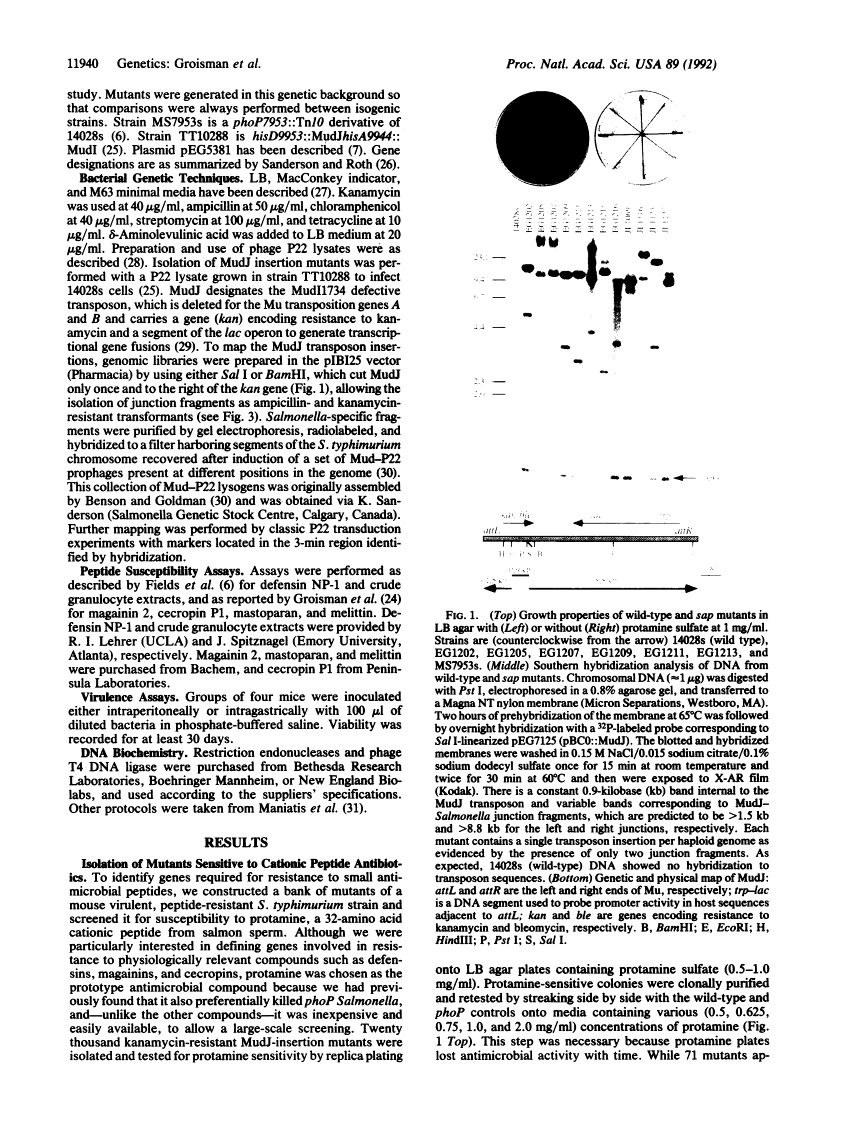
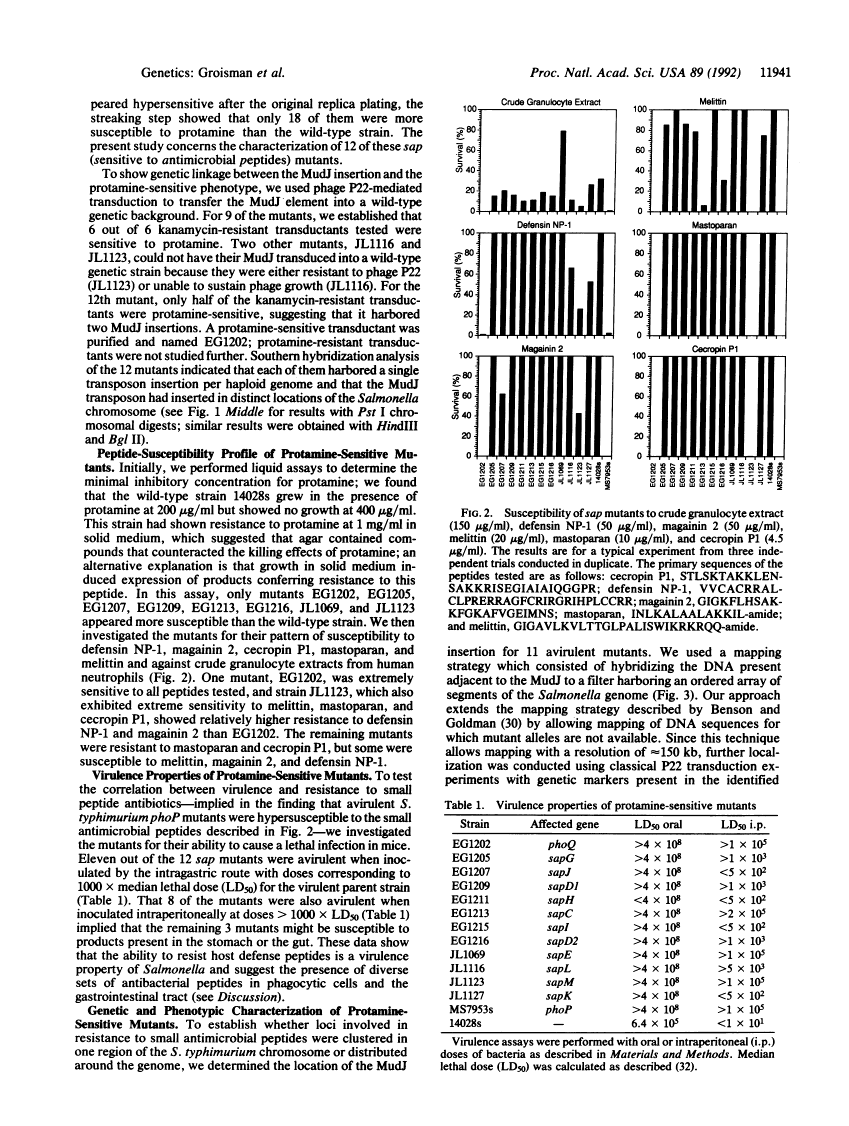
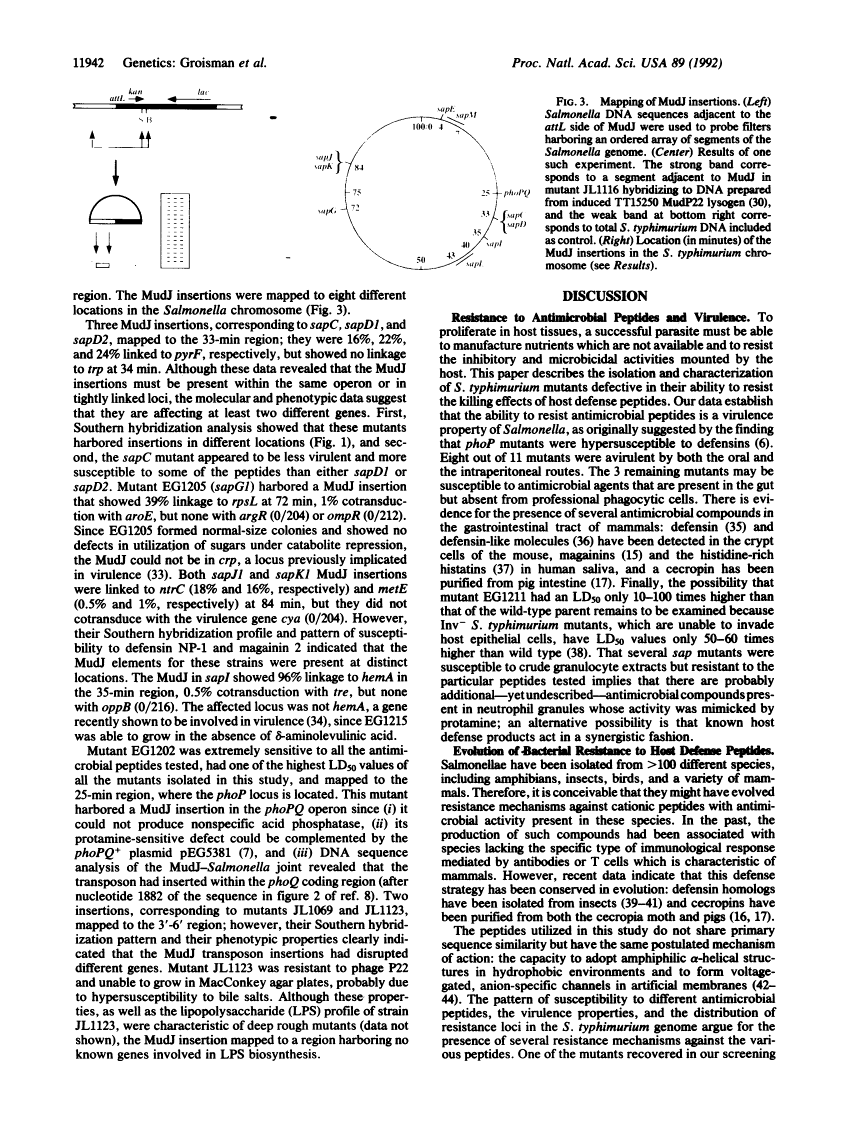
Abstract
Free full text

Resistance to host antimicrobial peptides is necessary for Salmonella virulence.
Abstract
The production of antibacterial peptides is a host defense strategy used by various species, including mammals, amphibians, and insects. Successful pathogens, such as the facultative intracellular bacterium Salmonella typhimurium, have evolved resistance mechanisms to this ubiquitous type of host defense. To identify the genes required for resistance to host peptides, we isolated a library of 20,000 MudJ transposon insertion mutants of a virulent peptide-resistant S. typhimurium strain and screened it for hypersensitivity to the antimicrobial peptide protamine. Eighteen mutants had heightened susceptibility to protamine and 12 of them were characterized in detail. Eleven mutants were attenuated for virulence in vivo when inoculated into BALB/c mice by the intragastric route, and 8 of them were also avirulent following intraperitoneal inoculation. The mutants fell into different phenotypic classes with respect to their susceptibility to rabbit defensin NP-1, frog magainin 2, pig cecropin P1, and the insect venom-derived peptides mastoparan and melittin. The resistance loci mapped to eight distinct locations in the genome. Characterization of the mutants showed that one had a defective lipopolysaccharide and another mutant harbored a mutation in phoP, a locus previously shown to control expression of Salmonella virulence genes. Our data indicate that the ability to resist the killing effect of host antimicrobial peptides is a virulence property and that several resistance mechanisms operate in S. typhimurium.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.3M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Groisman EA, Saier MH., Jr Salmonella virulence: new clues to intramacrophage survival. Trends Biochem Sci. 1990 Jan;15(1):30–33. [Abstract] [Google Scholar]
- Lehrer RI, Ganz T, Selsted ME. Defensins: endogenous antibiotic peptides of animal cells. Cell. 1991 Jan 25;64(2):229–230. [Abstract] [Google Scholar]
- Ganz T, Metcalf JA, Gallin JI, Boxer LA, Lehrer RI. Microbicidal/cytotoxic proteins of neutrophils are deficient in two disorders: Chediak-Higashi syndrome and "specific" granule deficiency. J Clin Invest. 1988 Aug;82(2):552–556. [Europe PMC free article] [Abstract] [Google Scholar]
- Fields PI, Groisman EA, Heffron F. A Salmonella locus that controls resistance to microbicidal proteins from phagocytic cells. Science. 1989 Feb 24;243(4894 Pt 1):1059–1062. [Abstract] [Google Scholar]
- Groisman EA, Chiao E, Lipps CJ, Heffron F. Salmonella typhimurium phoP virulence gene is a transcriptional regulator. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7077–7081. [Europe PMC free article] [Abstract] [Google Scholar]
- Miller SI, Kukral AM, Mekalanos JJ. A two-component regulatory system (phoP phoQ) controls Salmonella typhimurium virulence. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5054–5058. [Europe PMC free article] [Abstract] [Google Scholar]
- Fields PI, Swanson RV, Haidaris CG, Heffron F. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5189–5193. [Europe PMC free article] [Abstract] [Google Scholar]
- Foster JW, Hall HK. Adaptive acidification tolerance response of Salmonella typhimurium. J Bacteriol. 1990 Feb;172(2):771–778. [Europe PMC free article] [Abstract] [Google Scholar]
- Miller SI, Pulkkinen WS, Selsted ME, Mekalanos JJ. Characterization of defensin resistance phenotypes associated with mutations in the phoP virulence regulon of Salmonella typhimurium. Infect Immun. 1990 Nov;58(11):3706–3710. [Europe PMC free article] [Abstract] [Google Scholar]
- Zasloff M. Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5449–5453. [Europe PMC free article] [Abstract] [Google Scholar]
- Moore KS, Bevins CL, Brasseur MM, Tomassini N, Turner K, Eck H, Zasloff M. Antimicrobial peptides in the stomach of Xenopus laevis. J Biol Chem. 1991 Oct 15;266(29):19851–19857. [Abstract] [Google Scholar]
- Moore KS, Bevins CL, Tomassini N, Huttner KM, Sadler K, Moreira JE, Reynolds J, Zasloff M. A novel peptide-producing cell in Xenopus: multinucleated gastric mucosal cell strikingly similar to the granular gland of the skin. J Histochem Cytochem. 1992 Mar;40(3):367–378. [Abstract] [Google Scholar]
- Wolff A, Moreira JE, Bevins CL, Hand AR, Fox PC. Magainin-like immunoreactivity in human submandibular and labial salivary glands. J Histochem Cytochem. 1990 Nov;38(11):1531–1534. [Abstract] [Google Scholar]
- Boman HG, Faye I, Gudmundsson GH, Lee JY, Lidholm DA. Cell-free immunity in Cecropia. A model system for antibacterial proteins. Eur J Biochem. 1991 Oct 1;201(1):23–31. [Abstract] [Google Scholar]
- Lee JY, Boman A, Sun CX, Andersson M, Jörnvall H, Mutt V, Boman HG. Antibacterial peptides from pig intestine: isolation of a mammalian cecropin. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9159–9162. [Europe PMC free article] [Abstract] [Google Scholar]
- Romeo D, Skerlavaj B, Bolognesi M, Gennaro R. Structure and bactericidal activity of an antibiotic dodecapeptide purified from bovine neutrophils. J Biol Chem. 1988 Jul 15;263(20):9573–9575. [Abstract] [Google Scholar]
- Selsted ME, Novotny MJ, Morris WL, Tang YQ, Smith W, Cullor JS. Indolicidin, a novel bactericidal tridecapeptide amide from neutrophils. J Biol Chem. 1992 Mar 5;267(7):4292–4295. [Abstract] [Google Scholar]
- Diamond G, Zasloff M, Eck H, Brasseur M, Maloy WL, Bevins CL. Tracheal antimicrobial peptide, a cysteine-rich peptide from mammalian tracheal mucosa: peptide isolation and cloning of a cDNA. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3952–3956. [Europe PMC free article] [Abstract] [Google Scholar]
- Shigenaga T, Muta T, Toh Y, Tokunaga F, Iwanaga S. Antimicrobial tachyplesin peptide precursor. cDNA cloning and cellular localization in the horseshoe crab (Tachypleus tridentatus). J Biol Chem. 1990 Dec 5;265(34):21350–21354. [Abstract] [Google Scholar]
- Casteels P, Ampe C, Jacobs F, Vaeck M, Tempst P. Apidaecins: antibacterial peptides from honeybees. EMBO J. 1989 Aug;8(8):2387–2391. [Europe PMC free article] [Abstract] [Google Scholar]
- Fujiwara S, Imai J, Fujiwara M, Yaeshima T, Kawashima T, Kobayashi K. A potent antibacterial protein in royal jelly. Purification and determination of the primary structure of royalisin. J Biol Chem. 1990 Jul 5;265(19):11333–11337. [Abstract] [Google Scholar]
- Groisman EA, Heffron F, Solomon F. Molecular genetic analysis of the Escherichia coli phoP locus. J Bacteriol. 1992 Jan;174(2):486–491. [Europe PMC free article] [Abstract] [Google Scholar]
- Hughes KT, Roth JR. Transitory cis complementation: a method for providing transposition functions to defective transposons. Genetics. 1988 May;119(1):9–12. [Europe PMC free article] [Abstract] [Google Scholar]
- Sanderson KE, Roth JR. Linkage map of Salmonella typhimurium, edition VII. Microbiol Rev. 1988 Dec;52(4):485–532. [Europe PMC free article] [Abstract] [Google Scholar]
- Castilho BA, Olfson P, Casadaban MJ. Plasmid insertion mutagenesis and lac gene fusion with mini-mu bacteriophage transposons. J Bacteriol. 1984 May;158(2):488–495. [Europe PMC free article] [Abstract] [Google Scholar]
- Benson NR, Goldman BS. Rapid mapping in Salmonella typhimurium with Mud-P22 prophages. J Bacteriol. 1992 Mar;174(5):1673–1681. [Europe PMC free article] [Abstract] [Google Scholar]
- Curtiss R, 3rd, Kelly SM. Salmonella typhimurium deletion mutants lacking adenylate cyclase and cyclic AMP receptor protein are avirulent and immunogenic. Infect Immun. 1987 Dec;55(12):3035–3043. [Europe PMC free article] [Abstract] [Google Scholar]
- Benjamin WH, Jr, Hall P, Briles DE. A hemA mutation renders Salmonella typhimurium avirulent in mice, yet capable of eliciting protection against intravenous infection with S. typhimurium. Microb Pathog. 1991 Oct;11(4):289–295. [Abstract] [Google Scholar]
- Ouellette AJ, Greco RM, James M, Frederick D, Naftilan J, Fallon JT. Developmental regulation of cryptdin, a corticostatin/defensin precursor mRNA in mouse small intestinal crypt epithelium. J Cell Biol. 1989 May;108(5):1687–1695. [Europe PMC free article] [Abstract] [Google Scholar]
- Ouellette AJ, Lualdi JC. A novel mouse gene family coding for cationic, cysteine-rich peptides. Regulation in small intestine and cells of myeloid origin. J Biol Chem. 1990 Jun 15;265(17):9831–9837. [Abstract] [Google Scholar]
- Oppenheim FG, Xu T, McMillian FM, Levitz SM, Diamond RD, Offner GD, Troxler RF. Histatins, a novel family of histidine-rich proteins in human parotid secretion. Isolation, characterization, primary structure, and fungistatic effects on Candida albicans. J Biol Chem. 1988 Jun 5;263(16):7472–7477. [Abstract] [Google Scholar]
- Galán JE, Curtiss R., 3rd Cloning and molecular characterization of genes whose products allow Salmonella typhimurium to penetrate tissue culture cells. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6383–6387. [Europe PMC free article] [Abstract] [Google Scholar]
- Lambert J, Keppi E, Dimarcq JL, Wicker C, Reichhart JM, Dunbar B, Lepage P, Van Dorsselaer A, Hoffmann J, Fothergill J, et al. Insect immunity: isolation from immune blood of the dipteran Phormia terranovae of two insect antibacterial peptides with sequence homology to rabbit lung macrophage bactericidal peptides. Proc Natl Acad Sci U S A. 1989 Jan;86(1):262–266. [Europe PMC free article] [Abstract] [Google Scholar]
- Dimarcq JL, Zachary D, Hoffmann JA, Hoffmann D, Reichhart JM. Insect immunity: expression of the two major inducible antibacterial peptides, defensin and diptericin, in Phormia terranovae. EMBO J. 1990 Aug;9(8):2507–2515. [Europe PMC free article] [Abstract] [Google Scholar]
- Bulet P, Cociancich S, Dimarcq JL, Lambert J, Reichhart JM, Hoffmann D, Hetru C, Hoffmann JA. Insect immunity. Isolation from a coleopteran insect of a novel inducible antibacterial peptide and of new members of the insect defensin family. J Biol Chem. 1991 Dec 25;266(36):24520–24525. [Abstract] [Google Scholar]
- Cruciani RA, Barker JL, Zasloff M, Chen HC, Colamonici O. Antibiotic magainins exert cytolytic activity against transformed cell lines through channel formation. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3792–3796. [Europe PMC free article] [Abstract] [Google Scholar]
- Christensen B, Fink J, Merrifield RB, Mauzerall D. Channel-forming properties of cecropins and related model compounds incorporated into planar lipid membranes. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5072–5076. [Europe PMC free article] [Abstract] [Google Scholar]
- Kagan BL, Selsted ME, Ganz T, Lehrer RI. Antimicrobial defensin peptides form voltage-dependent ion-permeable channels in planar lipid bilayer membranes. Proc Natl Acad Sci U S A. 1990 Jan;87(1):210–214. [Europe PMC free article] [Abstract] [Google Scholar]
- Macias EA, Rana F, Blazyk J, Modrzakowski MC. Bactericidal activity of magainin 2: use of lipopolysaccharide mutants. Can J Microbiol. 1990 Aug;36(8):582–584. [Abstract] [Google Scholar]
- Benjamin WH, Jr, Yother J, Hall P, Briles DE. The Salmonella typhimurium locus mviA regulates virulence in Itys but not Ityr mice: functional mviA results in avirulence; mutant (nonfunctional) mviA results in virulence. J Exp Med. 1991 Nov 1;174(5):1073–1083. [Europe PMC free article] [Abstract] [Google Scholar]
- Blight MA, Holland IB. Structure and function of haemolysin B,P-glycoprotein and other members of a novel family of membrane translocators. Mol Microbiol. 1990 Jun;4(6):873–880. [Abstract] [Google Scholar]
- Juretić D, Chen HC, Brown JH, Morell JL, Hendler RW, Westerhoff HV. Magainin 2 amide and analogues. Antimicrobial activity, membrane depolarization and susceptibility to proteolysis. FEBS Lett. 1989 Jun 5;249(2):219–223. [Abstract] [Google Scholar]
- McHugh GL, Miller CG. Isolation and characterization of proline peptidase mutants of Salmonella typhimurium. J Bacteriol. 1974 Oct;120(1):364–371. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.89.24.11939
Read article for free, from open access legal sources, via Unpaywall:
https://www.pnas.org/content/pnas/89/24/11939.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
Atomic insights into the signaling landscape of E. coli PhoQ histidine kinase from molecular dynamics simulations.
Sci Rep, 14(1):17659, 26 Jul 2024
Cited by: 0 articles | PMID: 39085378 | PMCID: PMC11291726
Role of cold shock proteins B and D in Aeromonas salmonicida subsp. salmonicida physiology and virulence in lumpfish (Cyclopterus lumpus).
Infect Immun, 92(8):e0001124, 26 Jun 2024
Cited by: 0 articles | PMID: 38920386 | PMCID: PMC11320987
Molecular Mechanisms of Bacterial Resistance to Antimicrobial Peptides in the Modern Era: An Updated Review.
Microorganisms, 12(7):1259, 21 Jun 2024
Cited by: 3 articles | PMID: 39065030 | PMCID: PMC11279074
Review Free full text in Europe PMC
Determinants of bacterial survival and proliferation in blood.
FEMS Microbiol Rev, 48(3):fuae013, 01 May 2024
Cited by: 0 articles | PMID: 38734892
Review
Antimicrobial Peptide Recognition Motif of the Substrate Binding Protein SapA from Nontypeable <i>Haemophilus influenzae</i>.
Biochemistry, 63(3):294-311, 08 Jan 2024
Cited by: 0 articles | PMID: 38189237 | PMCID: PMC10851439
Go to all (163) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
PhoP-PhoQ activates transcription of pmrAB, encoding a two-component regulatory system involved in Salmonella typhimurium antimicrobial peptide resistance.
J Bacteriol, 178(23):6857-6864, 01 Dec 1996
Cited by: 273 articles | PMID: 8955307 | PMCID: PMC178586
Mechanisms and physiological effects of protamine resistance in Salmonella enterica serovar Typhimurium LT2.
J Antimicrob Chemother, 65(5):876-887, 16 Mar 2010
Cited by: 23 articles | PMID: 20233778
Outer membrane structure in smooth and rough strains of Salmonella typhimurium and their susceptibility to the antimicrobial peptides, magainins and defensins.
Prog Clin Biol Res, 292:77-85, 01 Jan 1989
Cited by: 3 articles | PMID: 2657770
S. Typhimurium strategies to resist killing by cationic antimicrobial peptides.
Biochim Biophys Acta, 1848(11 pt b):3021-3025, 30 Jan 2015
Cited by: 36 articles | PMID: 25644871 | PMCID: PMC4520786
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NIAID NIH HHS (2)
Grant ID: AI22933
Grant ID: AI29554