoplasmic reticulum and chloroplasts in Vallisneria mesophyll cells. Biol. Cell, 85, 207–222. [Abstract] [Google Scholar]
- Ma Y. and Hendershot L.M. (2001) The unfolding tale of the unfolded protein response. Cell, 107, 827–830. [Abstract] [Google Scholar]
- Matlack K.E., Mothes W. and Rapoport T.A. (1998) Protein translocation: tunnel vision. Cell, 92, 381–390. [Abstract] [Google Scholar]
- McMaster C.R. (2001) Lipid metabolism and vesicle trafficking: more than just greasing the transport machinery. Biochem. Cell Biol., 79, 681–692. [Abstract] [Google Scholar]
- Meldolesim J. and Pozzan T. (1998). The endoplasmic reticulum Ca2+ store: a view from the lumen. Trends Biochem. Sci., 23, 10–14. [Abstract] [Google Scholar]
- Palade G. (1975) Intracellular aspects of the process of protein synthesis. Science, 189, 347–358. [Abstract] [Google Scholar]
- Pan X., Roberts P., Chen Y., Kvam E., Shulga N., Huang K., Lemmons S. and Goldfarb D.S. (2000) Nucleus–vacuole junctions in Saccharomyces cerevisiae are formed through the direct interaction of Vac8p with Nvj1p. Mol. Biol. Cell, 11, 2445–2457. [Europe PMC free article] [Abstract] [Google Scholar]
- Prinz W.A., Grzyb L., Veenhuis M., Kahana J.A., Silver P.A. and Rapoport T.A. (2000) Mutants affecting the structure of the cortical endoplasmic reticulum in Saccharomyces cerevisiae. J. Cell Biol., 150, 461–474. [Europe PMC free article] [Abstract] [Google Scholar]
- Rizzuto R., Pinton P., Carrington W., Fay F.S., Fogarty K.E., Lifshitz L.M., Tuft R.A. and Pozzan T. (1998) Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+. Science, 280, 1763–1766. [Abstract] [Google Scholar]
- Rolls M.M., Stein P.S., Taylor S.S., Ha E., McKeon F. and Rapoport T.A. (1999) A visual screen of a GFP-fusion library identifies a new type of nuclear envelope membrane protein. J. Cell Biol., 146, 29–43. [Europe PMC free article] [Abstract] [Google Scholar]
- Rolls M.M., Hall D.H., Victor M., Stelzer E.H.K. and Rapoport T.A. (2002) Targeting of rough endoplasmic reticulum membrane proteins and ribosomes in invertebrate neurons. Mol. Biol. Cell, 13, 1778–1791. [Europe PMC free article] [Abstract] [Google Scholar]
- Roy L. et al. . (2000) Role of p97 and Syntaxin 5 in the assembly of transitional endoplasmic reticulum. Mol. Cell. Biol., 11, 2529–2542. [Europe PMC free article] [Abstract] [Google Scholar]
- Soullam B. and Worman H.J. (1995) Signals and structural features involved in integral membrane protein targeting to the inner nuclear envelope. J. Cell Biol., 130, 15–27. [Europe PMC free article] [Abstract] [Google Scholar]
- Sprong H., van der Sluijs P. and van Meer G. (2001) How proteins move lipids and lipids move proteins. Nat. Rev. Mol. Cell Biol., 2, 504–513. [Abstract] [Google Scholar]
- Staehelin L.A. (1997) The plant ER: a dynamic organelle composed of a large number of discrete functional domains. Plant J., 11, 1151–1165. [Abstract] [Google Scholar]
- Subramanian K. and Meyer T. (1997) Calcium-induced restructuring of nuclear envelope and endolasmic reticulum calcium stores. Cell, 89, 963–971. [Abstract] [Google Scholar]
- Takeshima H., Komazaki S., Nishi M., Iino M. and Kangawa K. (2000) Junctophilins: a novel family of junctional membrane complex proteins. Mol. Cell, 6, 11–22. [Abstract] [Google Scholar]
- Terasaki M. (2000) Dynamics of the endoplasmic reticulum and Golgi apparatus during early sea urchin development. J. Cell Biol., 114, 929–940. [Europe PMC free article] [Abstract] [Google Scholar]
- Terasaki M. and Jaffe L.A. (1991) Organization of the sea urchin egg endoplasmic reticulum and its reorganization at fertilization. J. Cell Biol., 114, 929–940. [Europe PMC free article] [Abstract] [Google Scholar]
- Terasaki M., Chen L.B. and Fujiwara K. (1986) Microtubules and the endoplasmic reticulum are highly interdependent structures. J. Cell Biol., 103, 1557–1568. [Europe PMC free article] [Abstract] [Google Scholar]
- Terasaki M., Slater N.T., Fein A., Schmidek A. and Reese T.S. (1994) Continuous network of endoplasmic reticulum in cerebellar Purkinje neurons. Proc. Natl Acad. Sci. USA, 91, 7510–7514. [Europe PMC free article] [Abstract] [Google Scholar]
- Voelker D.R. (2000) Interorganelle transport of aminoglycerophospholipids. Biochim. Biophys. Acta, 1486, 97–107. [Abstract] [Google Scholar]
- Vogel F., Hartmann E., Gorlich D. and Rapoport T.A. (1990) Segregation of the signal sequence receptor protein in the rough endoplasmic reticulum membrane. Eur. J. Cell Biol., 53, 197–202. [Abstract] [Google Scholar]
- Waterman-storer and Salmon E.D. (1998) Endoplasmic reticulum membrane tubules are distributed by microtubules in living cells using three distinct mechanisms. Curr. Biol., 8, 798–806. [Abstract] [Google Scholar]
- Yang L., Guan T. and Gerace L. (1997) Integral membrane proteins of the nuclear envelope are dispersed throughout the endoplasmic reticulum during mitosis. J. Cell Biol., 137, 1199–1210. [Europe PMC free article] [Abstract] [Google Scholar]
- Ye Q. and Worman H.J. (1994) Primary structure analysis and lamin B and DNA binding of human LBR, an integral protein of the nuclear envelope inner membrane. J. Biol. Chem., 269, 11306–11311. [Abstract] [Google Scholar]
- Zaar K., Bolkl A. and Fahimi H.D. (1987) Association of isolated bovine kidney cortex peroxisomes with endoplasmic reticulum. Biochim. Biophys. Acta, 897, 135–142. [Abstract] [Google Scholar]
Articles from EMBO Reports are provided here courtesy of Nature Publishing Group
Full text links
Read article at publisher's site: https://doi.org/10.1093/embo-reports/kvf202
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc1307613?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
Beyond fission and fusion-Diving into the mysteries of mitochondrial shape.
PLoS Biol, 22(7):e3002671, 01 Jul 2024
Cited by: 0 articles | PMID: 38949997 | PMCID: PMC11216622
Review Free full text in Europe PMC
Enzymatic control of intermolecular interactions for generating synthetic nanoarchitectures in cellular environment.
Sci Technol Adv Mater, 25(1):2373045, 28 Jun 2024
Cited by: 0 articles | PMID: 39011064 | PMCID: PMC11249168
Review Free full text in Europe PMC
Different ER-plasma membrane tethers play opposing roles in autophagy of the cortical ER.
Proc Natl Acad Sci U S A, 121(24):e2321991121, 05 Jun 2024
Cited by: 1 article | PMID: 38838012
Crosstalk between mitotic reassembly and repair of the nuclear envelope.
Nucleus, 15(1):2352203, 23 May 2024
Cited by: 0 articles | PMID: 38780365 | PMCID: PMC11123513
Review Free full text in Europe PMC
Mechanism of Decision Making between Autophagy and Apoptosis Induction upon Endoplasmic Reticulum Stress.
Int J Mol Sci, 25(8):4368, 15 Apr 2024
Cited by: 5 articles | PMID: 38673953 | PMCID: PMC11050573
Review Free full text in Europe PMC
Go to all (294) article citations
Other citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Peripheral ER structure and function.
Curr Opin Cell Biol, 21(4):596-602, 15 May 2009
Cited by: 115 articles | PMID: 19447593 | PMCID: PMC2753178
Review Free full text in Europe PMC
Subcompartments of the endoplasmic reticulum.
Semin Cell Biol, 3(5):325-341, 01 Oct 1992
Cited by: 10 articles | PMID: 1457776
Review
Endoplasmic reticulum of animal cells and its organization into structural and functional domains.
Int Rev Cytol, 205:149-214, 01 Jan 2001
Cited by: 274 articles | PMID: 11336391
Review
Shaping the endoplasmic reticulum into the nuclear envelope.
J Cell Sci, 121(pt 2):137-142, 01 Jan 2008
Cited by: 39 articles | PMID: 18187447








