Abstract
Free full text

Gene dosage as a possible major determinant for equal expression levels of genes encoding RNA polymerase subunits in the hypotrichous ciliate Euplotes octocarinatus.
Abstract
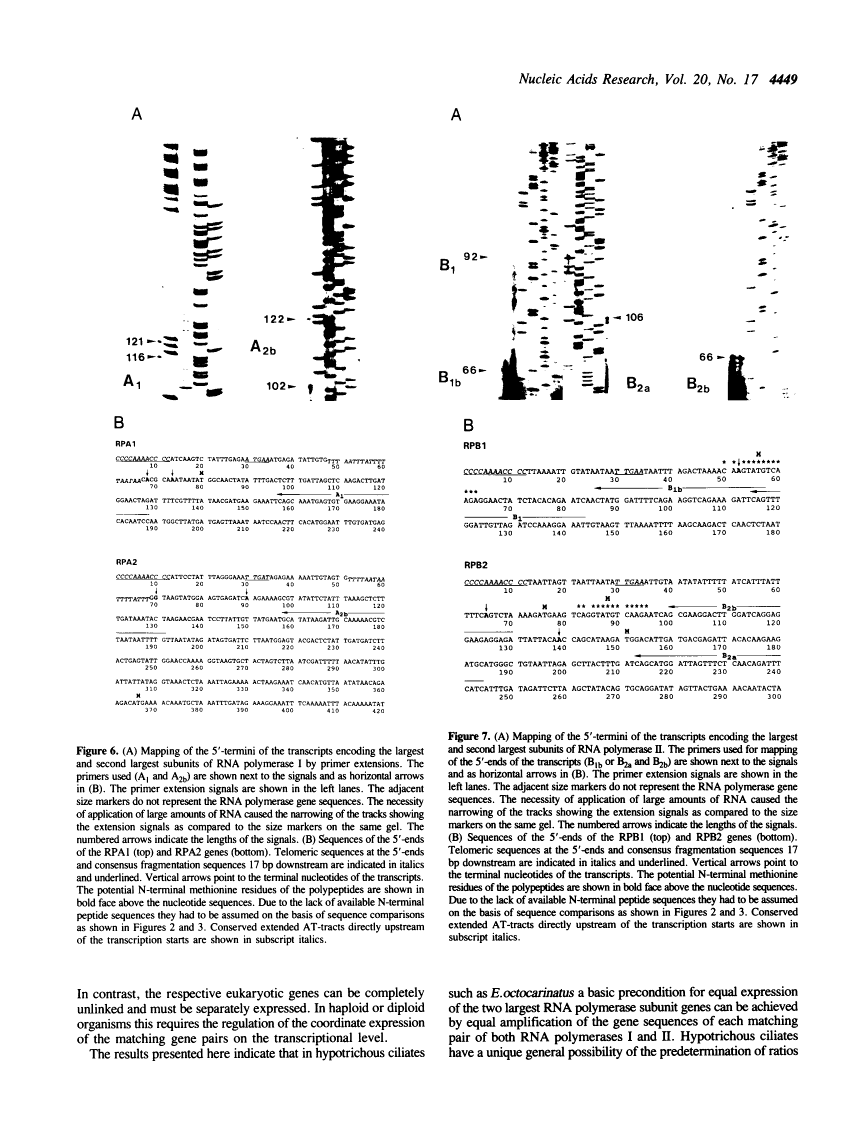
Ciliated protozoa harbor two different types of nuclei in each cell. The diploid micronucleus is the transcriptionally inactive generative nucleus, while the macronuclous contains a highly amplified transcriptionally active genome of lower complexity. The macronuclear genes encoding the two largest subunits of both RNA polymerases I and II of Euplotes octocarinatus were identified by a novel method of two step PCR walking, employing primer pairs derived from telomeric sequences of the organism and known conserved RNA polymerase polypeptide sequences, respectively. The relative gene dosage was determined. The genes are present in equal copy numbers for the respective matching subunits. Northern hybridizations showed comparable amounts of transcripts, as well, within the matching pairs. Mapping of the 5'-termini of the transcripts of the gene sized chromosomes showed that the upstream nontranscribed regions are very short and contain characteristic sequence motifs which could be the determinants of equal promoter strengths for subunits of a common RNA polymerase.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.4M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Swanton MT, Heumann JM, Prescott DM. Gene-sized DNA molecules of the macronuclei in three species of hypotrichs: size distributions and absence of nicks. DNA of ciliated protozoa. VIII. Chromosoma. 1980;77(2):217–227. [Abstract] [Google Scholar]
- Swanton MT, Greslin AF, Prescott DM. Arrangement of coding and non-coding sequences in the DNA molecules coding for rRNAs in Oxytricha sp. DNA of ciliated protozoa. VII. Chromosoma. 1980;77(2):203–215. [Abstract] [Google Scholar]
- Wünning IU, Lipps HJ. A transformation system for the hypotrichous ciliate Stylonychia mytilus. EMBO J. 1983;2(10):1753–1757. [Europe PMC free article] [Abstract] [Google Scholar]
- Baird SE, Klobutcher LA. Differential DNA amplification and copy number control in the hypotrichous ciliate Euplotes crassus. J Protozool. 1991 Mar-Apr;38(2):136–140. [Abstract] [Google Scholar]
- Larson DD, Umthun AR, Shaiu WL. Copy number control in the Tetrahymena macronuclear genome. J Protozool. 1991 May-Jun;38(3):258–263. [Abstract] [Google Scholar]
- Kolodziej PA, Woychik N, Liao SM, Young RA. RNA polymerase II subunit composition, stoichiometry, and phosphorylation. Mol Cell Biol. 1990 May;10(5):1915–1920. [Europe PMC free article] [Abstract] [Google Scholar]
- Casanova JL, Pannetier C, Jaulin C, Kourilsky P. Optimal conditions for directly sequencing double-stranded PCR products with sequenase. Nucleic Acids Res. 1990 Jul 11;18(13):4028–4028. [Europe PMC free article] [Abstract] [Google Scholar]
- Schulze Dieckhoff H, Freiburg M, Heckmann K. The isolation of gamones 3 and 4 of Euplotes octocarinatus. Eur J Biochem. 1987 Oct 1;168(1):89–94. [Abstract] [Google Scholar]
- Ammermann D, Steinbrück G, von Berger L, Hennig W. The development of the macronucleus in the ciliated protozoan Stylonychia mytilus. Chromosoma. 1974 May 10;45(4):401–429. [Abstract] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. [Abstract] [Google Scholar]
- Mémet S, Gouy M, Marck C, Sentenac A, Buhler JM. RPA190, the gene coding for the largest subunit of yeast RNA polymerase A. J Biol Chem. 1988 Feb 25;263(6):2830–2839. [Abstract] [Google Scholar]
- Cornelissen AW, Evers R, Köck J. Structure and sequence of genes encoding subunits of eukaryotic RNA polymerases. Oxf Surv Eukaryot Genes. 1988;5:91–131. [Abstract] [Google Scholar]
- Pühler G, Leffers H, Gropp F, Palm P, Klenk HP, Lottspeich F, Garrett RA, Zillig W. Archaebacterial DNA-dependent RNA polymerases testify to the evolution of the eukaryotic nuclear genome. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4569–4573. [Europe PMC free article] [Abstract] [Google Scholar]
- Falkenburg D, Dworniczak B, Faust DM, Bautz EK. RNA polymerase II of Drosophila. Relation of its 140,000 Mr subunit to the beta subunit of Escherichia coli RNA polymerase. J Mol Biol. 1987 Jun 20;195(4):929–937. [Abstract] [Google Scholar]
- Sweetser D, Nonet M, Young RA. Prokaryotic and eukaryotic RNA polymerases have homologous core subunits. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1192–1196. [Europe PMC free article] [Abstract] [Google Scholar]
- Kontermann R, Sitzler S, Seifarth W, Petersen G, Bautz EK. Primary structure and functional aspects of the gene coding for the second-largest subunit of RNA polymerase III of Drosophila. Mol Gen Genet. 1989 Nov;219(3):373–380. [Abstract] [Google Scholar]
- Yano R, Nomura M. Suppressor analysis of temperature-sensitive mutations of the largest subunit of RNA polymerase I in Saccharomyces cerevisiae: a suppressor gene encodes the second-largest subunit of RNA polymerase I. Mol Cell Biol. 1991 Feb;11(2):754–764. [Europe PMC free article] [Abstract] [Google Scholar]
- James P, Whelen S, Hall BD. The RET1 gene of yeast encodes the second-largest subunit of RNA polymerase III. Structural analysis of the wild-type and ret1-1 mutant alleles. J Biol Chem. 1991 Mar 25;266(9):5616–5624. [Abstract] [Google Scholar]
- Feinberg AP, Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. [Abstract] [Google Scholar]
- Feinberg AP, Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. [Abstract] [Google Scholar]
- Mémet S, Saurin W, Sentenac A. RNA polymerases B and C are more closely related to each other than to RNA polymerase A. J Biol Chem. 1988 Jul 25;263(21):10048–10051. [Abstract] [Google Scholar]
- Baird SE, Klobutcher LA. Characterization of chromosome fragmentation in two protozoans and identification of a candidate fragmentation sequence in Euplotes crassus. Genes Dev. 1989 May;3(5):585–597. [Abstract] [Google Scholar]
- Corden J, Wasylyk B, Buchwalder A, Sassone-Corsi P, Kedinger C, Chambon P. Promoter sequences of eukaryotic protein-coding genes. Science. 1980 Sep 19;209(4463):1406–1414. [Abstract] [Google Scholar]
- Furter-Graves EM, Hall BD. DNA sequence elements required for transcription initiation of the Schizosaccharomyces pombe ADH gene in Saccharomyces cerevisiae. Mol Gen Genet. 1990 Sep;223(3):407–416. [Abstract] [Google Scholar]
- Struhl K. Promoters, activator proteins, and the mechanism of transcriptional initiation in yeast. Cell. 1987 May 8;49(3):295–297. [Abstract] [Google Scholar]
- Guarente L. Regulatory proteins in yeast. Annu Rev Genet. 1987;21:425–452. [Abstract] [Google Scholar]
- Struhl K. Naturally occurring poly(dA-dT) sequences are upstream promoter elements for constitutive transcription in yeast. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8419–8423. [Europe PMC free article] [Abstract] [Google Scholar]
- Mahadevan S, Struhl K. Tc, an unusual promoter element required for constitutive transcription of the yeast HIS3 gene. Mol Cell Biol. 1990 Sep;10(9):4447–4455. [Europe PMC free article] [Abstract] [Google Scholar]
- Brunk CF, Sadler LA. Characterization of the promoter region of Tetrahymena genes. Nucleic Acids Res. 1990 Jan 25;18(2):323–329. [Europe PMC free article] [Abstract] [Google Scholar]
- Leffers H, Gropp F, Lottspeich F, Zillig W, Garrett RA. Sequence, organization, transcription and evolution of RNA polymerase subunit genes from the archaebacterial extreme halophiles Halobacterium halobium and Halococcus morrhuae. J Mol Biol. 1989 Mar 5;206(1):1–17. [Abstract] [Google Scholar]
- Spradling AC. The organization and amplification of two chromosomal domains containing Drosophila chorion genes. Cell. 1981 Nov;27(1 Pt 2):193–201. [Abstract] [Google Scholar]
- Osheim YN, Miller OL., Jr Novel amplification and transcriptional activity of chorion genes in Drosophila melanogaster follicle cells. Cell. 1983 Jun;33(2):543–553. [Abstract] [Google Scholar]
- Delidakis C, Kafatos FC. Amplification enhancers and replication origins in the autosomal chorion gene cluster of Drosophila. EMBO J. 1989 Mar;8(3):891–901. [Europe PMC free article] [Abstract] [Google Scholar]
- Allison LA, Moyle M, Shales M, Ingles CJ. Extensive homology among the largest subunits of eukaryotic and prokaryotic RNA polymerases. Cell. 1985 Sep;42(2):599–610. [Abstract] [Google Scholar]
Associated Data
Articles from Nucleic Acids Research are provided here courtesy of Oxford University Press
Full text links
Read article at publisher's site: https://doi.org/10.1093/nar/20.17.4445
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc334170?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Article citations
The molecular evolution of spiggin nesting glue in sticklebacks.
Mol Ecol, 24(17):4474-4488, 03 Aug 2015
Cited by: 6 articles | PMID: 26173374 | PMCID: PMC4989455
Chromosome copy number variation and control in the ciliate Chilodonella uncinata.
PLoS One, 8(2):e56413, 20 Feb 2013
Cited by: 5 articles | PMID: 23437129 | PMCID: PMC3577910
alpha-tubulin minichromosome promoters in the stichotrichous ciliate Stylonychia lemnae.
Eukaryot Cell, 6(1):28-36, 03 Nov 2006
Cited by: 4 articles | PMID: 17085637 | PMCID: PMC1800363
Coding properties of Oxytricha trifallax (Sterkiella histriomuscorum) macronuclear chromosomes: analysis of a pilot genome project.
Chromosoma, 113(2):69-76, 16 Jul 2004
Cited by: 23 articles | PMID: 15258807
The expression of the HSP70 gene in Moneuplotes crassus is controlled by a two-step process at the transcript level.
J Eukaryot Microbiol, 51(3):344-350, 01 May 2004
Cited by: 1 article | PMID: 15218705
Go to all (17) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
TGA cysteine codons and intron sequences in conserved and nonconserved positions are found in macronuclear RNA polymerase genes of Euplotes octocarinatus.
Nucleic Acids Res, 20(22):5985-5989, 01 Nov 1992
Cited by: 10 articles | PMID: 1461731 | PMCID: PMC334464
The hypotrichous ciliate Euplotes octocarinatus has only one type of tRNACys with GCA anticodon encoded on a single macronuclear DNA molecule.
Nucleic Acids Res, 26(20):4557-4565, 01 Oct 1998
Cited by: 21 articles | PMID: 9753721 | PMCID: PMC147889
Cloning and sequence analysis of the micronuclear and macronuclear gene encoding Rab protein of Euplotes octocarinatus.
Biosci Biotechnol Biochem, 69(3):649-652, 01 Mar 2005
Cited by: 2 articles | PMID: 15785000
Two introns in the pheromone 3-encoding gene of Euplotes octocarinatus.
Gene, 109(2):233-237, 01 Dec 1991
Cited by: 8 articles | PMID: 1765269