Abstract
Free full text

A Multiplicity of Coactivators Is Required by Gcn4p at Individual Promoters In Vivo
Abstract
Transcriptional activators interact with multisubunit coactivators that modify chromatin structure or recruit the general transcriptional machinery to their target genes. Budding yeast cells respond to amino acid starvation by inducing an activator of amino acid biosynthetic genes, Gcn4p. We conducted a comprehensive analysis of viable mutants affecting known coactivator subunits from the Saccharomyces Genome Deletion Project for defects in activation by Gcn4p in vivo. The results confirm previous findings that Gcn4p requires SAGA, SWI/SNF, and SRB mediator (SRB/MED) and identify key nonessential subunits of these complexes required for activation. Among the numerous histone acetyltransferases examined, only that present in SAGA, Gcn5p, was required by Gcn4p. We also uncovered a dependence on CCR4-NOT, RSC, and the Paf1 complex. In vitro binding experiments suggest that the Gcn4p activation domain interacts specifically with CCR4-NOT and RSC in addition to SAGA, SWI/SNF, and SRB/MED. Chromatin immunoprecipitation experiments show that Mbf1p, SAGA, SWI/SNF, SRB/MED, RSC, CCR4-NOT, and the Paf1 complex all are recruited by Gcn4p to one of its target genes (ARG1) in vivo. We observed considerable differences in coactivator requirements among several Gcn4p-dependent promoters; thus, only a subset of the array of coactivators that can be recruited by Gcn4p is required at a given target gene in vivo.
Eukaryotic activator proteins stimulate transcription by binding to their target genes and carrying out two general functions: (i) altering the locations or structures of nucleosomes and (ii) recruiting TATA-binding protein (TBP), other general transcription factors (GTFs), and RNA polymerase II (RNA PolII) to the promoter. Most activators carry out these functions indirectly by recruiting multisubunit complexes, collectively called coactivators (39, 70, 90). One class of coactivators uses ATP hydrolysis to displace nucleosomes and thereby expose or obscure protein binding sites in the promoter (91, 124). Each of the nucleosome remodeling complexes of Saccharomyces cerevisiae, known as SWI/SNF, RSC, ISW1, and ISW2, contains a different subunit harboring the ATPase activity of the complex (reviewed in references 70 and 91). Although each has been implicated in transcriptional activation in vivo (5, 38, 51, 85, 123), only the nonessential SWI/SNF complex has been shown to interact physically with activators (93, 139) and be recruited to a promoter for nucleosome remodeling and transcriptional activation in vitro (45, 96, 139). Recruitment of the SWI/SNF complex by yeast activators has also been demonstrated in living yeast cells by chromatin immunoprecipitation (ChIP) assays (24, 126).
Another class of coactivators alters chromatin structure by acetylation of lysines in the amino-terminal tails of histones. This modification destabilizes higher-order chromatin structure (116) and also may stimulate binding of other coactivator proteins containing a bromodomain (9, 91, 120, 135). The SAGA complex is the best-characterized yeast coactivator in this class (109, 118), and its histone acetyltransferase (HAT) subunit, Gcn5p, acetylates nucleosomal H3 and H2B (43). SAGA also binds to TBP in vitro (8, 119) and can promote TBP recruitment in vivo (31). Purified SAGA interacts with several yeast activators (30, 42, 92) and can be recruited to a chromatin template for transcriptional stimulation in a HAT-dependent manner (132). Activator recruitment of Gcn5p HAT activity or SAGA subunits was also demonstrated in vivo (12, 31, 63, 66).
SAGA shares a subset of TBP-associated factors (TAFs) with the general transcription factor TFIID, which also recruits TBP to certain promoters (104). All but one of the non-TAF subunits of SAGA are dispensable (109, 118); however, the majority of TAFs are essential proteins (84, 103). Transcriptome analysis indicates that SAGA and TFIID have redundant coactivator functions (67). Recruitment of TBP by the activator Gcn4p appears to involve the single polypeptide Mbf1p, which serves as an adaptor between the DNA binding domain of Gcn4p and TBP (127).
Other high-molecular-weight HAT complexes in yeast include NuA4, which acetylates predominantly nucleosomal H4, and NuA3, which acetylates exclusively nucleosomal H3 (42). NuA4 contains only one known nonessential subunit, Eaf3p (4, 33), which is dispensable for acetylation by NuA4 in vitro but required for wild-type (WT) basal expression of PHO5, HIS4, and TRP4 in vivo. These data indicate a role for NuA4 in transcription, as suggested by its interactions with activators in vitro (42, 132). The NuA3 complex, in contrast, has displayed no activator interactions, and mutation of its HAT subunit, Sas3p (57), seems to affect only silenced chromatin (105).
A third class of coactivators, of which the best characterized example is SRB mediator (SRB/MED), interacts with RNA PolII and the GTFs TFIIF and TFIIB (13, 89). The Srb subunits of SRB/MED interact genetically with the C-terminal domain (CTD) of the largest subunit of RNA PolII (128). Many SRB/MED subunits have been implicated in positive or negative transcriptional control (13, 89). Most notably, Gal11p, Med2p, Pgd1p, and Sin4p seem to comprise a subcomplex (27, 88) that is required for SRB/MED-dependent transcriptional activation (44, 71, 88) and for activator binding to SRB/MED in vitro (98). SRB/MED associates specifically with the CTD nonphosphorylated form of RNA PolII and is absent from the phosphorylated elongating enzyme (102, 125). Thus, SRB/MED may function primarily in recruitment of nonphosphorylated RNA PolII to the promoter.
A distinct RNA PolII holoenzyme has been identified (114, 133) that contains TFIIF and TFIIB, the SRB/MED subunits Rgr1p, Sin4p, and Gal11p (70), and other subunits specific to this form of holoenzyme, including Paf1p and Cdc73p (17, 86, 114, 117). Although this Paf1 complex associates with nonphosphorylated RNA PolII and contains GTFs, its genetic and physical interactions with Spt4p-Spt5p and the Spt16p-Pob3p-containing CP complex suggest a role in transcriptional elongation (117). Consistent with the latter, Paf1p was found to be associated with the transcribed regions as well as promoters of several yeast genes in vivo (102). Furthermore, the Paf1 complex interacts with Hpr1p, a stoichiometric component of the THO/TREX complex that was implicated in transcriptional elongation and transcription-induced recombination (3, 19, 121).
The Paf1 complex also interacts with Ccr4p, a protein with positive and negative regulatory functions in gene expression (17, 25, 74). A large fraction of the Ccr4p resides in a 1.2-MDa complex, distinct from the Paf1 complex, containing Caf1p/Pop2p, Caf40p, Caf130p, and the five NOT proteins (21, 74). NOT1 to NOT5 were identified genetically as negative regulators that prevent transcription from the noncanonical TATA element at HIS3 in the absence of Gcn4p (Not− phenotype) (23). Consistent with a role in transcriptional repression, mutations in NOT1, NOT3, NOT5, and CAF1 can suppress the lethal phenotype of an srb4 mutation (69). However, mutations in CCR4, CAF1, NOT2, and NOT3 impair CYC1 derepression in nonfermentable carbon sources, implicating these subunits in gene activation (74). Numerous other proteins are associated with the core CCR4-NOT complex, including a subset of SRB proteins associated with RNA PolII holoenzyme (75). The CCR4-NOT complex has been shown to interact with TBP and certain TAFs, most likely in the context of TFIID, possibly to inhibit TFIID binding to nonconsensus TATA elements (6, 73).
Gcn4p is a transcriptional activator of amino acid, vitamin, and purine biosynthetic genes in yeast (94) and is induced at the translational level by starvation for any amino acid (49). Gcn4p function is dependent on clusters of bulky hydrophobic residues distributed throughout its acidic activation domain (29, 54). Because of their functional redundancy, multiple hydrophobic clusters must be mutated simultaneously to impair Gcn4p function in vivo and abolish its interactions with coactivators in vitro (30). Gcn4p binds in vitro to SAGA, SWI/SNF, SRB/MED, and NuA4 (30, 42, 92, 93, 96, 132), and mutations were isolated in subunits of the first three complexes that decrease activation by Gcn4p in vivo (10, 40, 93, 96, 100). There is also ChIP evidence that Gcn4p recruits SWI/SNF (126) and Gcn5p HAT activity (63, 64) to target promoters in vivo.
In the present study, we conducted a comprehensive analysis of Gcn4p coactivator requirements by testing viable mutants from the Saccharomyces Genome Deletion Project for defects in activation by Gcn4p in vivo. Our results confirm previous findings that Gcn4p function requires SAGA, SWI/SNF, and SRB/MED and also reveal a dependence on CCR4-NOT, RSC, and the Paf1 complex for full activation by Gcn4p in vivo. In vitro binding experiments suggest that Gcn4p interacts specifically with CCR4-NOT and RSC, in addition to SAGA, SWI/SNF, and SRB/MED. ChIP assays indicate that SAGA, SWI/SNF, SRB/MED, CCR4-NOT, RSC, the Paf1 complex, and Mbf1p are recruited to the ARG1 promoter by Gcn4p. Although Gcn4p can recruit an array of coactivators to the same promoter in vivo, not all of these factors are required for WT activation at every Gcn4p target gene.
MATERIALS AND METHODS
Yeast strains, genetic methods, and plasmids.
All strains were grown at 25°C. Strains from the Saccharomyces Genome Deletion Project were purchased from Research Genetics, and most are listed in Table Table11 (exceptions are noted below). Strain LSO2 was generated from a cross of strain 13701 (BY4742 med2Δ) with strain 5489 (BY4741 med1Δ). HAT double mutants were generated by crossing strains 17285 (BY4742 gcn5Δ) or 14518 (BY4742 nut1Δ) with strains 2742 (BY4741 elp3Δ), 1551 (BY4741 ayt1Δ), 5608 (BY4741 hpa2Δ), 308 (BY4741 hpa3Δ), 6568 (BY4741 sas2Δ) or 4518 (BY4741 nut1Δ).
TABLE 1.
SM resistance and UASGCRE-CYC1-lacZ expression phenotypes of cofactor mutants
| Name | Parenta | Relevant genotypeb | Cofactor complex | SM resistance (% of WT)c | β-Gal activity (% of WT) ford:
| |
|---|---|---|---|---|---|---|
| Uninduced UASGCRE-CYC1-lacZ | Induced UASGCRE-CYC1-lacZ | |||||
| BY4741 | NA | MATahis3Δ1 leu2Δ0 met15Δ0 ura3Δ0 | NAe | 100 | 100 | 100 |
| BY4742 | NA | MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 | NA | |||
| BY4743 | NA | MATa/MATα his3Δ1/his3Δ1 leu2Δ0/leu2Δ0 MET15/met15Δ0 LYS2/lys2Δ0 ura3Δ0/ura3Δ0 | NA | 100 | 100 | 100 |
| 249 | BY4741 | gcn4Δ::kanMX4 | Activator | 5 | 10 | 1.0 |
| 30249 | BY4743 | gcn4Δ::kanMX4/gcn4Δ::kanMX4 | Activator | 5 | 1.1 | 0.2 |
| 6753 | BY4741 | mbf1Δ::kanMX4 | NA | 100 | ||
| 7335 | BY4741 | mbf1Δ::kanMX4 | NA | 100 | 92 | 180 |
| 1799 | BY4741 | ahc1Δ::kanMX4 | ADA | 100 | 86 | 145 |
| 4282 | BY4741 | ada2Δ::kanMX4 | ADA, SAGA | 51 | 54 | 22 |
| 3534 | BY4741 | ada3Δ::kanMX4 | ADA, SAGA | 46 | 86 | 30 |
| 7285 | BY4741 | gcn5Δ::kanMX4 | ADA, SAGA | 41 | 46 | 29 |
| 1038 | BY4741 | ada1Δ::kanMX4 | SAGA | 26 | 8.8 | 5.1 |
| 7309 | BY4741 | ada5Δ::kanMX4 | SAGA | 26 | 13 | 3.1 |
| 4228 | BY4741 | spt3Δ::kanMX4 | SAGA | 89 | 330 | 100 |
| 3218 | BY4741 | spt7Δ::kanMX4 | SAGA | 24 | 100 | 25 |
| 2666 | BY4741 | spt8Δ::kanMX4 | SAGA | 95 | 380 | 110 |
| 184 | BY4741 | yer049Δ::kanMX4 | NuA3 | 100 | 110 | 59 |
| 2150 | BY4741 | yp1101Δ::kanMX4 | NuA3 | 100 | 340 | 220 |
| 3078 | BY4741 | sas3Δ::kanMX4 | NuA3 | 100 | 67 | 68 |
| 7143 | BY4741 | eaf3Δ::kanMX4 | NuA4 | 100 | 28 | 100 |
| 5308 | BY4741 | bdf1Δ::kanMX4 | TFIID | 62 | 74 | 65 |
| 3767 | BY4741 | bdf2Δ::kanMX4 | TFIID | 100 | 100 | 140 |
| 2742 | BY4741 | elp3Δ::kanMX4 | HAT | 89 | ||
| 1551 | BY4741 | ayt1Δ::kanMX4 | HAT | 100 | ||
| 5608 | BY4741 | hpa2Δ::kanMX4 | HAT | 100 | ||
| 308 | BY4741 | hpa3Δ::kanMX4 | HAT | 100 | ||
| 6568 | BY4741 | sas2Δ::kanMX4 | HAT | 100 | ||
| 1114 | BY4741 | rpd3Δ::kanMX4 | HDAC | 100 | ||
| 5347 | BY4741 | hda1Δ::kanMX4 | HDAC | 91 | ||
| 5487 | BY4741 | hos1Δ::kanMX4 | HDAC | 100 | ||
| 4561 | BY4741 | hos2Δ::kanMX4 | HDAC | 100 | ||
| 2136 | BY4741 | hos3Δ::kanMX4 | HDAC | 100 | ||
| 3738 | BY4741 | sir2Δ::kanMX4 | HDAC | 100 | ||
| 1760 | BY4741 | hst1Δ::kanMX4 | HDAC | 100 | ||
| 2813 | BY4741 | hst2Δ::kanMX4 | HDAC | 100 | ||
| 1801 | BY4741 | hst3Δ::kanMX4 | HDAC | 100 | ||
| 3550 | BY4741 | hst4Δ::kanMX4 | HDAC | 100 | ||
| 2123 | BY4741 | tfg3Δ::kanMX4 | Multiplef | 28 | 40 | 22 |
| 1586 | BY4741 | swi2Δ::kanMX4 | SWI/SNF | 35 | 200 | 40 |
| 1250 | BY4741 | swi3Δ::kanMX4 | SWI/SNF | 62 | 170 | 33 |
| 7175 | BY4741 | snf5Δ::kanMX4 | SWI/SNF | 55 | 120 | 28 |
| 6409 | BY4741 | snf6Δ::kanMX4 | SWI/SNF | 55 | 110 | 27 |
| 4008 | BY4741 | snf11Δ::kanMX4 | SWI/SNF | 103 | 140 | 61 |
| 15398 | BY4742 | swp73Δ::kanMX4 | SWI/SNF | 41 | 160 | 39 |
| 4686 | BY4741 | rsc1Δ::kanMX4 | RSC | 92 | 140 | 100 |
| 5266 | BY4741 | rsc2Δ::kanMX4 | RSC | 70 | 45 | 42 |
| 3385 | BY4741 | isw1Δ::kanMX4 | ISW1 | 100 | ||
| 1601 | BY4741 | isw2Δ::kanMX4 | ISW2 | 100 | ||
| 4500 | BY4741 | itc1Δ::kanMX4 | ISW2 | 100 | ||
| 6160 | BY4741 | chd1Δ::kanMX4 | Homodimer | 100 | ||
| 1431 | BY4741 | not3Δ::kanMX4 | CCR4-NOT | 100 | 70 | 140 |
| 207 | BY4741 | not4Δ::kanMX4 | CCR4-NOT | 100 | 150 | 100 |
| 5491 | BY4741 | not5Δ::kanMX4 | CCR4-NOT | 42 | 56 | 17 |
| 7123 | BY4741 | caf1Δ::kanMX4 | CCR4-NOT | 27 | 44 | 8.2 |
| 7048 | BY4741 | caf4Δ::kanMX4 | CCR4-NOT | 97 | 100 | 110 |
| 5647 | BY4741 | caf16Δ::kanMX4 | CCR4-NOT | 100 | 62 | 110 |
| 6925 | BY4741 | caf17Δ::kanMX4 | CCR4-NOT | 91 | 280 | 80/PICK> |
| 1156 | BY4741 | caf40Δ::kanMX4 | CCR4-NOT | 100 | 130 | 120 |
| 6405 | BY4741 | caf130Δ::kanMX4 | CCR4-NOT | 100 | 71 | 110 |
| 6990 | BY4741 | dbf2Δ::kanMX4 | CCR4-NOT | 70 | 46 | 93 |
| 3858 | BY4741 | dhh1Δ::kanMX4 | CCR4-NOT | 64 | 11 | 6.3 |
| 4279 | BY4741 | srb9Δ::kanMX4 | SRB/MED, CCR4-NOT | 84 | 25 | 69 |
| 2786 | BY4741 | srb10Δ::kanMX4 | SRB/MED, CCR4-NOT | 58 | 170 | 35 |
| 5351 | BY4741 | srb11Δ::kanMX4 | SRB/MED, CCR4-NOT | 92 | 23 | 91 |
| 6611 | BY4741 | srb2Δ::kanMX4 | SRB/MED | 76 | 74 | 62 |
| 4734 | BY4741 | srb5Δ::kanMX4 | SRB/MED | 73 | 140 | 47 |
| 5799 | BY4741 | srb8Δ::kanMX4 | SRB/MED | 95 | 46 | 100 |
| 5489 | BY4741 | med1Δ::kanMX4 | SRB/MED | 88 | 120 | 100 |
| 5385 | BY4741 | med9Δ::kanMX4 | SRB/MED | 84 | 210 | 87 |
| 4518 | BY4741 | nut1Δ::kanMX4 | SRB/MED | 100 | 140 | 120 |
| 4393 | BY4741 | pgd1Δ::kanMX4 | SRB/MED | 58 | 50 | 26 |
| 3119 | BY4741 | rox3Δ::kanMX4 | SRB/MED | 12 | 230 | 35 |
| 1976 | BY4741 | sin4Δ::kanMX4 | SRB/MED, Paf1 | 85 | 500 | 100 |
| 1742 | BY4741 | gal11Δ::kanMX4 | SRB/MED, Paf1 | 24 | 98 | 27 |
| 5326 | BY4741 | cdc73Δ::kanMX4 | Paf1 complex | 94 | 19 | 92 |
| 4611 | BY4741 | rtf1Δ::kanMX4 | Paf1 complex | 100 | ||
| 2379 | BY4741 | leo1Δ::kanMX4 | Paf1 complex | 100 | ||
| 35727 | BY4743 | paf1Δ::kanMX4/paf1Δ::kanMX4 | Paf1 complex | 50 | 7.2 | 26 |
| 387 | BY4741 | ccr4Δ::kanMX4 | CCR4-NOT, Paf1 | 36 | 8.5 | 4.3 |
| 4072 | BY4741 | hpr1Δ::kanMX4 | Paf1, THO/TREX | 27 | 5.1 | 1.3 |
| 508 | BY4741 | mft1Δ::kanMX4 | THO/TREX | 100 | ||
| 1191 | BY4741 | tex1Δ::kanMX4 | THO/TREX | 100 | ||
| 2861 | BY4741 | thp2Δ::kanMX4 | THO/TREX | 100 | ||
| 4411 | BY4741 | dst1Δ::kanMX4 | TFIIS | 100 | ||
| 6986 | BY4741 | spt4Δ::kanMX4 | SPT | 100 | ||
| LSO2 | MATahis3Δ1 leu2Δ0 met15Δ0 ura3Δ0 med2Δ::kanMX4 | SRB/MED | 36 | 120 | 32 | |
All strains used for ChIP analysis containing Myc13-tagged alleles of coactivator genes were created in the gcn4Δ strain (strain 249) by a PCR-based method for tagging chromosomal genes by yeast transformation (77). The pFA6a-13Myc-His3MX6 plasmid was used as a template, and transformants were selected on synthetic complete medium lacking histidine (SC−His medium). His+ colonies were analyzed by colony PCR to verify the presence of the tag in the gene of interest and by Western analysis to verify expression of the tagged protein. Colony PCRs were performed as described previously (2). Strains used for glutathione S-transferase (GST) pull-down experiments containing Myc13-tagged alleles of coactivator genes were created in the same manner in the WT strain BY4741.
Plasmids containing CAF1, PAF1, SRB10, NOT5, or SWI3 were made by PCR amplification of the relevant gene followed by restriction digestion and insertion of the fragments into YCplac33 for CAF1 and PAF1 (41) or pRS316 for SRB10, NOT5, and SWI3 (115). Plasmids p2382 and pHQ1239, encoding HA3-Gcn4p, were produced as follows. Plasmid pCD48 (29) contains a GCN4 allele with a BglII site just before the stop codon. Into this site, a BglII fragment encoding three tandem hemagglutinin (HA) epitopes was cloned to generate plasmid p2382. A SalI-EcoRI fragment from p2382 containing GCN4-HA3 was subcloned into the high-copy-number URA3 vector YEplac195 (41) to generate pHQ1239. Plasmid pSK-1 containing GCN4-Myc13 was created by inserting a BglII-BamHI fragment encoding 13 Myc epitopes into the BglII site of plasmid pCD48.
For reporter gene assays, deletion mutants and the isogenic WT and gcn4Δ strains were transformed with pHYC2 carrying the UASGCRE-CYC1-lacZ reporter (50) or pKN7 carrying the HIS3-GUS fusion (92). Three independent transformants were replica plated to sulfometuron methyl (SM)-containing medium and compared to untransformed cells to ensure that the SMs phenotype was unchanged. All strains were grown to saturation in SC−Ura medium and diluted to an optical density at 600 nm of ~0.5 in two identical cultures. After 2.5 h of growth, one set of cultures was harvested and resuspended in SC medium lacking Ile and Val and also lacking uracil (SC−ILV−Ura medium) containing 0.5 μg of SM per ml. Uninduced cultures were grown for a total of 6 h, and induced cultures were grown in the presence of SM for 6 h. Enzyme assays were performed as previously described for β-galactosidase (83) and β-glucuronidase (92).
GST pull-down, Western blot, and Northern blot analyses.
Bacterial extracts containing GST proteins were prepared from transformants of Escherichia coli strain BL21 (30). Yeast whole-cell extracts (WCEs) were prepared (138) and GST pull-down assays were performed (30) as described previously. Samples were resolved on 4 to 12% bis-Tris NuPAGE gels (Invitrogen) with MOPS (morpholinepropanesulfonic acid) buffer according to the manufacturer's protocol. Proteins were transferred to nitrocellulose membranes and detected by immunoblotting. Rabbit polyclonal antisera have been described for Srb7p (47), Swp73p (15), Caf16p (75), and Taf40p (59). Purified rabbit polyclonal Sth1p antibodies were a generous gift from Brad Cairns. Myc-tagged proteins were detected with mouse monoclonal c-Myc (9E10) antibodies from Santa Cruz Biotechnologies (catalog no. sc-40) at a 1:500 dilution.
Yeast cultures for Western analysis of HA3-Gcn4p were grown and induced as described above for the reporter gene assays. Extracts were prepared as described previously (29) except that additional protease inhibitors were added (Roche complete inhibitor used at a 1× concentration), and the cells were lysed in 14-ml tubes by using 10 cycles of 30 s of vortexing with 1.5 min of cooling on ice between cycles. Protein concentration was determined with a bicinchoninic acid protein assay kit (Pierce). Proteins were resolved on 10% bis-Tris NuPAGE gels (Invitrogen) in MOPS buffer at a constant voltage of 200 V for 1.5 h with the gel chamber submerged in ice. After transfer of the proteins to nitrocellulose, the membranes were cut and probed with rabbit polyclonal HA antibodies from Santa Cruz Biotechnologies (probe Y-11; catalog no. sc-805) at a dilution of 1:500 to detect HA3-Gcn4p and with Gcd6p antibodies (14).
Cultures were grown and induced for Northern analysis as described above or in some cases by using the inducing conditions described below for ChIP assays. Total RNA was extracted and subjected to Northern analysis as described previously (92). Probes were prepared from PCR amplified yeast genomic DNA or as restriction fragments from plasmid clones by random prime labeling using the RediPrime II system (Invitrogen).
Chromatin immunoprecipitations.
Yeast cells were grown as described above for reporter assays except that SM induction was carried out for 2 h. Living cells were fixed with 1% formaldehyde and broken by vortexing as described previously (62). Lysates were collected and sonicated to produce chromatin fragments of 200 to 1,000 bp, with an average size of ~500 bp. After sonication, the chromatin extracts were clarified by centrifugation for 1 h. Chromatin immunoprecipitation was conducted as described (24). Quantitative PCRs contained 1× Platinum Taq polymerase buffer (Invitrogen), 1.5 mM Mg2Cl2, 0.2 mM deoxynucleoside triphosphate, 1.6 μCi of [33P]dATP (Amersham), 0.5 μM POL1 primer pair, 0.15 μM ARG1UAS primer pair, 1.5 U of Platinum Taq polymerase (Invitrogen), and 1/10 of the immunoprecipitated chromatin sample or 1,000-fold diluted input DNA in 15-μl reaction volumes. PCR parameters were 94°C for 4 min; 94°C for 30 s, 52°C for 30 s, and 65°C for 1 min for 26 cycles; and then 65°C for 5 min. PCR products were resolved on 6% polyacrylamide gels and quantified by phosphorimaging analysis.
The sequences of the POL1 primer pair are 5′ GAC AAA ATG AAG AAA ATG CTG ATG CAC C 3′ (positions 2477 to 2504, with the A of the start codon as position 1) and 5′ TAA TAA CCT TGG TAA AAC ACC CTG 3′ (positions 2730 to 2707). The sequences of the ARG1UAS primer pair are 5′ ACG GCT CTC CAG TCA TTT AT 3′ (−378 to −359, with the A of the start codon as +1) and 5′ GCA GTC ATC AAT CTG ATC CA 3′ (−213 to −232).
RESULTS
A comprehensive analysis of nonessential coactivator subunits reveals novel requirements for activation by Gcn4p.
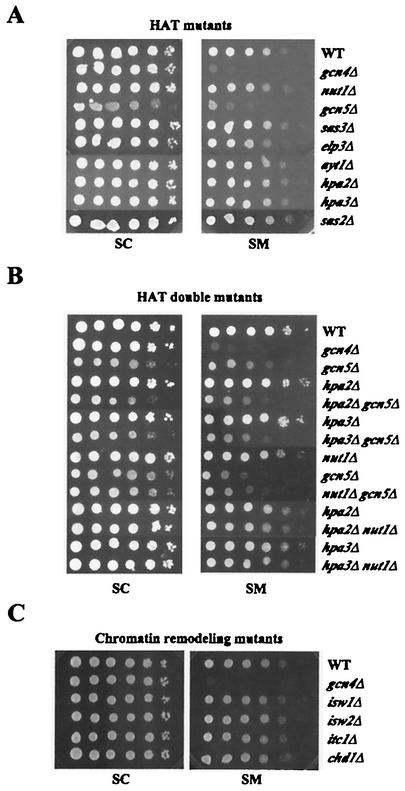
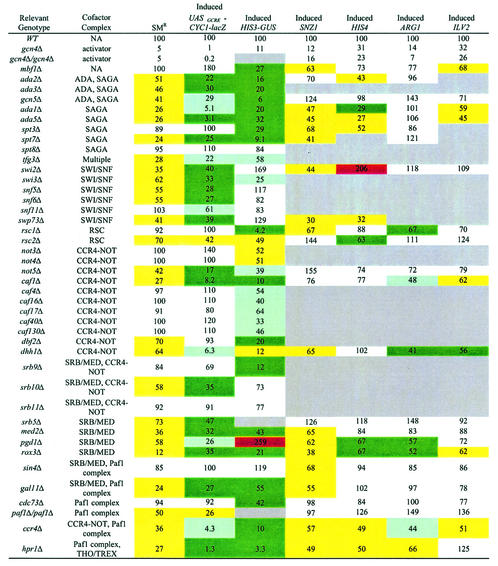
Mutants from the Saccharomyces Genome Deletion Project (136) harboring deletions in each of 80 genes encoding proteins implicated as coactivators or corepressors (Table (Table1)1) were tested for sensitivity to SM, an inhibitor of leucine, isoleucine, and valine biosynthesis (65). Mutants with defects in Gcn4p-mediated induction of enzymes in these pathways are unable to grow on SM-containing medium (Gcn− phenotype) (55, 93). Tenfold serial dilutions of each strain were spotted on SC−ILV medium and containing various concentrations of SM. Each strain was assigned a numerical score based on its degree of growth at each dilution for four different concentrations of SM, and the resulting scores were expressed as a percentage of that assigned to the WT. This analysis provided a semiquantitative assessment of SM resistance for each strain (SMr) (Fig. (Fig.1A).1A). We also analyzed the growth of the mutants on SC medium without SM to identify mutants with slow-growth (Slg−) phenotypes, and we adjusted the SMr scores accordingly (e.g., rox3Δ and not5Δ in Fig. Fig.1A).1A). The resulting SMr phenotypes of all of the strains are listed in Table Table11 and plotted in the histograms shown in Fig. 2A and B and Fig. 3B and C. For every mutant judged to have a level of SM resistance that was <85% of that of the WT, we confirmed that its SM-sensitive phenotype (SMs) was complemented by a plasmid-borne copy of the WT gene (Table (Table2;2; e.g., gal11Δ in Fig. Fig.1C).1C). PCR analysis of genomic DNA was carried out to verify the presence of the relevant deletion (Fig. (Fig.1B)1B) in the remaining mutants with SM resistance that was >85% of that of the WT.
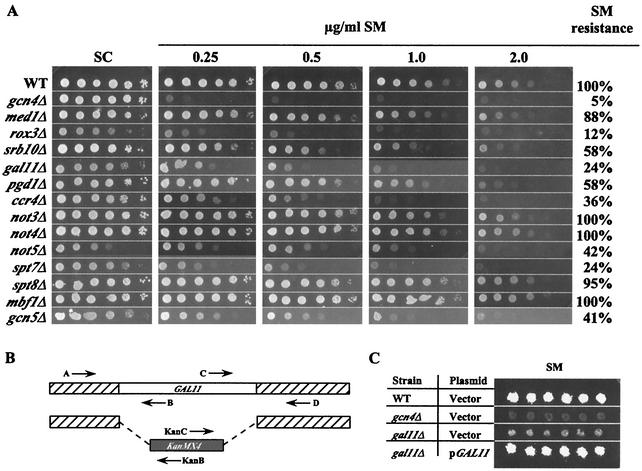
Analysis of SM resistance and physical and genetic assays used to confirm the identity of deletion strains. (A) Tenfold serial dilutions of the isogenic WT, gcn4Δ, and relevant deletion strains grown in SC medium were spotted to SC (control) medium or SC−ILV medium containing SM at 0.25, 0.5, 1.0, and 2.0 μg/ml. Growth on each plate after 3 days at 25°C was scored and adjusted for slow growth on SC medium, and these values were used to generate semiquantitative scores expressed as a percentage of the WT score on the same plate (SM resistance). (B) Schematic representation of PCR confirmation of deletions. For each gene of interest an upstream primer (A) or downstream primer (D) was used in conjunction with internal primers (B or C, respectively) to identify the WT allele or with primers specific for the kanMX4 sequences (primer KanB or KanC, respectively) to identify the deletion. All primer sequences were the same as those used by the Saccharomyces Genome Deletion Project for confirmation of the deletions (listed at http://www-sequence.stanford.edu/group/yeast_deletion_project/deletions3.html). (C) Example of the complementation assay used to confirm the identity of mutants displaying SM resistance less than 85% of that of the WT. A plasmid containing the WT gene corresponding to the relevant deletion (in this case GAL11; see Table Table22 for a complete list of plasmids), or the empty vector, was introduced into the mutant strain, and transformants were tested by replica plating for sensitivity to SM.
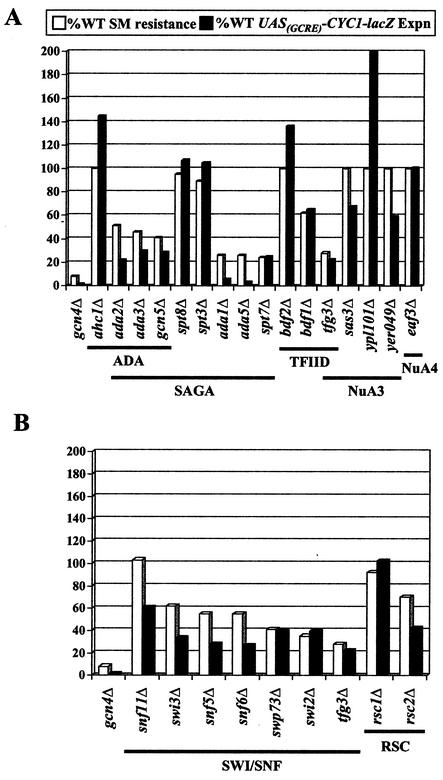
Phenotypes of deletion mutants lacking subunits of chromatin-modifying complexes. The SM resistance measured as described for Fig. Fig.1A1A and induced UASGCRE-CYC1-lacZ expression measured as described in Table Table11 for each deletion strain are shown graphically as percentages of the WT values. The values for the UASGCRE-CYC1-lacZ reporter are averages for three independent transformants induced with SM. Standard deviations, all less than 20% of the mean values, have been omitted for clarity. (A) HAT-containing complexes; (B) chromatin-remodeling complexes.
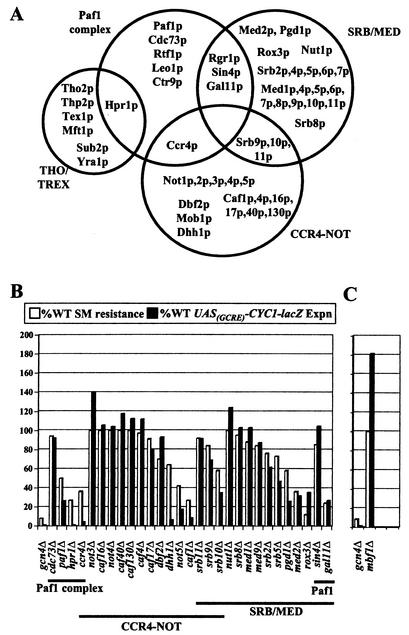
Phenotypes of mediator and related complexes and miscellaneous mutants. (A) Venn diagram depicting the relationships among the subunits of CCR4-NOT, SRB/MED, the Paf1 complex, and THO/TREX; (B) graphic representation of the phenotypes of deletion mutants lacking subunits of these complexes, as shown in Fig. Fig.2;2; (C) phenotypes of the mbf1Δ strain analyzed as for panel B.
TABLE 2.
Plasmids used for complementation assay
| Gene | Plasmid | Source |
|---|---|---|
| ADA1 | YCp50-ADA1 | 52 |
| ADA2 | pNS3.8 | 10 |
| ADA3 | pADA3-HHV | 100 |
| ADA5 | pSR36 | 108 |
| BDF1 | BDF1/pRS316 | B. Futcher |
| CAF1 | YCp33/CAF1 | This study |
| CCR4 | YEp213 + CCR4 | 46 |
| DHH1 | YEp213 + DHH1 | 46 |
| GAL11 | pJF111 | 37 |
| GCN5 | GCN5/pRS316 | 81 |
| HPR1 | pHK229 | H. Klein |
| MED2 | pGM26 | 87 |
| NOT5 | pRS316/NOT5 | This study |
| PAF1 | YCplac33/PAF1 | This study |
| PGD1 | p316HRS1-11 | 111 |
| ROX3 | YCp(33)ROX3H | 36 |
| RSC2 | 316.RSC2 | 16 |
| SPT7 | pFW127 | F. Winston |
| SNF5 | pAC153 | 1 |
| SNF6 | pEL3.10 | 35 |
| SRB2 | pCT24 | R. Young |
| SRB5 | pCT39 | 128 |
| SRB10 | pRM57 | This study |
| SW12 | pLN138-4 | 1 |
| SW13 | pRS316/SW13 | This study |
| SWP73 | pUCA-SWP73 | 15 |
| TFG3 | 316.TFG3 | B. Cairns |
Because Gcn4p target genes are often regulated by more than one transcriptional activator (48), the SMs phenotype could arise from defective activation of an isoleucine-valine biosynthetic (ILV) gene by an activator besides Gcn4p. To measure activation by Gcn4p more directly, we assayed expression of a Gcn4p-dependent lacZ reporter in cells treated with SM, in which Gcn4p synthesis is induced at the translational level (134). This UASGCRE-CYC1-lacZ reporter contains two copies of a Gcn4p binding site from HIS4 (UASGCRE) inserted at the CYC1 promoter in place of the endogenous upstream activation sequence (UAS) element. It is induced by a factor of 20 to 30 in WT cells (typically, from 75 to 2,000 U of β-galactosidase activity) and is almost completely dependent on Gcn4p (50), giving only 1% of WT expression in the gcn4Δ mutant (Table (Table11 and Fig. Fig.2A).2A). At least three independent transformants of each SMs strain harboring the reporter plasmid were assayed for β-galactosidase activity under inducing and noninducing conditions (i.e., presence and absence of SM), and the mean values are listed in Table Table11 and graphed in Fig. Fig.22 and and3.3. In all cases, the standard deviations were less than 20% of the means.
With only one exception (dbf2Δ), all of the mutants whose resistance to SM was less than 75% of that of the WT also were impaired for induction of the UASGCRE-CYC1-lacZ reporter, showing less than 65% of WT expression under inducing conditions. The results for the 27 mutants fulfilling these criteria are presented in Table Table11 and in Fig. Fig.22 and and3.3. Although the dbf2Δ mutant was not significantly impaired for UASGCRE-CYC1-lacZ expression, it was defective for induction of a HIS3-GUS reporter described below. In general, the SMs phenotype correlated reasonably well with the quantitative defects in UASGCRE-CYC1-lacZ induction (Fig. 2A and B and and3B).3B). In the hpr1Δ, ccr4Δ, and dhh1Δ strains, however, reporter induction was considerably more impaired than was expected from the severity of SM sensitivity (Fig. (Fig.3B3B and Table Table1).1). This discrepancy could be explained in several ways. One possibility is that other activators responsible for basal expression of the ILV genes are less dependent on Hpr1p, Ccr4p, or Dhh1p function than is Gcn4p. Alternatively, the mutations could have a greater effect on the function of the CYC1 core promoter in the UASGCRE-CYC1-lacZ construct than on the core promoters of ILV genes. Finally, it was shown previously that hpr1 mutations impede transcriptional elongation through lacZ sequences (18), and this could lead to a greater impairment of UASGCRE-CYC1-lacZ expression than with authentic Gcn4p target genes.
Expression of UASGCRE-CYC1-lacZ was reduced in the hpr1Δ strain by a factor of 77 under inducing conditions but by a factor of only 20 under noninducing conditions (Table (Table1).1). Thus, while the low level of UASGCRE-CYC1-lacZ expression in the hpr1Δ mutant probably reflects an elongation defect, the fact that this mutation had a greater effect on reporter expression under inducing than noninducing conditions suggests that transcriptional activation by Gcn4p is also impaired. If the effect of a coactivator mutation on UASGCRE-CYC1-lacZ expression was greater under inducing than noninducing conditions by a factor of 2.5 or more, we concluded that transcriptional activation was damaged in that strain, in addition to other defects in elongation or core promoter function that might exist. The group of mutants that satisfied this criterion is identified in Table Table1.1. In addition to hpr1Δ, this group also includes not5Δ, caf1Δ (affecting CCR4/NOT), and multiple mutations in subunits of SAGA, SWI/SNF, and SRB/MED.
A second group of mutants that did not satisfy this rigorous criterion but were comparable to gcn5Δ in their relative impairment of reporter expression under inducing and noninducing conditions are also shown in Table Table1.1. Mutants in this second group include ada1Δ (SAGA), ccr4Δ and dhh1Δ (affecting the Paf1 complex or CCR4/NOT), pgd1Δ (SRB/MED), snf11Δ (SWI/SNF), yer049Δ (NuA3), and tfg3Δ (affecting multiple complexes) strains. Given the considerable evidence that Gcn5p is a bone fide coactivator for Gcn4p (63, 64, 126), it seems likely that many mutants in this second class also are defective for transcriptional activation by Gcn4p. A third group of five mutants in Table Table11 reduced UASGCRE-CYC1-lacZ expression to the same, or even greater, extent under noninducing versus inducing conditions, making it unclear whether activation by Gcn4p was defective in these strains.
Finally, there were mutations affecting subunits of SAGA (spt3Δ and spt8Δ), NuA3 (ypl101Δ), SWI/SNF (swi2Δ), CCR4/NOT (caf17Δ), and SRB/MED (med9Δ, rox3Δ, and sin4Δ) that led to expression levels two- to fivefold higher than WT levels under noninducing conditions (Table (Table1).1). Such mutations may eliminate a negative regulatory mechanism that represses CYC1 promoter activity at low levels of Gcn4p. Interestingly, the swi2Δ and rox3Δ mutations reduced UASGCRE-CYC1-lacZ expression under inducing conditions, indicating dual positive and negative functions at this promoter for these subunits of SWI/SNF and SRB/MED (Table (Table11).
The array of coactivator subunits required for activation by Gcn4p varies at different promoters.
In an effort to confirm the conclusions reached above, we assayed a subset of the mutants for expression of a second Gcn4p-dependent reporter containing the 5′ noncoding region of HIS3 from position −450 to −3 (relative to the ATG start codon) fused to GUS coding sequences. As above, at least three independent transformants of each strain harboring the reporter plasmid were assayed for β-glucuronidase activity under inducing and noninducing conditions, and the mean values are listed in Table Table3.3. In all cases, the standard errors were less than 20% of the mean values. The HIS3-GUS reporter is induced three- to eightfold by SM treatment of WT cells (data not shown), and ~90% of its expression under these conditions is dependent on Gcn4p (Table (Table3).3). The residual HIS3-GUS expression in the gcn4Δ mutant can be attributed to the AT-rich element that confers Gcn4p-independent promoter activity (122). Because HIS3-GUS expression is ~5-fold more dependent on Gcn4p under inducing than noninducing conditions (Table (Table3),3), by comparing the relative impairment of reporter expression under these conditions, we could evaluate whether the coactivator mutants are defective for activation of HIS3-GUS by Gcn4p. The data are coded according to criteria similar to those employed above for the UASGCRE-CYC1-lacZ reporter (see the legend to Table Table33 for details). The results suggest that Hpr1p, the Cdc73p subunit of the Paf1 complex, RSC subunit Rsc1p, and multiple subunits of SAGA, SRB/MED, and CCR4/NOT are all required for full activation of the HIS3 promoter by Gcn4p (Table (Table33).
TABLE 3.
HIS3-GUS expression phenotypes of cofactor mutants
| Relevant genotype | Cofactor complex | β-Glucuronidase activity (% of WT) fora:
| |
|---|---|---|---|
| Uninduced HIS3-GUS | Induced HIS3-GUS | ||
| gcn4Δ | Activator | 55 | 11 |
| mbf1Δ | NAb | 76 | 27 |
| ada2Δ | ADA, SAGA | 86 | 16 |
| ada3Δ | ADA, SAGA | 143 | 20 |
| gcn5Δ | ADA, SAGA | 29 | 6.4 |
| spt8Δ | SAGA | 289 | 84 |
| spt3Δ | SAGA | 141 | 29 |
| ada1Δ | SAGA | 149 | 20 |
| ada5Δ | SAGA | 235 | 32 |
| spt7Δ | SAGA | 46 | 9.1 |
| tfg3Δ | Multiplec | 113 | 58 |
| rsc1Δ | RSC | 15 | 4.2 |
| rsc2Δ | RSC | 23 | 49 |
| swi2Δ | SWI/SNF | 523 | 169 |
| swi3Δ | SWI/SNF | 43 | 25 |
| snf5Δ | SWI/SNF | 363 | 117 |
| snf6Δ | SWI/SNF | 147 | 82 |
| snf11Δ | SWI/SNF | 112 | 83 |
| swp73Δ | SWI/SNF | 437 | 129 |
| cdc73Δ | Paf1 complex | 117 | 42 |
| hpr1Δ | Paf1 complex, THO/TREX | 13 | 3.3 |
| ccr4Δ | CCR4-NOT, Paf1 complex | 25 | 10 |
| not3Δ | CCR4-NOT | 58 | 52 |
| caf16Δ | CCR4-NOT | 76 | 40 |
| not4Δ | CCR4-NOT | 67 | 51 |
| caf40Δ | CCR4-NOT | 58 | 33 |
| caf130Δ | CCR4-NOT | 56 | 46 |
| caf4Δ | CCR4-NOT | 86 | 54 |
| caf17Δ | CCR4-NOT | 97 | 64 |
| dbf2Δ | CCR4-NOT | 40 | 20 |
| dhh1Δ | CCR4-NOT | 16 | 12 |
| not5Δ | CCR4-NOT | 67 | 39 |
| caf1Δ | CCR4-NOT | 53 | 10 |
| srb11Δ | SRB/MED, CCR4-NOT | 140 | 77 |
| srb9Δ | SRB/MED, CCR4-NOT | 53 | 12 |
| srb10Δ | SRB/MED, CCR4-NOT | 227 | 73 |
| pgd1Δ | SRB/MED | 469 | 259 |
| med2Δ | SRB/MED | 97 | 43 |
| rox3Δ | SRB/MED | 92 | 21 |
| sin4Δ | SRB/MED, Paf1 complex | 253 | 119 |
| ga111Δ | SRB/MED, Paf1 complex | 145 | 55 |
Interestingly, the spt3Δ mutant was impaired for HIS3-GUS induction (Table (Table3),3), even though it showed WT induction of UASGCRE-CYC1-lacZ (Table (Table1).1). Hence, the requirement for this SAGA subunit in activation by Gcn4p seems to vary between these two promoters. This last phenomenon also holds for the CCR4/NOT subunit Dbf2p, the SRB/MED subunit Srb9p, Rsc1p, and Mbf1p. The SRB/MED subunit Pgd1p showed the opposite behavior, as pgd1Δ cells were strongly impaired for UASGCRE-CYC1-lacZ induction (Table (Table1)1) but showed greater-than-WT induction of HIS3-GUS. Thus, Pgd1p may have a role in negative control that overrides its importance as a coactivator for Gcn4p at HIS3-GUS. This conclusion is consistent with previous results indicating dual positive and negative functions for Pgd1p at other promoters (101). Whereas the rsc1Δ mutation had a greater effect than rsc2Δ on HIS3-GUS expression (Table (Table3),3), the opposite was true for the UASGCRE-CYC1-lacZ reporter (Table (Table1).1). There are two forms of RSC that contain either Rsc1p or Rsc2p (16). Thus, it seems that Gcn4p utilizes both forms of RSC, but the relative importance of the Rsc1p- and Rsc2p-containing complexes depends on the promoter.
It was also important to analyze the effects of coactivator mutations on induction of authentic mRNAs by Gcn4p. Accordingly, we prepared total RNA from mutant and WT strains and conducted Northern analysis using probes specific for the Gcn4p target genes SNZ1, HIS4, ARG1, and ILV2 (94) and for ACT1, analyzed as an internal control. RNA was prepared from two independent cultures of each strain, and the Northern signals for the Gcn4p-regulated mRNAs were quantified and normalized to the ACT1 signals. Typical Northern data are presented in Fig. Fig.44 for the duplicate determinations of selected mutants to illustrate the reproducibility of these data. The mean normalized mRNA levels for the entire set of mutants are given in Table Table44.
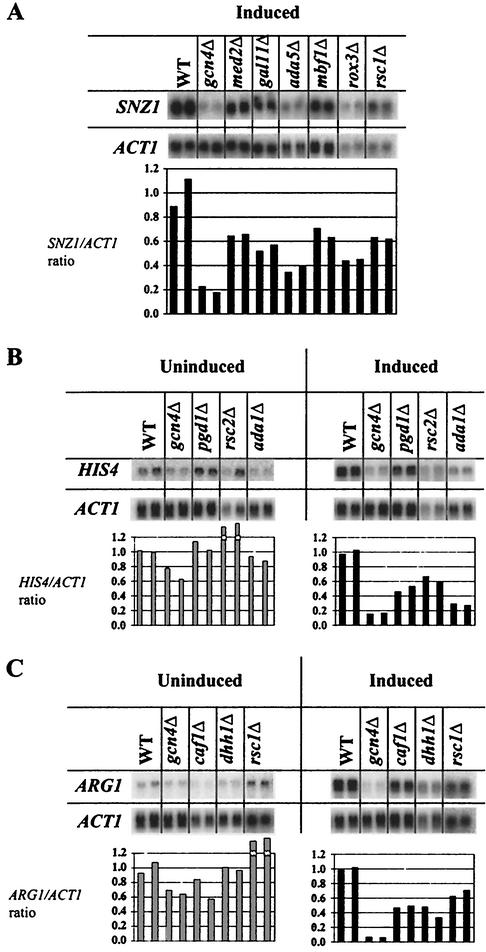
Northern analysis of authentic Gcn4p target genes in a typical subset of deletion mutants. Total RNA was isolated for each strain under the inducing and noninducing conditions described in Table Table1,1, and equal amounts of RNA were subjected to Northern analysis, probing for ACT1, ARG1, HIS4, ILV2, and SNZ1 mRNAs. Adjacent lanes contain RNA samples isolated from two independent cultures for each strain. The hybridization signals were quantified with a PhosphorImager (Molecular Dynamics, with ImageQuant 5.2 software), and the values obtained for SNZ1 (A), HIS4 (B), and ARG1 (C) were normalized to the corresponding ACT1 signals. The resulting ratios calculated for the mutant strains were normalized to the ratio measured in the WT, and the normalized ratios are plotted in histograms beneath the corresponding lanes of each blot.
TABLE 4.
Northern blot analysis of cofactor mutants
| Relevant genotype | Cofactor complex | Mean normalized signal ratio (% of WT)a
| ||||||
|---|---|---|---|---|---|---|---|---|
| SNZ1, induced | HIS4
| ILV2
| ARG1
| |||||
| Uninduced | Induced | Uninduced | Induced | Uninduced | Induced | |||
| gcn4Δ | Activator | 12b,c | 67b,c | 31b,c | 78c | 32c | 51b,c | 14b,c |
| mbf1Δ | NAd | 63c | 73c | 68c | 77c | |||
| ada2Δ | ADA, SAGA | 70b | 47b | 43b | 207b | 96b | ||
| gcn5Δ | ADA, SAGA | 124b,c | 105b,c | 98b,c | 38c | 71c | 313b,c | 143b,c |
| ada1Δ | SAGA | 47c | 90c | 29 c c | 69c | 59c | 915c | 101c |
| ada5Δ | SAGA | 45c | 27c | 45c | 106c | |||
| spt3Δ | SAGA | 68b | 69b | 52b | 130b | 86b | ||
| spt7Δ | SAGA | 41b | 458b | 121b | ||||
| swi2Δ | SWI/SNF | 44b,c | 139b | 206b | 109c | 118b,c | ||
| swp73Δ | SWI/SNF | 30b | 32b | |||||
| rsc1Δ | RSC | 67c | 118c | 88c | 88c | 70b,c | 198c | 67 c c |
| rsc2Δ | RSC | 144b,c | 242c | 63c | 99c | 124c | >600c | 111c |
| not5Δ | CCR4-NOT | 155c | 118c | 74c | 85c | 79c | 91c | 72c |
| caf1Δ | CCR4-NOT | 76b,c | 103c | 77c | 51c | 62c | 71c | 48c |
| dhh1Δ | CCR4-NOT | 65b,c | 135b,c | 102b,c | 96c | 56 c c | 99c | 41 c c |
| srb5Δ | SRB/MED | 126b,c | 307c | 118c | 152c | 92c | >300b,c | 148b,c |
| pgd1Δ | SRB/MED | 62c | 144b,c | 67 b,c b,c | 114c | 72c | 474c | 57 c c |
| rox3Δ | SRB/MED | 38b,c | 129b,c | 67 b,c b,c | 83c | 62c | 105b,c | 52 b,c b,c |
| sin4Δ | SRB/MED, Paf1 | 68c | 125c | 94c | 91c | 86c | 379c | 85c |
| gal11Δ | SRB/MED, Paf1 | 55c | 78b,c | 102b,c | 118c | 78c | 213b,c | 97b,c |
| med2Δ | SRB/MED | 65c | 76c | 84c | 168c | 88c | 171c | 83c |
| cdc73Δ | Paf1 complex | 98c | 84c | 77c | 100c | |||
| ccr4Δ | CCR4-NOT, Paf1 | 57b,c | 70b,c | 49b,c | 60c | 51c | 70c | 44c |
| hpr1Δ | Paf1, THO/TREX | 49b,c | 59b,c | 50b,c | 54c | 125c | 65c | 66c |
| gcn4Δ/gcn4Δ | Activator | 16c | 57c | 23c | 81c | 26c | 54c | 7c |
| paf1Δ/paf1Δ | Paf1 complex | 97c | 327c | 126c | 120c | 136c | 2,770c | 149c |
| snf5Δ/snf5Δ | SWI/SNF | 75c | 93c | 85c | 261c | 162c | 500c | 104c |
SNZ1 mRNA is an interesting case because it resembles the UASGCRE-CYC1-lacZ construct in showing very low levels of expression in noninducing conditions and a strong dependence on Gcn4p under inducing conditions (94). As shown in Fig. Fig.4A4A and Table Table4,4, mutations in multiple subunits of SAGA, SWI/SNF, and SRB/MED, Rsc1p (RSC), Dhh1p (CCR4/NOT), Ccr4p (CCR4/NOT and Paf1), Hpr1p (Paf1 and THO/TREX), and Mbf1p all lowered the induced level of SNZ1 mRNA to below 68% of WT levels. Surprisingly, deletion of GCN5 led to slightly greater than WT levels of SNZ1 mRNA. Although WT transcriptional induction of SNZ1 by Gcn4p requires the same coactivator complexes needed for high-level expression of the reporter constructs, the subunits most critically required differ somewhat among the SNZ1, HIS3-GUS, and UASGCRE-CYC1-lacZ promoters.
Efficient induction of HIS4 mRNA was dependent on multiple subunits of SAGA and SRB/MED and also required Rsc2p, Swp73p (SWI/SNF), Ccr4p, and Hpr1p (Paf1p and THO/TREX) (Fig. (Fig.4B4B and Table Table4).4). Surprisingly, deleting the ATPase subunit of SWI/SNF (Swi2p) led to greater-than-WT HIS4 expression, whereas inactivation of the Swp73p subunit significantly reduced HIS4 induction. It was shown recently that Swi2p can function as a repressor independently of other SWI/SNF subunits (82). Thus, perhaps Swi2p acts in this manner as a repressor at HIS4 and also in conjunction with other SWI/SNF subunits in activation and the former activity has the greater impact on HIS4 transcription. Again, the array of subunits of SAGA, SRB/MED, CCR4/NOT, SWI/SNF, and RSC critical for activation by Gcn4p differs somewhat between SNZ1 and HIS4: Swi2p, Rsc1p, Dhh1p, Med2p, Sin4p, and Gal11p are required at SNZ1 but not at HIS4, whereas Ada2p and Rsc2p are more important at HIS4 than at SNZ1.
ILV2 mRNA induction was substantially impaired by mutations in several subunits of SAGA and CCR4/NOT and in MBF1 and by deletion of ROX3 (SRB/MED) (Table (Table4).4). Thus, with the possible exception of SWI/SNF, high-level activation of ILV2 requires at least one subunit of the same coactivator complexes needed for activation of other Gcn4p-dependent promoters. We noted that a number of mutants with a SMs phenotype displayed WT or greater levels of ILV2 mRNA (Table (Table4).4). Presumably, these mutants are defective for induction of one of the other four ILV genes rather than ILV2 (58).
The coactivator requirements for activation of ARG1 transcription are unique. As for certain other Gcn4p target genes, high-level induction was impaired by mutations in multiple subunits of CCR4/NOT and SRB/MED, Rsc1p, and Hpr1p (Table (Table44 and Fig. Fig.4C).4C). However, ARG1 induction showed no significant dependence on any SAGA subunit. Moreover, nearly all of the SAGA mutations led to higher ARG1 expression under noninducing conditions (Table (Table4).4). The latter data are consistent with the finding that SAGA is required for arginine-specific repression of ARG1 by the ArgR/Mcm1p repressor complex (107). The fact that SAGA mutations did not produce higher-than-WT ARG1 expression under inducing conditions (Table (Table4)4) may indicate that activation of this promoter by Gcn4p is SAGA dependent, as observed for all other Gcn4p target genes. In this view, the SAGA subunit deletions have offsetting positive and negative effects on transcription by simultaneously impairing repression by ArgR/Mcm1p and activation by Gcn4p. Considering the other coactivator mutations that derepressed ARG1 mRNA under noninducing conditions (Table (Table4),4), we suggest that RSC, SWI/SNF, SRB/MED, and the Paf1 complex may also be required for ARG1 repression. The derepressing effect of the paf1Δ mutation on ARG1 mRNA is particularly striking.
Coactivator mutants with SMs phenotypes generally do not have reduced levels of Gcn4p.
A mutation may impair the activation of Gcn4p target genes by lowering the induced level of Gcn4p. To distinguish such mutants from those truly defective in activation, we measured the steady-state levels of an HA epitope-tagged version of Gcn4p by Western analysis in each SMs mutant. The relevant strains were transformed with a single-copy plasmid expressing HA3-Gcn4p from the native promoter (CEN/GCN4-HA3), and WCEs were prepared following growth in the presence of SM to induce HA3-Gcn4p expression. Western analysis using anti-HA antibodies showed that, as expected, HA3-Gcn4p levels increased dramatically on treatment of WT transformants with SM (data not shown). In 20 of 27 mutants tested, the levels of HA3-Gcn4p were greater than or equal to those in the WT strain. An example of the data obtained for one such mutant (ccr4Δ) is shown in Fig. Fig.5A5A (see Table Table55 for results on all mutants).
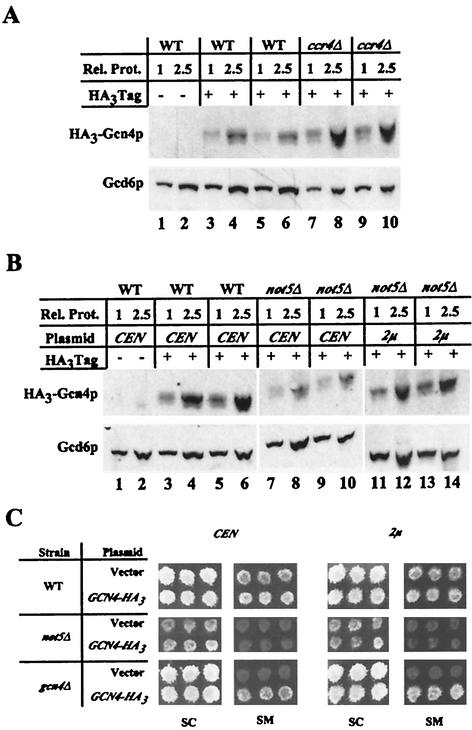
Western analysis of Gcn4p levels in Gcn− mutants. Single-copy plasmid p2382 (CEN) or high-copy-number plasmid pHQ1239 (2μm) harboring the GCN4-HA3 allele was introduced into the WT and Gcn− deletion strains, and the WT strain was also transformed with empty CEN or 2μm vector. (A and B) Extracts were prepared from two transformants of each strain induced with SM. Two amounts (20 and 50 μg) of total protein, labeled as relative protein (Rel. Prot.) amounts of 1 and 2.5, respectively, were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and probed with Gcd6p antibodies and anti-HA antibodies. Lanes 1 and 2 contain samples from the WT strain bearing the empty vector. All Western blotting was carried out two or more times, and representative results are presented for the ccr4Δ (A) and not5Δ (B) mutants. All results are summarized in Table Table5.5. (C) Most mutants with lowered levels of HA3-Gcn4p, such as the not5Δ strain, maintain their SMs phenotypes after transformation with the high-copy-number GCN4-HA3 plasmid (2μm). The not5Δ and gcn4Δ mutants and the WT strain containing p2382 (CEN), pHQ1239 (2μm), or empty vector were replica plated to SC−ILV medium containing 1 μg of SM per ml and incubated at 25°C for 3 days.
TABLE 5.
Summary of HA3-Gcn4p levels in Gcn− mutants
| Mutant allele | HA3-Gcn4p level (% of WT)a
| Complementationb of SMS phenotype by 2μm/GCN4-HA3 | |
|---|---|---|---|
| With CEN/GCN4-HA3 | With 2μm/GCN4-HA3 | ||
| ada1Δ | 40 | 100 | No |
| ada2Δ | 250 | ||
| ada3Δ | 200 | ||
| ada5Δ | 40 | 100 | No |
| bdf1Δ | 40 | 200 | Partial |
| caf1Δ | 100 | ||
| ccr4Δ | 250 | ||
| dhh1Δ | 20 | 200 | No |
| gal11Δ | 100 | ||
| gcn5Δ | 300 | ||
| hpr1Δ | 40 | 100 | No |
| med2Δ | 100 | ||
| not5Δ | 40 | 150 | No |
| paf1Δ | 100 | ||
| pgd1Δ | 100 | ||
| rox3Δ | 100 | ||
| rsc2Δ | 100 | ||
| snf5Δ | 150 | ||
| snf6Δ | 100 | ||
| spt7Δ | 100 | ||
| srb10Δ | 100 | ||
| srb2Δ | 100 | ||
| srb5Δ | 100 | ||
| swi2Δ | 400 | ||
| swi3Δ | 40 | 100 | Partial |
| swp73Δ | 250 | ||
| tfg3Δ | 120 | ||
In the remaining seven mutants, we saw a significant reduction in the levels of HA3-Gcn4p relative to the WT strain (Table (Table5),5), ranging from 20% (dhh1Δ) to 40% (ada1Δ, ada5Δ/spt20Δ, bdf1Δ, hpr1Δ, not5Δ, and swi3Δ) of the WT level. To determine whether the SMs phenotypes of this class resulted from low levels of the activator, we increased expression of HA3-Gcn4p by introducing GCN4-HA3 on a high-copy-number plasmid (2μm/GCN4-HA3). In five of these mutants (ada1Δ, ada5Δ/spt20Δ, dhh1Δ, hpr1Δ, and not5Δ), the levels of HA3-Gcn4p equaled or exceeded that in the WT strain containing single-copy GCN4-HA3, and the SM sensitivity remained unchanged. An example of this behavior is illustrated in Fig. 5B and C for the not5Δ mutant. We conclude that the SMs phenotypes of these five strains cannot be accounted for by reduced levels of Gcn4p and that Gcn4p activation function is impaired. It is also noteworthy that hpr1Δ cells showed no activation defect at ILV2, that dhh1Δ did not impair HIS4 induction, and that not5Δ cells showed normal induction of SNZ1 mRNA (Table (Table4).4). Thus, the reductions in Gcn4p levels observed in these three mutants are not great enough to impair the activation of all Gcn4p-dependent promoters. Transformants of the bdf1Δ and swi3Δ mutants harboring the 2μm/GCN4-HA3 plasmid had levels of HA3-Gcn4p exceeding that of the WT strain bearing CEN/GCN4-HA3; however, their SMs phenotypes were partially complemented (Table (Table5).5). Thus, the activation defects in the bdf1Δ and swi3Δ strains may result partly from reduced Gcn4p expression.
Mutations in enzymes affecting chromatin structure generally do not disrupt activation by Gcn4p.
Since Gcn4p requires a number of complexes involved in chromatin modification, it was possible that any perturbation of chromatin structure might affect activation by Gcn4p. To address this possibility, we examined mutants with deletions of other nonessential HATs, histone deacetylases and ATP-dependent chromatin-remodeling enzymes for growth in the presence of SM. Among the known HAT mutants, only the gcn5Δ strain showed sensitivity to SM (Fig. (Fig.6A).6A). Interestingly, deletion of the HAT subunit of SRB/MED, Nut1p, or the transcription Elongator complex, Elp1p, had no effect on SM resistance, as did deletions of two other members of Elongator, Elp2p and Elp3p (Fig. (Fig.6A6A and data not shown). To address the possibility of redundant contributions of different HATs to activation by Gcn4p, we generated double deletions of various HATs with gcn5Δ or nut1Δ and assayed growth on SM. Double mutants containing a deletion of GCN5 displayed phenotypes nearly identical to that of the gcn5Δ single mutant (Fig. (Fig.6B6B and data not shown). The gcn5Δ sas3Δ strain was not viable, consistent with previous findings (53). Combining deletions of various HATs with the nut1Δ mutation also did not reveal any additive SMs phenotypes (Fig. (Fig.6B6B and data not shown). Although histone deacetylases are normally associated with transcriptional repression, some deacetylase mutants show decreased transcription of specific genes (11); however, we detected no SM sensitivity for any deacetylase mutant (Table (Table11).
As Gcn4p requires both SWI/SNF and the highly related RSC complex for WT activation (Fig. (Fig.2B),2B), we assayed deletions of the nonessential subunits of other chromatin-remodeling complexes. As shown in Fig. Fig.6C,6C, we observed no SM sensitivity in mutants lacking nonessential subunits of the ISWI complexes (isw1Δ, isw2Δ, and itc1Δ) and Chd1p, which functions as a homodimer and can affect transcription both positively and negatively (129). Thus, the SMs phenotypes of the SWI/SNF and RSC mutants reflect a specific requirement for these complexes in activation by Gcn4p rather than a general perturbation of chromatin structure.
Gcn4p interacts with the RSC and CCR4-NOT complexes dependent on hydrophobic residues in the activation domain.
Previously, we showed that SAGA, SWI/SNF, and SRB/MED in WCEs bind specifically to a full-length GST-Gcn4p fusion, dependent on clusters of hydrophobic amino acids in the activation domain required for activation by Gcn4p in vivo (29, 30, 54, 93). Here, we performed similar experiments probing for subunits in the complexes newly determined to be required for Gcn4p activity: RSC, CCR4-NOT, and the Paf1 complex. Sth1p, the catalytic subunit of RSC, coprecipitated with WT GST-Gcn4p but not with the mutant protein containing 10 alanine substitutions in four hydrophobic clusters (10Ala) that destroy activation by Gcn4p in vivo (54) (Fig. (Fig.7A,7A, lanes 3 and 4). Similar results were obtained by probing for Swp73p of SWI/SNF and Srb7p of SRB/MED. As described above, Rsc1p and Rsc2p form mutually exclusive RSC complexes. To determine whether Gcn4p interacts with both forms of RSC, we used extracts from the rsc1Δ and rsc2Δ strains in pull-down assays. The Sth1p subunit in both extracts was precipitated by the WT but not by 10Ala GST-Gcn4p (Fig. (Fig.7A,7A, lanes 7 and 8 and lanes 11 and 12, respectively). Moreover, pull-down assays using extracts from strains in which either Rsc1p or Rsc2p is tagged with 13 copies of the Myc epitope show that WT GST-Gcn4p can precipitate both Rsc1p and Rsc2p (Fig. (Fig.7B,7B, lane 3). We conclude that both forms of the RSC complex can interact specifically with the Gcn4p activation domain in vitro, consistent with our data showing that both forms of RSC are required for WT activation of various Gcn4p-dependent promoters (Fig. (Fig.44).
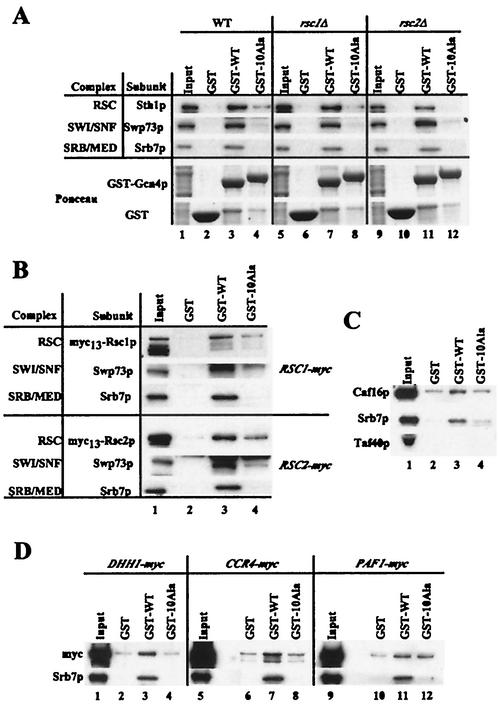
GST-Gcn4p interacts specifically with the RSC and CCR4-NOT complexes but not with the Paf1 complex in cell extracts. Equal amounts of GST, GST-Gcn4p (GST-WT), and GST-Gcn4p containing 10 alanine substitutions in the activation domain (GST-10Ala) were incubated with WCEs from yeast strains grown in YPD medium. The GST proteins were precipitated with glutathione Sepharose, resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and detected by Western analysis. (A) Equal amounts of WCEs from WT (BY4741), rsc1Δ (strain 4686), and rsc2Δ (strain 5266) strains were incubated with the GST proteins. Blots were probed with antibodies against the proteins listed on the left of the upper three panels. The bottom two panels depict Ponceau S staining of total proteins. Input lanes contained 1% of the WCEs used in pull-down assays. (B) Same as panel A except that WCEs were from derivatives of WT strain BY4741 expressing Myc13-Rsc1p (top three panels) or Myc13-Rsc2p (lower three panels), detected with Myc antibodies. (C) GST proteins were incubated with yeast WCE from WT strain BY4741, and the pull-down assays were probed with antibodies against the proteins listed beside the three panels, subunits of CCR4-NOT (Caf16p), SRB/MED (Srb7), and TFIID (TAF11/TAF40). (D) The GST proteins were incubated with WCEs from strains derived from BY4741 expressing Myc13-tagged forms of Dhh1p (DHH1-myc), Ccr4p (CCR4-myc), or Paf1p (PAF1-myc). The tagged proteins were detected with Myc antibody, and SRB/MED interaction was detected with Srb7p antibody. Input lanes contained 10% of the WCEs used in the pull-down assays.
The results of pull-down assays shown in Fig. 7C and D suggest that CCR4-NOT also interacts specifically with the Gcn4p activation domain but that the Paf1 complex does not. Probing the pull-down assays from a WT extract with antibodies against the Caf16p subunit of CCR4-NOT revealed a higher level of binding to the WT than to 10Ala GST-Gcn4p or GST alone, comparable to the results obtained for the SRB/MED subunit Srb7p (Fig. (Fig.7C,7C, lanes 2 to 4). As expected, the TFIID subunit Taf40p did not precipitate with GST-Gcn4p (30, 93). Similarly, using extracts from strains expressing Myc13-tagged forms of Dhh1p or Ccr4p in pull-down assays, we observed specific binding by both of these CCR4-NOT subunits to WT GST-Gcn4p (Fig. (Fig.7D,7D, lanes 1 to 8). By contrast, Myc13-tagged Paf1p did not interact with GST-Gcn4p dependent on the activation domain (Fig. (Fig.7D,7D, lanes 11 and 12). As Ccr4p is a component of CCR4-NOT and the Paf1 complex, it most likely precipitates with GST-Gcn4p as a component of the former complex.
Gcn4p recruits the coactivator complexes required for full activation function to a single promoter in vivo.
The results above indicate that SAGA, SWI/SNF, RSC, SRB/MED, CCR4-NOT, and Paf1 complexes and Mbf1p all play a role in activation by Gcn4p in vivo. To determine whether each of these coactivators is physically recruited by Gcn4p to one of its target genes, ARG1, we performed ChIP experiments. A panel of 15 strains was constructed from the gcn4Δ mutant (strain 249), each containing a different coactivator subunit tagged with 13 Myc epitopes and transformed with either the GCN4 plasmid pHQ1239 or an empty vector. An additional strain was produced by introducing a plasmid expressing Myc13-tagged Gcn4p in the gcn4Δ strain. None of the epitope-tagged strains exhibited any growth phenotypes that would signify an effect of the Myc13 tag on coactivator function (data not shown). Cells were induced with SM, and the tagged proteins were immunoprecipitated with Myc antibody. The relative amount of precipitating DNA containing the ARG1 UAS or the POL1 open reading frame (ORF) (analyzed as a negative control) was determined by quantitative PCR (Fig. (Fig.8A).8A). The amounts of precipitated ARG1 DNA, after normalization for the amount of precipitating POL1 DNA, in the WT and gcn4Δ strains were compared in three independent experiments. The results are presented in Fig. 8B and C as the average ratios of precipitated ARG1 DNA in WT versus gcn4Δ cells. As expected, Myc13-Gcn4p bound specifically to the ARG1 promoter in cells starved with SM (Fig. (Fig.8A,8A, lanes 1 and 2). Strains with tagged subunits unique to SWI/SNF (Swi2p), SAGA (Spt7p), or SRB/MED (Srb6p) all showed a strong dependence on Gcn4p for immunoprecipitation of the ARG1 UAS, with GCN4/gcn4Δ ratios of 7.5 or greater. Similar results were obtained for Gal11p and Sin4p, which reside in both SRB/MED and the Paf1 complex (Fig. (Fig.8B).8B). Less pronounced but significant Gcn4p-dependent immunoprecipitation of the ARG1 UAS was observed for the strains containing Myc13-Mbf1p or Myc13-tagged subunits of the Paf1 complex (Paf1p) and CCR4-NOT (Ccr4p and Not2p) (P values of 0.02, 0.04, 0.06, and 0.014, respectively, in an unpaired Student's t test) (Fig. 8A and C). The Gcn4p-dependence of ARG1 UAS immunoprecipitation was not statistically significant for any of the remaining tagged strains in Fig. Fig.8C8C when considered individually. However, significant Gcn4p dependence was observed when all of the data from strains with tagged Not2p, Dhh1p, Not5p, or Caf1p were analyzed as a group representing the CCR4-NOT complex (P value of 0.05; P = 0.007 with Myc13-Ccr4p results also included). Similarly, significant Gcn4p-dependence of ARG1 UAS immunoprecipitation was observed when the data from strains with tagged Rsc8p and Sth1p were analyzed as a group representing RSC (P value of 0.03). We observed no GCN4-dependent immunoprecipitation of the ARG1 UAS in a number of other strains containing Myc13-tagged Tho2p, Rpn6p, or Rpn11p (data not shown), supporting the specificity of our findings on Gcn4p-dependent recruitment of coactivator subunits.
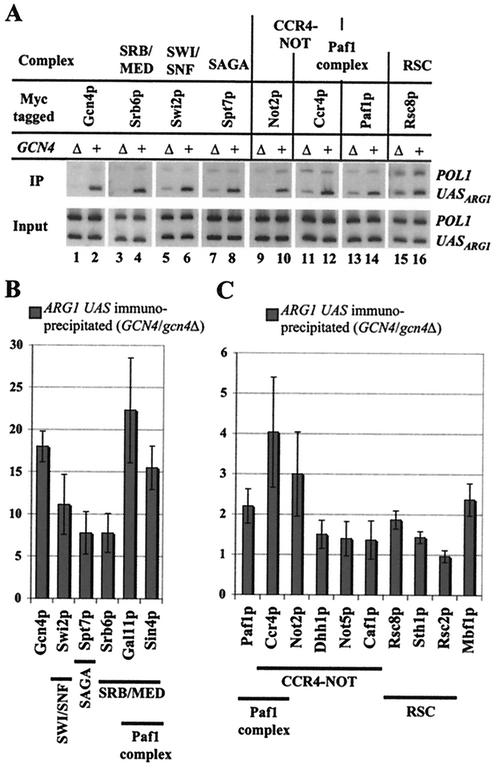
Evidence from ChIP analysis that Gcn4p recruits seven coactivators to the ARG1 promoter in vivo. (A) gcn4Δ strains expressing different Myc-tagged coactivator subunits, all derived from the gcn4Δ strain 249, were transformed with the high-copy-number GCN4 plasmid pHQ1239 or empty vector. The resulting transformants and gcn4Δ strain 249 containing vector or the GCN4-myc plasmid pSK-1 were induced with SM and treated with formaldehyde. Chromatin was sheared, heated to reverse the cross-links, and immunoprecipitated with anti-Myc antibodies. The amounts of coprecipitated DNA containing the ARG1 UAS or POL1 ORF (upper panels [IP]) and the corresponding amounts in the input chromatin samples (lower panels) were measured by quantitative PCR. Representative results are shown in lanes 3 to 16 for the high-copy-number GCN4 (even-numbered lanes) and vector (odd-numbered lanes) transformants of strains containing the indicated Myc13-tagged subunits. Lanes 1 and 2 show representative results from the gcn4Δ strain transformed with vector or the GCN4-myc plasmid. (B and C) For each ChIP experiment, the PCR products were quantified by phosphorimaging analysis, and the ratio of UASARG1 signals in the immunoprecipitated samples to those in the input samples was calculated and normalized for the corresponding ratio calculated for the POL1 signals. The resulting normalized ratio (IPUASARG1/inputUASARG1)/(IPPOL1/inputPOL1) obtained for the GCN4 strain was divided by the corresponding normalized ratio calculated for the gcn4Δ strain. The resulting values obtained in three or more independent experiments for each tagged strain were averaged, and the mean values and standard errors were plotted in the histograms as the ratios of the amounts of UASARG1 specifically associated with each tagged subunit in GCN4 versus gcn4Δ cells.
The fact that certain tagged subunits of CCR4-NOT and RSC showed weaker Gcn4p-dependent binding to ARG1 than others may indicate that the former subunits are not efficiently cross-linked to chromatin and dissociate from the rest of the chromatin-bound complexes during immunoprecipitation. Alternatively, the Myc13 epitopes on these subunits may be inaccessible to antibodies in the initiation complexes formed at ARG1 by Gcn4p. The greater Gcn4p-dependent association of SRB/MED, SWI/SNF, and SAGA with ARG1 (Fig. (Fig.8B)8B) compared to that seen for Mbf1p, CCR4-NOT, the Paf1 complex, and RSC (Fig. (Fig.8C)8C) could indicate that relatively larger amounts of the first three complexes are recruited by Gcn4p to the ARG1 promoter. Alternatively, the last three complexes and Mbf1p may undergo relatively higher levels of Gcn4p-independent binding at ARG1. The former explanation is favored by the fact that the absolute amounts of precipitated ARG1 DNA (normalized for POL1 DNA precipitation) for Mbf1p, CCR4-NOT, the Paf1 complex, and RSC subunits were relatively smaller in the GCN4 strains than the amounts that precipitated with the SRB/MED, SWI/SNF, and SAGA subunits (Fig. (Fig.8A8A and data not shown). However, it is possible that comparable amounts of all six coactivators are recruited to the promoter and that Mbf1p, CCR4-NOT, the Paf1 complex, and RSC dissociate more rapidly, or travel with RNA PolII into the ARG1 coding region. At odds with the last possibility, we did not see significant Gcn4p-dependent precipitation of ARG1 coding sequences when we used primers complementary to the 3′ end of the ORF in any of the ChIP assays whose results are shown in Fig. Fig.88 (data not shown). Thus, detectable Gcn4p-dependent binding of all six coactivator complexes was limited to the ARG1 5′ noncoding region.
DISCUSSION
Gcn4p requires and recruits a multiplicity of coactivators for transcriptional activation in vivo.
Several genetic screenings were conducted previously to identify gene products required for transcriptional activation of amino acid biosynthetic genes by Gcn4p (28, 93, 113, 137), but none had saturated this Gcn− class of mutants. We used strains from the Saccharomyces Genome Deletion Project to conduct a more systematic analysis of nonessential genes implicated previously in transcriptional control for their involvement in activation by Gcn4p. We defined a Gcn− mutant as one showing sensitivity to Ile/Val starvation imposed by SM, indicating reduced expression of one or more ILV genes, and also diminished activation of the UASGCRE-CYC1-lacZ reporter, which is wholly dependent on Gcn4p. We identified 27 such mutants that showed reductions of >25% in resistance to SM and >35% in levels of UASGCRE-CYC1-lacZ expression relative to WT levels (Table (Table1).1). We showed that most of these Gcn− mutants were also defective for induced expression of a HIS3-GUS reporter or one of the authentic Gcn4p target genes SNZ1, HIS4, ILV2, and ARG1 (Tables (Tables33 and and4),4), consistent with a defect in transcriptional activation by Gcn4p. These additional assays revealed that most coactivator mutations do not impair activation of every Gcn4p-dependent promoter we examined. Indeed, we identified three additional mutants that were defective for transcriptional activation of HIS3-GUS and one or more authentic Gcn4p target genes but did not show strong sensitivity to SM or impaired induction of UASGCRE-CYC1-lacZ reporter, namely, mbf1Δ, spt3Δ, and rsc1Δ. It is noteworthy that transcriptional induction of SNZ1, HIS4, ILV2, and ARG1 was virtually unaffected by deletion of Gcn5p, perhaps the best-characterized coactivator for Gcn4p. Thus, there is a strong precedent for the promoter specificity exhibited by the new coactivators identified here.
In addition to a defect in Gcn4p activation, a Gcn− phenotype could result from an impairment of core promoter function, reduced activity of a different required activator, or a defect in transcriptional elongation through the coding sequences of the genes under study. By considering the relative effects of the Gcn− mutations on induced versus uninduced expression of reporter constructs and authentic mRNAs, we identified mutants in which the phenotype could be attributed at least partly to impaired activation by Gcn4p. Figure Figure99 summarizes these results (data diagnostic of a Gcn4p activation defect are highlighted in dark green). Inspection of these data shows that mutations in the following 29 coactivator proteins impaired Gcn4p-mediated activation of one or more promoters: (i) Mbf1p, (ii) seven of the eight SAGA subunits examined, (iii) five of the six SWI/SNF subunits examined, (iv) Rsc1p and Rsc2p of RSC, (v) Not5p, Caf1p, Dbf2p, Dhh1p, and Ccr4p of CCR4-NOT, (vi) Srb9p, Srb10, Srb5p, Med2p, Pgd1p, Rox3p, and Gal11p of SRB/MED, (vii) Cdc73 of the Paf1 complex, and (viii) Hpr1p of THO/TREX and the Paf1 complex. Less definitive results were obtained for mutations in Tfg3p and five other subunits of CCR4-NOT (Caf4p, Caf16p, Caf17p, Caf40p, and Caf130p) (Fig. (Fig.9).9). Thus, in addition to confirming previous findings that SWI/SNF, SAGA, SRB/MED, and Mbf1p are required for WT activation by Gcn4p, our results implicate CCR4-NOT, RSC, and the Paf1 complex in this process. Moreover, they reveal which nonessential subunits in each of these complexes are crucial for activation by Gcn4p, allowing future genetic studies to focus on these key proteins.
Summary of effects of coactivator deletions on induction by Gcn4p of multiple target genes. The table includes only those mutants for which we assayed expression of HIS3-GUS or Gcn4p target gene transcripts by Northern analysis. The data shown here are from Table Table11 (SMr and induced UASGCRE-CYC1-lacZ expression), Table Table33 (induced HIS3-GUS expression), and Table Table44 (induced SNZ1, HIS4, ARG1, and ILV2 mRNAs). Data that are in italics in the tables are highlighted in red here; those underlined and in boldface with an asterisk, underlined and in boldface, or underlined only in the tables are shown in dark green, light green, or yellow, respectively. Briefly, data highlighted in dark and light green provide strong and suggestive evidence, respectively, that Gcn4p activation of the promoter is impaired by the mutation. Data highlighted in yellow indicate reduced promoter function in the mutant that cannot be attributed specifically to a defect in activation by Gcn4p. Data highlighted in red indicate greater-than-WT expression of the promoter in the mutant.
Recombinant Gcn4p can interact with the SAGA, SWI/SNF, SRB/MED, and NuA4 complexes, present in cell extracts or in purified form, dependent on hydrophobic clusters in the activation domain (30, 93, 98, 132). In addition, the DNA binding domain of Gcn4p binds specifically to Mbf1p in vitro (127). Here we showed that Gcn4p can also interact specifically with CCR4-NOT and both forms of RSC (containing Rsc1p or Rsc2p) in cell extracts.
Previous ChIP experiments provided strong evidence that Gcn4p recruits Gcn5p to the HIS3 promoter in living cells (63, 64). Our ChIP data showing Gcn4p-dependent binding of Spt7p to the ARG1 promoter are consistent with the idea that Gcn4p recruits Gcn5p as a component of SAGA. It was also shown previously that Gcn4p recruits the Snf5p subunit of SWI/SNF to a modified PHO5 promoter containing a UASGCRE (126). Here, we confirmed that Gcn4p recruits Swi2p/Snf2p to the authentic target gene ARG1. ChIP results reported by Park et al. indicated that Rgr1p is recruited to the HIS4 promoter in vivo (98); however, it was unknown whether Gcn4p or another activator functioning at this promoter (Bas1p, Bas2p, or Rap1p) was responsible for recruiting this shared subunit of the SRB/MED and Paf1 complexes. Our ChIP data demonstrate high-level recruitment of the SRB/MED-specific subunit Srb6p by Gcn4p to the ARG1 promoter (Fig. (Fig.88).
We also observed a lower level of recruitment of Mbf1p, several subunits of CCR4-NOT, Rsc8p and Sth1p of RSC, and Paf1p by Gcn4p to the ARG1 promoter (Fig. (Fig.8).8). Thus, it appears that Gcn4p recruits both SRB and Paf1 mediators to the same promoter, as reported for other activators (102). It also recruits two different ATP-dependent chromatin-remodeling complexes, RSC and SWI/SNF, and two different coactivators that function at least partly as adaptors for TBP (SAGA and Mbf1p). Except for the Paf1 complex, all of these coactivators showed specific binding to Gcn4p in WCEs and hence may be recruited to ARG1 through direct contact with the Gcn4p activation domain. Perhaps the Paf1 complex is recruited indirectly by Gcn4p through promoter-bound RNA PolII. Considering that the Paf1 complex was found to be associated with promoters and coding regions of various yeast genes while SRB/MED was restricted to the promoters, it is possible that the Paf1 complex is exchanged for SRB/MED during the transition from initiation to elongation (102). However, we could not detect binding of Paf1p to ARG1 coding sequences and found it only in the 5′ noncoding region of ARG1.
Although Gcn4p recruits seven different coactivators to the ARG1 promoter, it does not seem to require SAGA or SWI/SNF for WT activation of this promoter (Fig. (Fig.9).9). As noted above, SAGA may have offsetting positive and negative functions at ARG1, being required for activation by Gcn4p and for arginine-mediated repression by the ArgR/Mcm1p repressor. Nevertheless, given the idiosyncratic coactivator requirements at different Gcn4p target genes (Fig. (Fig.9),9), it is likely that Gcn4p frequently recruits more coactivators to a given target gene than are needed for high-level induction of that promoter. Because it activates hundred of genes (68, 94), Gcn4p may have evolved to interact effectively with many coactivators to counteract a wide range of repressive chromatin structures and sequence-specific repressors throughout the genome, and also to provide redundant pathways for recruitment of TBP, GTFs, and RNA PolII.
Gcn4p requires subunits of the HAT complex SAGA for transcriptional activation in vivo.
Our finding that WT activation of the UASGCRE-CYC1-lacZ and HIS3-GUS reporters by Gcn4p requires the HAT Gcn5p agrees with previous results showing impaired induction of certain Gcn4p target genes (HIS3, ILV1, and TRP3) in a gcn5 mutant (40). By contrast, we and others found that HIS4, SNZ1, ARG1, and ILV2 transcription is Gcn5p independent (Fig. (Fig.9),9), and there is evidence that the Gcn5p requirement at HIS3 can be diminished by changes in the Gcn4p binding site (40). Since UASGCRE-CYC1-lacZ contains tandem copies of a Gcn4p binding site from HIS4 and is clearly Gcn5p dependent (Fig. (Fig.2),2), other elements in the HIS4 promoter may reduce its Gcn5p dependence compared to other Gcn4p target genes like HIS3.
We found that the nonessential subunits of SAGA, Ada2p, Ada3p, Ada1p, Ada5p/Spt20p, and Spt7p are required for WT activation of multiple promoters by Gcn4p in vivo (Fig. (Fig.9),9), consistent with previous reports on ada2 (10), ada3 (100), ada5/spt20 (80), and ada1 (52) mutants. Overall, the deletions of SPT7, ADA5/SPT20, and ADA1 had greater effects on Gcn4p activation of multiple promoters than did deletion of ADA2, ADA3, GCN5, or SPT3, whereas deleting SPT8 had little or no effect (Fig. (Fig.9).9). Similar results were reported previously concerning the relative effects of gcn5Δ, spt7Δ, ada5Δ/spt20Δ, spt3Δ, and spt8Δ mutations on HIS3 activation by Gcn4p (8). Moreover, others have shown that spt7Δ, ada5Δ/spt20Δ, and ada1Δ mutants display a broader range and severity of growth phenotypes compared to gcn5Δ, ada2Δ, ada3Δ, spt3Δ, and spt8Δ mutants (52, 80, 119), which may be attributable to a requirement for Spt7p, Ada5p/Spt20p (42), and Ada1p (119) for SAGA integrity. These and other genetic findings indicate that SAGA performs an important function beyond the HAT activity of Gcn5p, such as TBP recruitment (34, 67, 78, 108, 119).
We observed higher-than-WT expression of the UASGCRE-CYC1-lacZ and HIS3-GUS reporters in spt3Δ and spt8Δ mutants under noninducing conditions (Tables (Tables11 and and3),3), in accordance with previous observations that HIS3 and TRP1 mRNA levels are elevated in such mutants (8). The negative function of SAGA at the latter genes has been attributed to inhibition of TBP binding. At ARG1, Gcn5p-dependent histone H3 acetylation, most likely in the context of SAGA, is required for transcriptional repression by ArgR/Mcm1p and is correlated with reduced TBP binding to the promoter (107).
The smaller HAT complex known as ADA shares Ada2p, Ada3p, and Gcn5p with SAGA, but ADA uniquely contains Ahc1p. The deletion of AHC1 had no effect on activation of the ILV genes (judging from the WT SM resistance of the mutant or UASGCRE-CYC1-lacZ (Table (Table1),1), consistent with the finding that purified ADA does not interact with Gcn4p in vitro (132). Because the ADA complex is unstable in the absence of Ahc1p (32), it seems likely that Ada2p, Ada3p, and Gcn5p promote activation by Gcn4p in the context of SAGA and not ADA.
Mutants lacking any one of seven nonessential HATs besides Gcn5p had little or no defect in activation of ILV genes by Gcn4p, as judged by their WT resistance to SM, including the HATs found in the SRB/MED and NuA3 coactivators, Nut1p and Sas3p, respectively (Fig. (Fig.6A).6A). Even in strains lacking Gcn5p, we observed no increase in SM sensitivity upon deleting Nut1p (Fig. (Fig.6B).6B). As the gcn5Δ sas3Δ strain is inviable, these two HATs could make overlapping contributions to activation by Gcn4p. Indeed, the modest effects of the sas3Δ and yer049Δ mutations on UASGCRE-CYC1-lacZ induction (Table (Table1)1) are consistent with a minor role for NuA3 in activation of this reporter. However, neither mutant has an SMs phenotype, and Gcn4p could not recruit NuA3 to chromatinized templates in vitro (132).
In vitro, Gcn4p can interact with NuA4, containing the essential HAT Esa1p (132). In addition, depletion of Esa1p in vivo reduced histone H4 acetylation at HIS3 and HIS4, along with other promoters analyzed in parallel. Except for ribosomal protein genes, however, Esa1p depletion was not associated with reduced transcription. Consistently, we observed no SMs phenotype in temperature-sensitive esa1 mutants at semipermissive growth temperatures (data not shown). Therefore, Gcn5p is the only HAT with an established in vivo function in transcriptional activation by Gcn4p.
Gcn4p requires SWI/SNF and RSC but not the ISWI chromatin-remodeling factors for transcriptional activation at certain promoters in vivo.
We confirmed our previous finding that Gcn4p requires the ATP-dependent chromatin-remodeling complex SWI/SNF for transcriptional activation in vivo. Except for SNF11, deletions of all SWI/SNF subunits produced SMs phenotypes and defects in UASGCRE-CYC1-lacZ induction comparable to those observed in the swi2Δ/snf2Δ mutant, which lacks the ATPase subunit. It was shown that deletions of SWI2/SNF2, SWI3, SNF5, or SNF6 affect the integrity of the SWI/SNF complex; however, Swi2p/Snf2p was still found in a high-molecular-weight complex of ca. 1 MDa that lacked Swi3p, Snf5p, and Snf6p in these mutants (99). Interestingly, the Swi2p/Snf2p-containing subcomplex present in a snf5Δ mutant was defective for binding to the SUC2 promoter in vivo, implicating Snf5p (or one of the other subunits lacking in this subcomplex) in recruitment of SWI/SNF by activators. Consistently, Gcn4p was cross-linked to Snf5p, Swi1p, and Swi2p/Snf2p in vitro (95), suggesting that interactions with multiple noncatalytic subunits may contribute to SWI/SNF recruitment by Gcn4p.
It was surprising that only Swi3p was required for activation of HIS3-GUS (Table (Table3),3), particularly since it was shown previously that transposon insertions in SWI2/SNF2, SWI1, and SWP73 all impaired induction of this reporter by Gcn4p in another strain background (93). Perhaps the deletion library background contains a genetic modifier of the activation defects conferred by certain swi/snf mutations. Mutations in Swi2p, Snf5p, and Swp73 did result in derepressed HIS3-GUS expression (Table (Table3),3), and the swi2Δ/snf2Δ strain had derepressed HIS4 mRNA levels (Table (Table4)4) under noninducing conditions. Thus, activation defects in these mutants may be obscured by offsetting defects in a repression mechanism.
Our results showed that the Rsc2p subunit of RSC is required for full activation by Gcn4p of UASGCRE-CYC1-lacZ, HIS3-GUS, and HIS4, whereas activation of HIS3-GUS, SNZ1, and ARG1 was reduced by deletion of RSC1 (Fig. (Fig.9).9). Rsc2p is more abundant than Rsc1p (16), and we found that a 2μm/RSC1 plasmid can suppress the SMs phenotype of the rsc2Δ strain (data not shown). Thus, the SMs phenotype of rsc2Δ cells may reflect the fact that the Rsc1p complex is not abundant enough to support full activation by Gcn4p at one of the ILV promoters. However, the strong defect in HIS3-GUS, SNZ1, and ARG1 activation in rsc1Δ cells suggests that the Rsc1p complex is required to alter the chromatin structure at these genes in a way that cannot be performed by the Rsc2p complex.
It may seem surprising that Gcn4p requires both SWI/SNF and RSC for full activation of certain promoters, including UASGCRE-CYC1-lacZ, SNZ1, and HIS4. Whereas both complexes are recruited by Hir1p and Hir2p to the HTA1/HTB1 histone genes, SWI/SNF functions as a coactivator while RSC recruitment was correlated with repression (26, 97). Not all ATP-dependent chromatin-remodeling complexes are required for activation by Gcn4p, however, as deletion of the ATPase subunits of the ISWI complexes and of Chd1p had no effect on activation of ILV genes by Gcn4p (Fig. (Fig.6C6C).
Gcn4p shows differential requirements for Gal11 module subunits in SRB/MED.
The Gal11 module of SRB/MED, containing Gal11p, Med2p, Pgd1p, and Sin4p, has been implicated as a target of activators through biochemical analysis of mutant mediator complexes. SRB/MED complexes purified from pgd1Δ, med2Δ, or gal11Δ strains are devoid of two or all three of these subunits, and they exhibit quantitative reductions in transcriptional activation by Gcn4p in vitro (71, 88, 98). Consistently, the purified pgd1Δ mediator complex, lacking Gal11p and Med2p, failed to bind recombinant Gcn4p, and both recombinant Gal11p and Pgd1p can interact with Gcn4p in vitro. However, apart from the med2 insertion described previously (93), deletions of GAL11, PGD1, and MED2 were reported to have little or no effect on activation by Gcn4p in vivo (88, 98). By contrast, in the strain background employed here, deletions of all three genes led to significant defects in activation by Gcn4p. Thus, our genetic data support the idea that the Gal11 module provides an important binding site for Gcn4p in SRB/MED.
The purified sin4Δ mediator is devoid of all four Gal11 module subunits (27) and was shown to be more defective than the SRB/MED complexes purified from pgd1Δ, med2Δ, or gal11Δ strains in promoting transcriptional stimulation by Gcn4p in vitro (88). These results suggested that Sin4p is critically required for activation by Gcn4p. Surprisingly, transcriptional activation of HIS3 (56), HIS3-GUS, UASGCRE-CYC1-lacZ, HIS4, ARG1, and ILV2 (Fig. (Fig.9)9) was not diminished in the sin4Δ mutant. To account for this discrepancy between in vitro and in vivo findings, it could be proposed that the absence of Gal11p, Pgd1p, and Med2p from the sin4Δ mediator is an artifact of purification that does not prevail in vivo and that the latter proteins can support strong activation by Gcn4p in the absence of Sin4p in SRB/MED (89). Another possibility is that Sin4p harbors both positive and negative regulatory functions, and elimination of its negative function can overcome the absence of the entire Gal11 module at certain promoters in the context of in vivo chromatin structures. Indeed, the sin4Δ mutation led to derepression of UASGCRE-CYC1-lacZ, HIS3-GUS, and ARG1 expression under noninducing conditions (Tables (Tables1,1, ,3,3, and and4)4) and was shown to restore partial function to the CYC1 promoter lacking the native UAS (56).
The activation defects conferred by the rox3Δ mutation are comparable to those produced by deletion of GAL11, PGD1, or MED2 (Fig. (Fig.9);9); however, Rox3p does not appear to be a component of the Gal11 module (71, 76, 88). Moreover, ROX3 is an essential gene in other strain backgrounds (89). Perhaps Rox3p provides a second binding site for Gcn4p in SRB/MED. Srb5p and Srb2p, and presumably the subcomplex comprised of these proteins and containing Srb4p and Srb6p (60), also contribute to activation by Gcn4p in vivo (Table (Table1).1). There is evidence that Srb5p facilitates a step in gene activation, possibly CTD phosphorylation, subsequent to activator recruitment (71).
Srb10p and Srb11p constitute a kinase-cyclin pair that is thought to impede assembly of the RNA PolII holoenzyme prior to promoter binding through phosphorylation of the CTD (89). Recently, Srb10p was also implicated in rapid degradation of Gcn4p (22). However, Gcn4p target genes were not constitutively induced in an srb10Δ mutant (51). Moreover, we observed a Gcn− phenotype for the srb10Δ mutant in our genetic background (Fig. (Fig.9).9). Presumably, the positive effects of Srb10p on Gcn4p function outweigh its role in Gcn4p degradation.
Evidence that the Paf1 complex functions in Gcn4p-mediated transcriptional activation.
We found that Paf1p is required for WT SM resistance and induction of the UASGCRE-CYC1-lacZ reporter, whereas the Cdc73p subunit of the Paf1 complex was required for normal induction of the HIS3-GUS reporter. However, we observed no defects in activation of authentic Gcn4p target genes in paf1Δ or cdc73Δ cells (Fig. (Fig.9).9). As the Paf1 complex has been implicated in transcriptional elongation (117), it might be required by Gcn4p only for transcriptional elongation through reporter constructs containing bacterial coding sequences. The same argument could apply to the hpr1Δ mutant, shown previously to be defective for transcriptional elongation through lacZ (18), as this strain shows very low reporter expression but only a modest defect in activation of authentic Gcn4p target genes (Fig. (Fig.9).9). However, both the cdc73Δ and hpr1Δ mutants impaired reporter gene expression to a greater extent under inducing than noninducing conditions, suggesting that they contribute to the mechanism of activation by Gcn4p. This observation, plus our finding that Paf1p was recruited by Gcn4p to ARG1 (Fig. (Fig.8),8), leads us to suggest that the Paf1 complex is a physiological coactivator required by Gcn4p for WT induction of a subset of target genes that harbor transcribed sequences that impede elongation.
Hpr1p belongs to the THO/TREX complex, also involved in elongation, mitotic recombination (18, 19), and nuclear export of mRNA (112, 121). We observed no SM sensitivity in mutants lacking other THO/TREX complex subunits, including Tex1p, Mft1p, and Thp2p (Table (Table1).1). However, it is possible that hpr1Δ impairs an overlapping function of the Paf1 and THO/TREX complexes in elongation that is critical for activation by Gcn4p. We did not detect any requirement for the Elongator complex or for elongation factors encoded by SPT4 (Spt complex) or DST1 (TFIIS) for activation of ILV genes by Gcn4p, judging from the WT SM resistance of the corresponding mutants (Table (Table1).1). Thus, Gcn4p may depend primarily on the functions of Hpr1p in the Paf1 or THO/TREX complexes for stimulating transcriptional elongation at its target genes.
Gcn4p requires the CCR4-NOT coactivator, which possesses cytoplasmic deadenylase activity.
There is a large body of evidence implicating the CCR4-NOT complex in transcriptional activation and repression, including the requirement for Ccr4p in transcriptional activation by Adr1p, its physical association with Srb9p, -10p, and -11p, and its genetic and physical interactions with TBP and associated factors (see the introduction). Our ChIP results provide the first evidence for activator-dependent recruitment of CCR4-NOT to an induced promoter. These results are significant because CCR4-NOT also functions in the cytoplasm as an mRNA deadenylase (130, 131), with Ccr4p itself serving as the catalytic subunit (20, 130). Deadenylation should diminish translation of an mRNA and also render it susceptible to decapping and 5′-3′ exonucleolytic degradation (7, 110). Thus, mutations that merely destroy the deadenylase activity should not lower transcript levels in the manner we observed for several Gcn4p target mRNAs in ccr4Δ cells (Fig. (Fig.9).9). Previously, others provided strong evidence that factors involved in mRNA processing (79) or export from the nucleus (72, 121) become associated with the nascent transcript. Perhaps recruitment of CCR4-NOT by Gcn4p allows subunits of this complex to associate with the nascent transcript and accompany it into the cytoplasm to control deadenylation, translation, and degradation of the mRNA.
MBF1 is required for activation of a subset of Gcn4p-dependent genes.
Previous results indicated that Mbf1p serves as an adaptor between the DNA binding domain of Gcn4p and TBP. Deletion of MBF1 eliminated HIS3 and HIS5 mRNAs in vivo, and point mutations in Gcn4p that reduced binding to Mbf1p and a point mutation in Mbf1p that reduced interaction with TBP both lowered HIS3 mRNA levels (127). The mbf1Δ mutation in our strain background did not produce SM sensitivity or impair UASGCRE-CYC1-lacZ expression. However, expression of the HIS3-GUS reporter was lower in the mbf1Δ strain, in agreement with previous findings, and induction of SNZ1 and ILV2 was also impaired. Together, the results indicate a promoter-specific role for Mbf1p in Gcn4p-activated transcription.
Concluding remarks.
The seven different coactivators recruited by Gcn4p to ARG1 could potentially provide a multiplicity of functions important for stimulating transcription initiation. SWI/SNF, RSC, or SAGA could displace or modify nucleosomes to expose binding sites for TBP and RNA PolII in the core promoter. Mbf1p, SAGA, CCR4/NOT, SRB/MED, or the Paf1 complex could help recruit TBP, other GTFs, or RNA PolII to stimulate preinitiation complex assembly. The Paf1 complex or THO/TREX may stimulate the elongation phase of transcription and even promote the export of transcripts from the nucleus (THO/TREX). Given the large size of these coactivator complexes, it seems unlikely that they could all reside at the promoter simultaneously; rather, they may bind transiently and dissociate after carrying out their specific functions at the promoter. Binding of one coactivator may facilitate association of another, e.g., by covalent modification of nucleosomes, augmenting or even replacing direct recruitment by the activator. For example, Swi5p must recruit SWI/SNF to the HO gene to permit subsequent binding of SAGA in the absence of activator (24, 61). On the other hand, HAT activity was shown to retain SWI/SNF at promoters in vitro (45) and to target particular nucleosomes for remodeling in vivo (106). In the case of Gcn4p, there is evidence that the bromodomain in Gcn5p is required for transferring SAGA from the activation domain to acetylated nucleosomes, freeing Gcn4p to recruit SWI/SNF (126). It will be interesting to see what other functional interactions exist between the numerous coactivators recruited to promoters by Gcn4p.
Acknowledgments
We thank Andres Aguilera, Brad Cairns, Marion Carlson, Jan Fassler, Bruce Futcher, Leonard Guarente, Young-Joon Kim, Hannah Klein, Lawrence Myers, Akira Sakai, Fred Winston, Rick Young, and Richard Zitomer for plasmids; Brad Cairns, Rick Young, Clyde Denis, and Tony Weil for generous gifts of antibodies; Maria Pia Cosma, Min-Hao Kuo, and Rohinton Kamakaka for advice on ChIP assays; Judith Jaehning for sharing unpublished data on the Paf1 complex; members of the Hinnebusch and Dever labs for helpful suggestions; and Bobbie Felix, Jane Lin, and Felecia Johnson for help in preparation of the manuscript.
M.J.S. and H.Q. contributed equally to this work.
REFERENCES
Articles from Molecular and Cellular Biology are provided here courtesy of Taylor & Francis
Full text links
Read article at publisher's site: https://doi.org/10.1128/mcb.23.8.2800-2820.2003
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc152555?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/102524404
Article citations
Commonly asked questions about transcriptional activation domains.
Curr Opin Struct Biol, 84:102732, 05 Dec 2023
Cited by: 3 articles | PMID: 38056064 | PMCID: PMC11193542
Review Free full text in Europe PMC
Disruption of the Mammalian Ccr4-Not Complex Contributes to Transcription-Mediated Genome Instability.
Cells, 12(14):1868, 17 Jul 2023
Cited by: 0 articles | PMID: 37508532 | PMCID: PMC10378556
Differential requirements for Gcn5 and NuA4 HAT activities in the starvation-induced versus basal transcriptomes.
Nucleic Acids Res, 51(8):3696-3721, 01 May 2023
Cited by: 2 articles | PMID: 36864781 | PMCID: PMC10164569
Biochemical Identification of a Nuclear Coactivator Protein Required for AtrR-Dependent Gene Regulation in Aspergillus fumigatus.
mSphere, 7(6):e0047622, 14 Nov 2022
Cited by: 7 articles | PMID: 36374043 | PMCID: PMC9769526
Distinct functions of three chromatin remodelers in activator binding and preinitiation complex assembly.
PLoS Genet, 18(7):e1010277, 06 Jul 2022
Cited by: 6 articles | PMID: 35793348 | PMCID: PMC9292117
Go to all (109) article citations
Other citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Recruitment of SWI/SNF by Gcn4p does not require Snf2p or Gcn5p but depends strongly on SWI/SNF integrity, SRB mediator, and SAGA.
Mol Cell Biol, 23(23):8829-8845, 01 Dec 2003
Cited by: 48 articles | PMID: 14612422 | PMCID: PMC262668
Simultaneous recruitment of coactivators by Gcn4p stimulates multiple steps of transcription in vivo.
Mol Cell Biol, 25(13):5626-5638, 01 Jul 2005
Cited by: 86 articles | PMID: 15964818 | PMCID: PMC1156971
An array of coactivators is required for optimal recruitment of TATA binding protein and RNA polymerase II by promoter-bound Gcn4p.
Mol Cell Biol, 24(10):4104-4117, 01 May 2004
Cited by: 70 articles | PMID: 15121833 | PMCID: PMC400468
Interdependent recruitment of SAGA and Srb mediator by transcriptional activator Gcn4p.
Mol Cell Biol, 25(9):3461-3474, 01 May 2005
Cited by: 57 articles | PMID: 15831453 | PMCID: PMC1084306