Abstract
Free full text

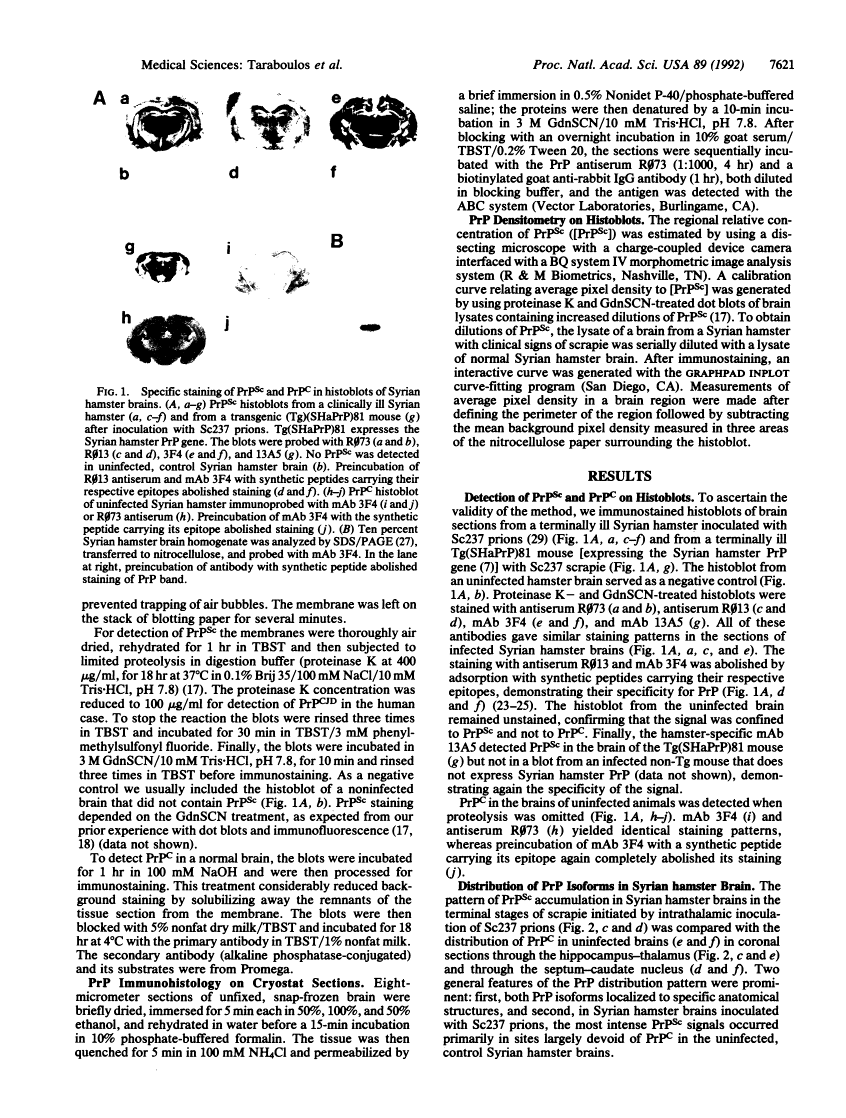
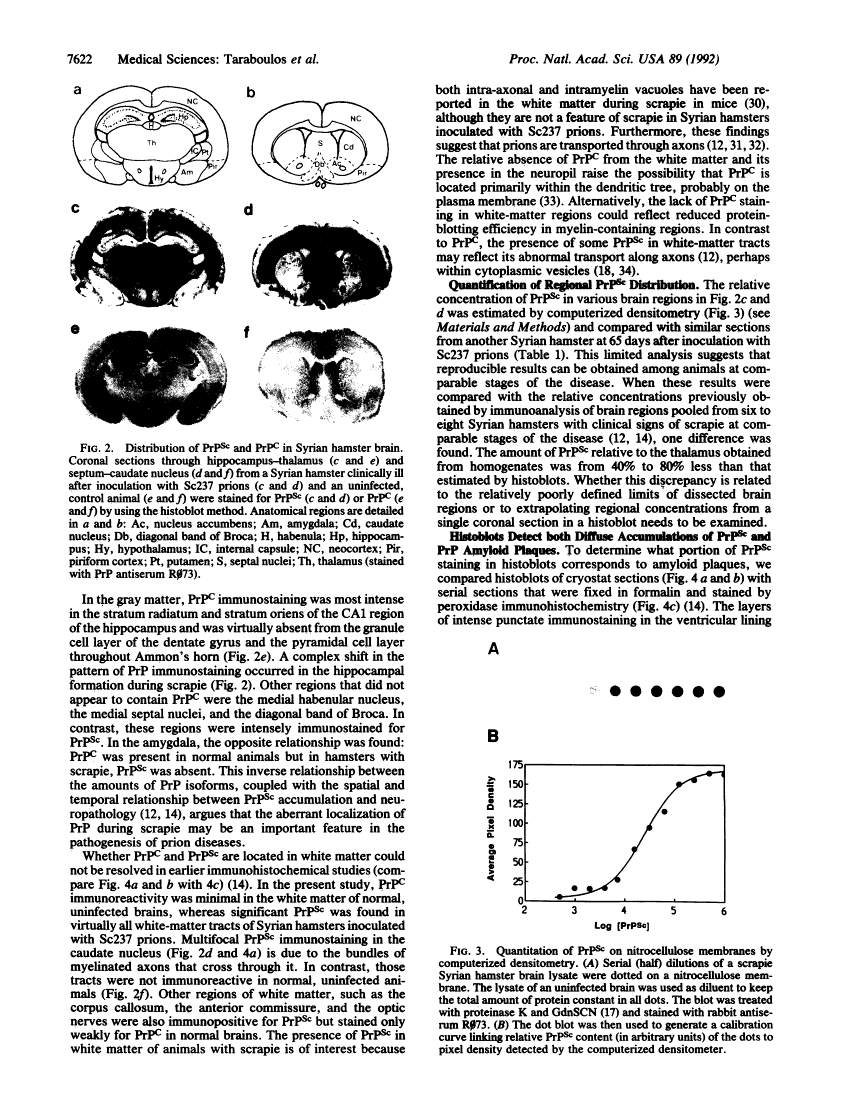
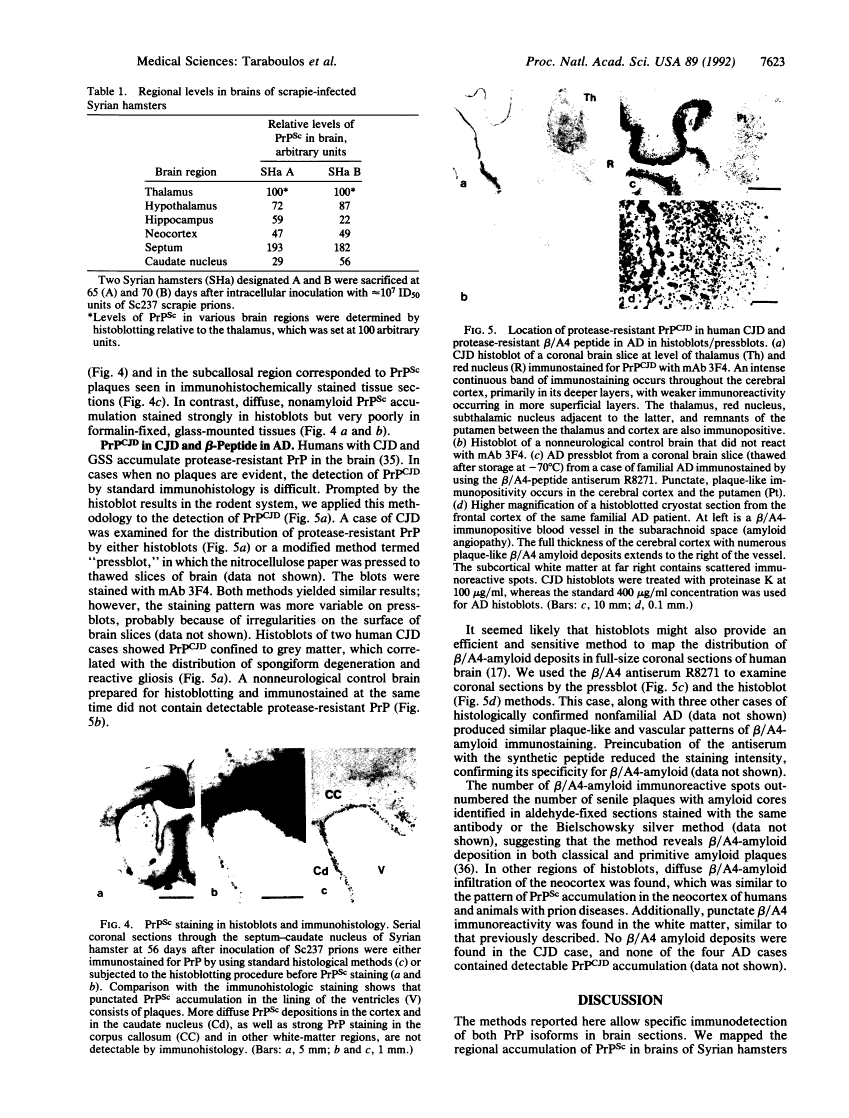
Regional mapping of prion proteins in brain.
Abstract
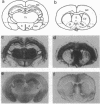
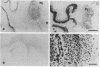
Scrapie is characterized by the accumulation of a protease-resistant isoform of the prion protein PrPSc. Limited proteolysis and chaotropes were used to map the distribution of PrPSc in cryostat sections of scrapie-infected brain blotted onto nitrocellulose membranes, designated histoblots. Proteolysis was omitted in order to map the cellular isoform of the prion protein (PrPC) in uninfected brains. Compared with immunohistochemistry, histoblots increased the sensitivity for PrPSc detection and showed different patterns of PrPSc accumulation. In Syrian hamsters with Sc237 scrapie, the most intense PrPSc signals occurred in sites with relatively little PrPC, suggesting that aberrant localization of prion protein may be an important feature in the pathogenesis of prion diseases. Immunostaining of PrPSc in white-matter tracts suggested that prions spread along neuroanatomical pathways. PrPSc immunostaining in histoblots was quantitated by densitometry, permitting assessment of the extent of PrPSc accumulation within specific structures. Histoblots were also useful in localizing PrPCJD and beta/A4-amyloid peptide in the brains of patients with Creutzfeldt-Jakob disease and Alzheimer disease, respectively.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.5M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science. 1982 Apr 9;216(4542):136–144. [Abstract] [Google Scholar]
- Prusiner SB. Molecular biology of prion diseases. Science. 1991 Jun 14;252(5012):1515–1522. [Abstract] [Google Scholar]
- Wilesmith JW, Wells GA, Cranwell MP, Ryan JB. Bovine spongiform encephalopathy: epidemiological studies. Vet Rec. 1988 Dec 17;123(25):638–644. [Abstract] [Google Scholar]
- Bolton DC, McKinley MP, Prusiner SB. Identification of a protein that purifies with the scrapie prion. Science. 1982 Dec 24;218(4579):1309–1311. [Abstract] [Google Scholar]
- Chesebro B, Race R, Wehrly K, Nishio J, Bloom M, Lechner D, Bergstrom S, Robbins K, Mayer L, Keith JM, et al. Identification of scrapie prion protein-specific mRNA in scrapie-infected and uninfected brain. Nature. 1985 May 23;315(6017):331–333. [Abstract] [Google Scholar]
- Oesch B, Westaway D, Wälchli M, McKinley MP, Kent SB, Aebersold R, Barry RA, Tempst P, Teplow DB, Hood LE, et al. A cellular gene encodes scrapie PrP 27-30 protein. Cell. 1985 Apr;40(4):735–746. [Abstract] [Google Scholar]
- Scott M, Foster D, Mirenda C, Serban D, Coufal F, Wälchli M, Torchia M, Groth D, Carlson G, DeArmond SJ, et al. Transgenic mice expressing hamster prion protein produce species-specific scrapie infectivity and amyloid plaques. Cell. 1989 Dec 1;59(5):847–857. [Abstract] [Google Scholar]
- McKinley MP, Bolton DC, Prusiner SB. A protease-resistant protein is a structural component of the scrapie prion. Cell. 1983 Nov;35(1):57–62. [Abstract] [Google Scholar]
- Gabizon R, McKinley MP, Groth D, Prusiner SB. Immunoaffinity purification and neutralization of scrapie prion infectivity. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6617–6621. [Europe PMC free article] [Abstract] [Google Scholar]
- Hsiao KK, Scott M, Foster D, Groth DF, DeArmond SJ, Prusiner SB. Spontaneous neurodegeneration in transgenic mice with mutant prion protein. Science. 1990 Dec 14;250(4987):1587–1590. [Abstract] [Google Scholar]
- Jendroska K, Heinzel FP, Torchia M, Stowring L, Kretzschmar HA, Kon A, Stern A, Prusiner SB, DeArmond SJ. Proteinase-resistant prion protein accumulation in Syrian hamster brain correlates with regional pathology and scrapie infectivity. Neurology. 1991 Sep;41(9):1482–1490. [Abstract] [Google Scholar]
- Prusiner SB, Scott M, Foster D, Pan KM, Groth D, Mirenda C, Torchia M, Yang SL, Serban D, Carlson GA, et al. Transgenetic studies implicate interactions between homologous PrP isoforms in scrapie prion replication. Cell. 1990 Nov 16;63(4):673–686. [Abstract] [Google Scholar]
- DeArmond SJ, Mobley WC, DeMott DL, Barry RA, Beckstead JH, Prusiner SB. Changes in the localization of brain prion proteins during scrapie infection. Neurology. 1987 Aug;37(8):1271–1280. [Abstract] [Google Scholar]
- Kascsak RJ, Rubenstein R, Merz PA, Tonna-DeMasi M, Fersko R, Carp RI, Wisniewski HM, Diringer H. Mouse polyclonal and monoclonal antibody to scrapie-associated fibril proteins. J Virol. 1987 Dec;61(12):3688–3693. [Europe PMC free article] [Abstract] [Google Scholar]
- Kitamoto T, Ogomori K, Tateishi J, Prusiner SB. Formic acid pretreatment enhances immunostaining of cerebral and systemic amyloids. Lab Invest. 1987 Aug;57(2):230–236. [Abstract] [Google Scholar]
- Serban D, Taraboulos A, DeArmond SJ, Prusiner SB. Rapid detection of Creutzfeldt-Jakob disease and scrapie prion proteins. Neurology. 1990 Jan;40(1):110–117. [Abstract] [Google Scholar]
- Taraboulos A, Serban D, Prusiner SB. Scrapie prion proteins accumulate in the cytoplasm of persistently infected cultured cells. J Cell Biol. 1990 Jun;110(6):2117–2132. [Europe PMC free article] [Abstract] [Google Scholar]
- DeArmond SJ, McKinley MP, Barry RA, Braunfeld MB, McColloch JR, Prusiner SB. Identification of prion amyloid filaments in scrapie-infected brain. Cell. 1985 May;41(1):221–235. [Abstract] [Google Scholar]
- Cassab GI, Varner JE. Immunocytolocalization of extensin in developing soybean seed coats by immunogold-silver staining and by tissue printing on nitrocellulose paper. J Cell Biol. 1987 Dec;105(6 Pt 1):2581–2588. [Europe PMC free article] [Abstract] [Google Scholar]
- Pont-Lezica RF, Varner JE. Histochemical localization of cysteine-rich proteins by tissue printing on nitrocellulose. Anal Biochem. 1989 Nov 1;182(2):334–337. [Abstract] [Google Scholar]
- Barry RA, Vincent MT, Kent SB, Hood LE, Prusiner SB. Characterization of prion proteins with monospecific antisera to synthetic peptides. J Immunol. 1988 Feb 15;140(4):1188–1193. [Abstract] [Google Scholar]
- Lowenstein DH, Butler DA, Westaway D, McKinley MP, DeArmond SJ, Prusiner SB. Three hamster species with different scrapie incubation times and neuropathological features encode distinct prion proteins. Mol Cell Biol. 1990 Mar;10(3):1153–1163. [Europe PMC free article] [Abstract] [Google Scholar]
- Rogers M, Serban D, Gyuris T, Scott M, Torchia T, Prusiner SB. Epitope mapping of the Syrian hamster prion protein utilizing chimeric and mutant genes in a vaccinia virus expression system. J Immunol. 1991 Nov 15;147(10):3568–3574. [Abstract] [Google Scholar]
- Barry RA, Prusiner SB. Monoclonal antibodies to the cellular and scrapie prion proteins. J Infect Dis. 1986 Sep;154(3):518–521. [Abstract] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. [Abstract] [Google Scholar]
- Prusiner SB, Bolton DC, Groth DF, Bowman KA, Cochran SP, McKinley MP. Further purification and characterization of scrapie prions. Biochemistry. 1982 Dec 21;21(26):6942–6950. [Abstract] [Google Scholar]
- Marsh RF, Kimberlin RH. Comparison of scrapie and transmissible mink encephalopathy in hamsters. II. Clinical signs, pathology, and pathogenesis. J Infect Dis. 1975 Feb;131(2):104–110. [Abstract] [Google Scholar]
- Liberski PP, Yanagihara R, Gibbs CJ, Jr, Gajdusek DC. White matter ultrastructural pathology of experimental Creutzfeldt-Jakob disease in mice. Acta Neuropathol. 1989;79(1):1–9. [Abstract] [Google Scholar]
- Kimberlin RH, Field HJ, Walker CA. Pathogenesis of mouse scrapie: evidence for spread of infection from central to peripheral nervous system. J Gen Virol. 1983 Mar;64(Pt 3):713–716. [Abstract] [Google Scholar]
- Stahl N, Borchelt DR, Hsiao K, Prusiner SB. Scrapie prion protein contains a phosphatidylinositol glycolipid. Cell. 1987 Oct 23;51(2):229–240. [Abstract] [Google Scholar]
- McKinley MP, Taraboulos A, Kenaga L, Serban D, Stieber A, DeArmond SJ, Prusiner SB, Gonatas N. Ultrastructural localization of scrapie prion proteins in cytoplasmic vesicles of infected cultured cells. Lab Invest. 1991 Dec;65(6):622–630. [Abstract] [Google Scholar]
- Bockman JM, Kingsbury DT, McKinley MP, Bendheim PE, Prusiner SB. Creutzfeldt-Jakob disease prion proteins in human brains. N Engl J Med. 1985 Jan 10;312(2):73–78. [Abstract] [Google Scholar]
- Wisniewski HM, Bancher C, Barcikowska M, Wen GY, Currie J. Spectrum of morphological appearance of amyloid deposits in Alzheimer's disease. Acta Neuropathol. 1989;78(4):337–347. [Abstract] [Google Scholar]
- Kretzschmar HA, Prusiner SB, Stowring LE, DeArmond SJ. Scrapie prion proteins are synthesized in neurons. Am J Pathol. 1986 Jan;122(1):1–5. [Europe PMC free article] [Abstract] [Google Scholar]
- Hecker R, Taraboulos A, Scott M, Pan KM, Yang SL, Torchia M, Jendroska K, DeArmond SJ, Prusiner SB. Replication of distinct scrapie prion isolates is region specific in brains of transgenic mice and hamsters. Genes Dev. 1992 Jul;6(7):1213–1228. [Abstract] [Google Scholar]
- McGrath CM, Grudzien JL, Decker DA, Robbins TO. Cytometrically coherent transfer of receptor proteins on microporous membranes. Biotechniques. 1991 Sep;11(3):352–361. [Abstract] [Google Scholar]
Associated Data
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.89.16.7620
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc49762?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1073/pnas.89.16.7620
Article citations
Abnormal synaptic architecture in iPSC-derived neurons from a multi-generational family with genetic Creutzfeldt-Jakob disease.
Stem Cell Reports, 19(10):1474-1488, 26 Sep 2024
Cited by: 0 articles | PMID: 39332406 | PMCID: PMC11561462
Propagation of distinct CWD prion strains during peripheral and intracerebral challenges of gene-targeted mice.
Proc Natl Acad Sci U S A, 121(32):e2402726121, 31 Jul 2024
Cited by: 0 articles | PMID: 39083420
Strain-Specific Targeting and Destruction of Cells by Prions.
Biology (Basel), 13(1):57, 20 Jan 2024
Cited by: 0 articles | PMID: 38275733 | PMCID: PMC10813089
Review Free full text in Europe PMC
Novel Prion Strain as Cause of Chronic Wasting Disease in a Moose, Finland.
Emerg Infect Dis, 29(2):323-332, 01 Feb 2023
Cited by: 10 articles | PMID: 36692340 | PMCID: PMC9881765
Histoblot: A sensitive method to quantify the expression of proteins in normal and pathological conditions.
Histol Histopathol, 38(7):725-737, 05 Jan 2023
Cited by: 1 article | PMID: 36648032
Review
Go to all (190) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Rapid detection of Creutzfeldt-Jakob disease and scrapie prion proteins.
Neurology, 40(1):110-117, 01 Jan 1990
Cited by: 141 articles | PMID: 1967489
Replication of distinct scrapie prion isolates is region specific in brains of transgenic mice and hamsters.
Genes Dev, 6(7):1213-1228, 01 Jul 1992
Cited by: 124 articles | PMID: 1628828
Three scrapie prion isolates exhibit different accumulation patterns of the prion protein scrapie isoform.
Proc Natl Acad Sci U S A, 90(14):6449-6453, 01 Jul 1993
Cited by: 55 articles | PMID: 8101989 | PMCID: PMC46949
Prion encephalopathies of animals and humans.
Dev Biol Stand, 80:31-44, 01 Jan 1993
Cited by: 13 articles | PMID: 8270114
Review
Funding
Funders who supported this work.
NIA NIH HHS (2)
Grant ID: AG08967
Grant ID: AG02132
NINDS NIH HHS (1)
Grant ID: NS14069