Abstract
Free full text

Enhanced production of monocyte chemoattractant protein-1 in rheumatoid arthritis.
Abstract
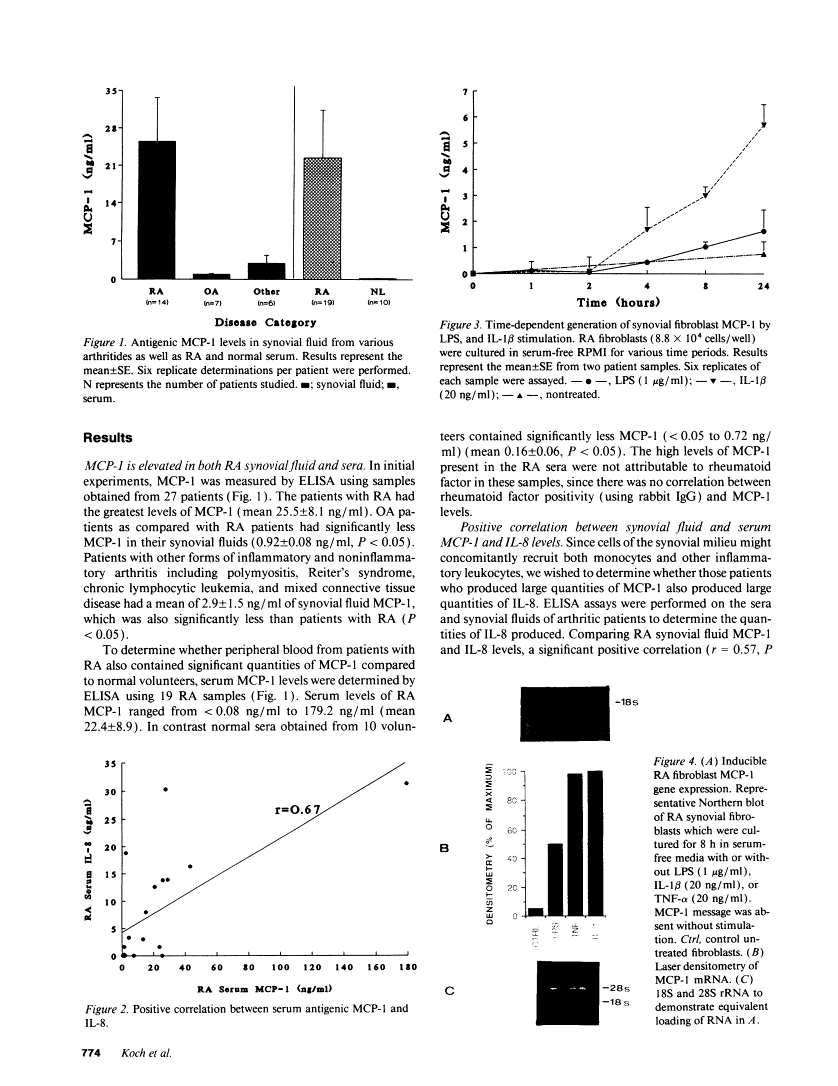
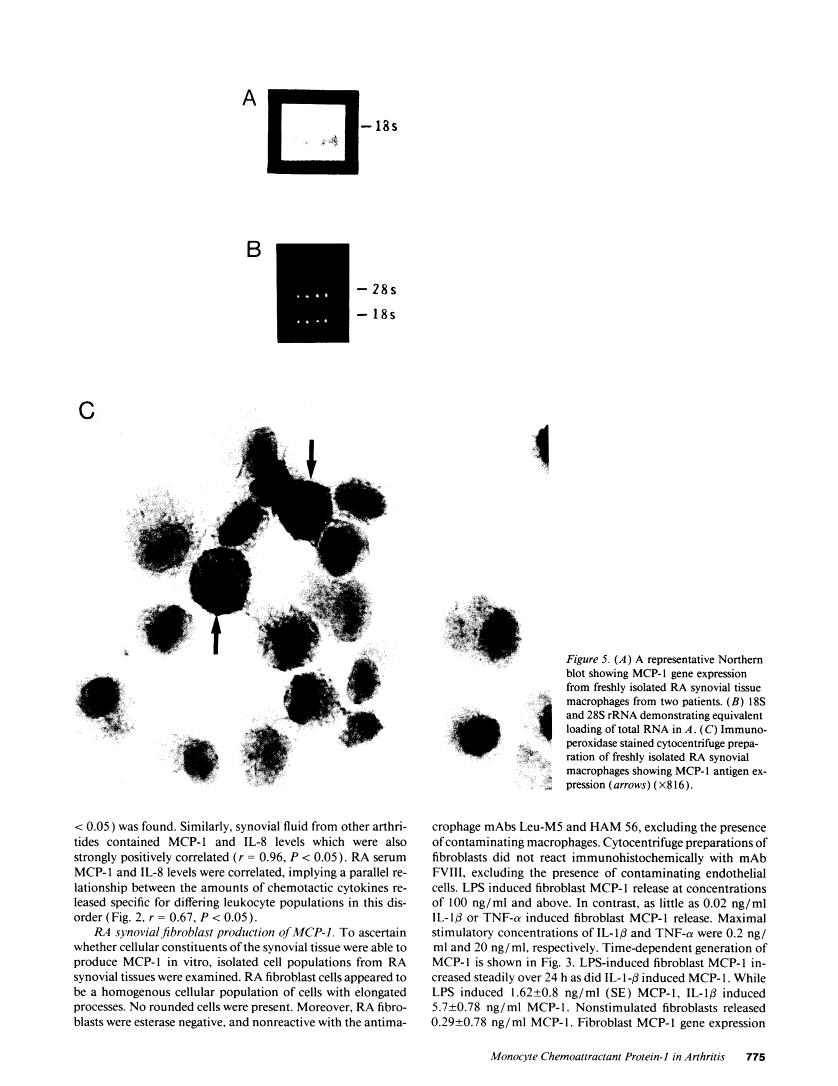
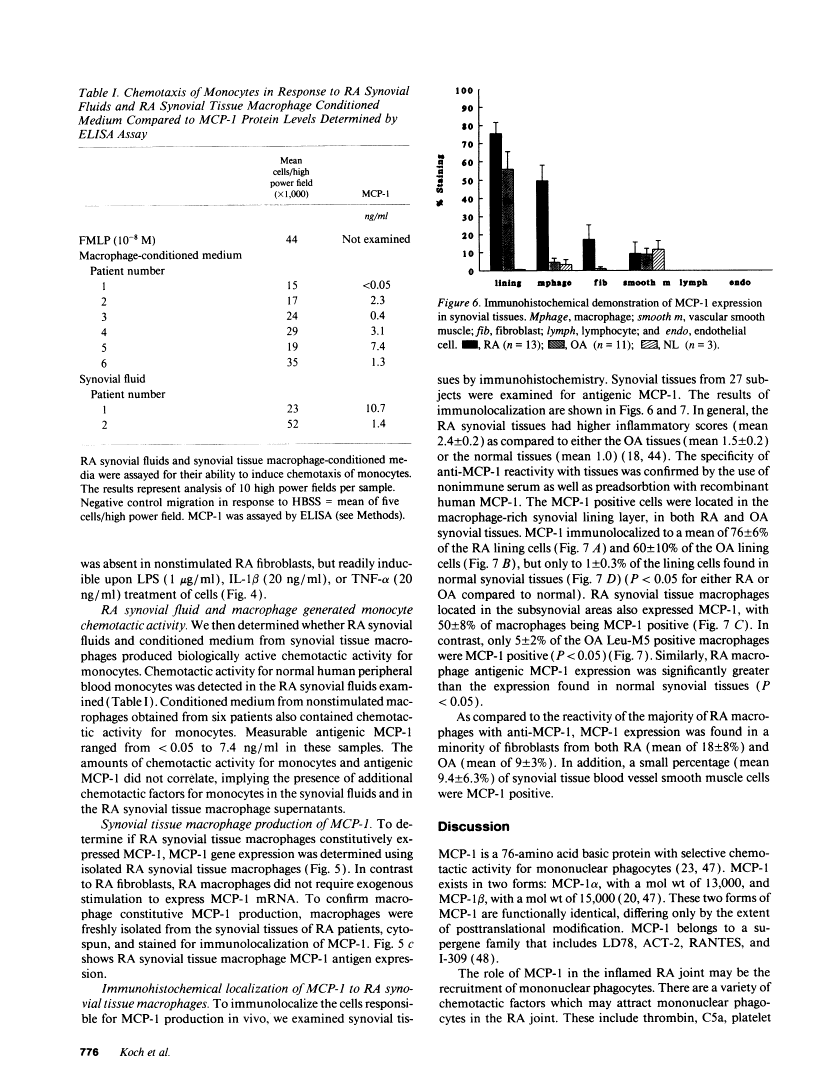
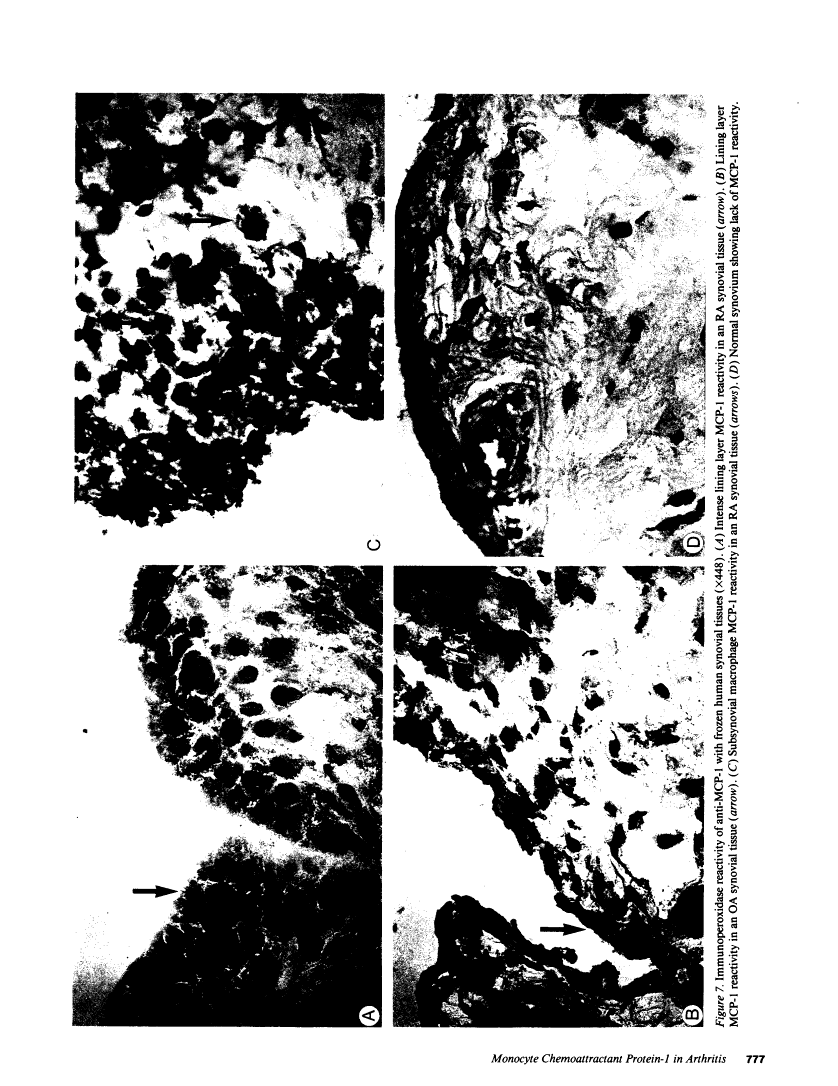
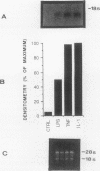
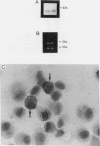
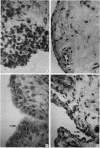
Cells within the synovial tissue may recruit mononuclear phagocytes into the synovial fluid and tissues of arthritic patients. We investigated the production of the chemotactic cytokine monocyte chemoattractant protein-1 (MCP-1) using sera, synovial fluid, synovial tissue, as well as macrophages and fibroblasts isolated from synovial tissues from 80 arthritic patients. MCP-1 levels were significantly higher (P less than 0.05) in synovial fluid from RA patients (mean 25.5 +/- 8.1 ng/ml [SE]) compared to synovial fluid from osteoarthritis (OA) patients (0.92 +/- 0.08), or from patients with other arthritides (2.9 +/- 1.5). MCP-1 levels in RA sera (8.44 +/- 2.33) were significantly greater than MCP-1 in normal sera (0.16 +/- 0.06). The quantities of RA synovial fluid IL-8, which is chemotactic for neutrophils and lymphocytes, and MCP-1 were strongly positively correlated (P less than 0.05). To examine the cellular source of MCP-1, RA synovial tissue macrophages and fibroblasts were isolated. Synovial tissue fibroblasts did not express MCP-1 mRNA, but could be induced to produce MCP-1 by stimulation with either IL-1 beta, tumor necrosis factor-alpha (TNF-alpha), or LPS. In contrast, unlike normal peripheral blood monocytes or alveolar macrophages, RA synovial tissue macrophages constitutively expressed MCP-1 mRNA and antigen. Immunohistochemical analysis of synovial tissue showed that a significantly greater percentage of RA macrophages (50 +/- 8%) as compared to either OA macrophages (5 +/- 2) or normal macrophages (1 +/- 0.3) reacted with anti-MCP-1 antibodies. In addition, the synovial lining layer reacted with MCP-1 in both RA and OA synovial tissues. In contrast, only a minority of synovial fibroblasts (18 +/- 8%) from RA synovium were positive for immunolocalization of MCP-1. These results suggest that synovial production of MCP-1 may play an important role in the recruitment of mononuclear phagocytes during inflammation associated with RA and that synovial tissue macrophages are the dominant source of this cytokine.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (2.9M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Koch AE, Kunkel SL, Burrows JC, Evanoff HL, Haines GK, Pope RM, Strieter RM. Synovial tissue macrophage as a source of the chemotactic cytokine IL-8. J Immunol. 1991 Oct 1;147(7):2187–2195. [Abstract] [Google Scholar]
- Wood DD, Ihrie EJ, Hamerman D. Release of interleukin-1 from human synovial tissue in vitro. Arthritis Rheum. 1985 Aug;28(8):853–862. [Abstract] [Google Scholar]
- Miyasaka N, Sato K, Goto M, Sasano M, Natsuyama M, Inoue K, Nishioka K. Augmented interleukin-1 production and HLA-DR expression in the synovium of rheumatoid arthritis patients. Possible involvement in joint destruction. Arthritis Rheum. 1988 Apr;31(4):480–486. [Abstract] [Google Scholar]
- Wood DD, Ihrie EJ, Dinarello CA, Cohen PL. Isolation of an interleukin-1-like factor from human joint effusions. Arthritis Rheum. 1983 Aug;26(8):975–983. [Abstract] [Google Scholar]
- Yocum DE, Esparza L, Dubry S, Benjamin JB, Volz R, Scuderi P. Characteristics of tumor necrosis factor production in rheumatoid arthritis. Cell Immunol. 1989 Aug;122(1):131–145. [Abstract] [Google Scholar]
- Husby G, Williams RC., Jr Synovial localization of tumor necrosis factor in patients with rheumatoid arthritis. J Autoimmun. 1988 Aug;1(4):363–371. [Abstract] [Google Scholar]
- Lipsky PE, Davis LS, Cush JJ, Oppenheimer-Marks N. The role of cytokines in the pathogenesis of rheumatoid arthritis. Springer Semin Immunopathol. 1989;11(2):123–162. [Abstract] [Google Scholar]
- Arend WP, Dayer JM. Cytokines and cytokine inhibitors or antagonists in rheumatoid arthritis. Arthritis Rheum. 1990 Mar;33(3):305–315. [Abstract] [Google Scholar]
- Dayer JM, de Rochemonteix B, Burrus B, Demczuk S, Dinarello CA. Human recombinant interleukin 1 stimulates collagenase and prostaglandin E2 production by human synovial cells. J Clin Invest. 1986 Feb;77(2):645–648. [Europe PMC free article] [Abstract] [Google Scholar]
- Dayer JM, Beutler B, Cerami A. Cachectin/tumor necrosis factor stimulates collagenase and prostaglandin E2 production by human synovial cells and dermal fibroblasts. J Exp Med. 1985 Dec 1;162(6):2163–2168. [Europe PMC free article] [Abstract] [Google Scholar]
- Firestein GS, Alvaro-Gracia JM, Maki R, Alvaro-Garcia JM. Quantitative analysis of cytokine gene expression in rheumatoid arthritis. J Immunol. 1990 May 1;144(9):3347–3353. [Abstract] [Google Scholar]
- Firestein GS. The immunopathogenesis of rheumatoid arthritis. Curr Opin Rheumatol. 1991 Jun;3(3):398–406. [Abstract] [Google Scholar]
- Firestein GS, Zvaifler NJ. How important are T cells in chronic rheumatoid synovitis? Arthritis Rheum. 1990 Jun;33(6):768–773. [Abstract] [Google Scholar]
- Alvaro-Gracia JM, Zvaifler NJ, Brown CB, Kaushansky K, Firestein GS. Cytokines in chronic inflammatory arthritis. VI. Analysis of the synovial cells involved in granulocyte-macrophage colony-stimulating factor production and gene expression in rheumatoid arthritis and its regulation by IL-1 and tumor necrosis factor-alpha. J Immunol. 1991 May 15;146(10):3365–3371. [Abstract] [Google Scholar]
- Alvaro-Gracia JM, Zvaifler NJ, Firestein GS. Cytokines in chronic inflammatory arthritis. IV. Granulocyte/macrophage colony-stimulating factor-mediated induction of class II MHC antigen on human monocytes: a possible role in rheumatoid arthritis. J Exp Med. 1989 Sep 1;170(3):865–875. [Europe PMC free article] [Abstract] [Google Scholar]
- Koch AE, Polverini PJ, Leibovich SJ. Stimulation of neovascularization by human rheumatoid synovial tissue macrophages. Arthritis Rheum. 1986 Apr;29(4):471–479. [Abstract] [Google Scholar]
- Koch AE, Polverini PJ, Leibovich SJ. Functional heterogeneity of human rheumatoid synovial tissue macrophages. J Rheumatol. 1988 Jul;15(7):1058–1063. [Abstract] [Google Scholar]
- Koch AE, Burrows JC, Haines GK, Carlos TM, Harlan JM, Leibovich SJ. Immunolocalization of endothelial and leukocyte adhesion molecules in human rheumatoid and osteoarthritic synovial tissues. Lab Invest. 1991 Mar;64(3):313–320. [Abstract] [Google Scholar]
- Carlos TM, Harlan JM. Membrane proteins involved in phagocyte adherence to endothelium. Immunol Rev. 1990 Apr;114:5–28. [Abstract] [Google Scholar]
- Yoshimura T, Robinson EA, Tanaka S, Appella E, Leonard EJ. Purification and amino acid analysis of two human monocyte chemoattractants produced by phytohemagglutinin-stimulated human blood mononuclear leukocytes. J Immunol. 1989 Mar 15;142(6):1956–1962. [Abstract] [Google Scholar]
- Furutani Y, Nomura H, Notake M, Oyamada Y, Fukui T, Yamada M, Larsen CG, Oppenheim JJ, Matsushima K. Cloning and sequencing of the cDNA for human monocyte chemotactic and activating factor (MCAF). Biochem Biophys Res Commun. 1989 Feb 28;159(1):249–255. [Abstract] [Google Scholar]
- Yoshimura T, Robinson EA, Tanaka S, Appella E, Kuratsu J, Leonard EJ. Purification and amino acid analysis of two human glioma-derived monocyte chemoattractants. J Exp Med. 1989 Apr 1;169(4):1449–1459. [Europe PMC free article] [Abstract] [Google Scholar]
- Matsushima K, Larsen CG, DuBois GC, Oppenheim JJ. Purification and characterization of a novel monocyte chemotactic and activating factor produced by a human myelomonocytic cell line. J Exp Med. 1989 Apr 1;169(4):1485–1490. [Europe PMC free article] [Abstract] [Google Scholar]
- Strieter RM, Wiggins R, Phan SH, Wharram BL, Showell HJ, Remick DG, Chensue SW, Kunkel SL. Monocyte chemotactic protein gene expression by cytokine-treated human fibroblasts and endothelial cells. Biochem Biophys Res Commun. 1989 Jul 31;162(2):694–700. [Abstract] [Google Scholar]
- Sica A, Wang JM, Colotta F, Dejana E, Mantovani A, Oppenheim JJ, Larsen CG, Zachariae CO, Matsushima K. Monocyte chemotactic and activating factor gene expression induced in endothelial cells by IL-1 and tumor necrosis factor. J Immunol. 1990 Apr 15;144(8):3034–3038. [Abstract] [Google Scholar]
- Larsen CG, Zachariae CO, Oppenheim JJ, Matsushima K. Production of monocyte chemotactic and activating factor (MCAF) by human dermal fibroblasts in response to interleukin 1 or tumor necrosis factor. Biochem Biophys Res Commun. 1989 May 15;160(3):1403–1408. [Abstract] [Google Scholar]
- Yoshimura T, Leonard EJ. Secretion by human fibroblasts of monocyte chemoattractant protein-1, the product of gene JE. J Immunol. 1990 Mar 15;144(6):2377–2383. [Abstract] [Google Scholar]
- Graves DT, Jiang YL, Williamson MJ, Valente AJ. Identification of monocyte chemotactic activity produced by malignant cells. Science. 1989 Sep 29;245(4925):1490–1493. [Abstract] [Google Scholar]
- Elner SG, Strieter RM, Elner VM, Rollins BJ, Del Monte MA, Kunkel SL. Monocyte chemotactic protein gene expression by cytokine-treated human retinal pigment epithelial cells. Lab Invest. 1991 Jun;64(6):819–825. [Abstract] [Google Scholar]
- Rollins BJ, Stier P, Ernst T, Wong GG. The human homolog of the JE gene encodes a monocyte secretory protein. Mol Cell Biol. 1989 Nov;9(11):4687–4695. [Europe PMC free article] [Abstract] [Google Scholar]
- Rollins BJ, Yoshimura T, Leonard EJ, Pober JS. Cytokine-activated human endothelial cells synthesize and secrete a monocyte chemoattractant, MCP-1/JE. Am J Pathol. 1990 Jun;136(6):1229–1233. [Europe PMC free article] [Abstract] [Google Scholar]
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. [Abstract] [Google Scholar]
- Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, Christy W, Cooke TD, Greenwald R, Hochberg M, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986 Aug;29(8):1039–1049. [Abstract] [Google Scholar]
- Koch AE, Burrows JC, Skoutelis A, Marder R, Domer PH, Anderson B, Leibovich SJ. Monoclonal antibodies detect monocyte/macrophage activation and differentiation antigens and identify functionally distinct subpopulations of human rheumatoid synovial tissue macrophages. Am J Pathol. 1991 Jan;138(1):165–173. [Europe PMC free article] [Abstract] [Google Scholar]
- Koch AE, Polverini PJ, Leibovich SJ. Induction of neovascularization by activated human monocytes. J Leukoc Biol. 1986 Feb;39(2):233–238. [Abstract] [Google Scholar]
- Evanoff HL, Burdick MD, Moore SA, Kunkel SL, Strieter RM. A sensitive ELISA for the detection of human monocyte chemoattractant protein-1 (MCP-1). Immunol Invest. 1992 Feb;21(1):39–45. [Abstract] [Google Scholar]
- Standiford TJ, Kunkel SL, Basha MA, Chensue SW, Lynch JP, 3rd, Toews GB, Westwick J, Strieter RM. Interleukin-8 gene expression by a pulmonary epithelial cell line. A model for cytokine networks in the lung. J Clin Invest. 1990 Dec;86(6):1945–1953. [Europe PMC free article] [Abstract] [Google Scholar]
- Chirgwin JM, Przybyla AE, MacDonald RJ, Rutter WJ. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. [Abstract] [Google Scholar]
- Jonas E, Sargent TD, Dawid IB. Epidermal keratin gene expressed in embryos of Xenopus laevis. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5413–5417. [Europe PMC free article] [Abstract] [Google Scholar]
- Koch AE, Haines GK, Rizzo RJ, Radosevich JA, Pope RM, Robinson PG, Pearce WH. Human abdominal aortic aneurysms. Immunophenotypic analysis suggesting an immune-mediated response. Am J Pathol. 1990 Nov;137(5):1199–1213. [Europe PMC free article] [Abstract] [Google Scholar]
- Hsu SM, Raine L, Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. [Abstract] [Google Scholar]
- Koch AE, Robinson PG, Radosevich JA, Pope RM. Distribution of CD45RA and CD45RO T-lymphocyte subsets in rheumatoid arthritis synovial tissue. J Clin Immunol. 1990 Jul;10(4):192–199. [Abstract] [Google Scholar]
- Koch AE, Burrows JC, Marder R, Domer PH, Leibovich SJ. Reactivity of human tissues with monoclonal antibodies to myeloid activation and differentiation antigens. An immunohistochemical study. Pathobiology. 1990;58(5):241–248. [Abstract] [Google Scholar]
- Matsushima K, Oppenheim JJ. Interleukin 8 and MCAF: novel inflammatory cytokines inducible by IL 1 and TNF. Cytokine. 1989 Nov;1(1):2–13. [Abstract] [Google Scholar]
- Schall TJ. Biology of the RANTES/SIS cytokine family. Cytokine. 1991 May;3(3):165–183. [Abstract] [Google Scholar]
- DeMarco D, Kunkel SL, Strieter RM, Basha M, Zurier RB. Interleukin-1 induced gene expression of neutrophil activating protein (interleukin-8) and monocyte chemotactic peptide in human synovial cells. Biochem Biophys Res Commun. 1991 Jan 31;174(2):411–416. [Abstract] [Google Scholar]
- Strieter RM, Chensue SW, Basha MA, Standiford TJ, Lynch JP, Baggiolini M, Kunkel SL. Human alveolar macrophage gene expression of interleukin-8 by tumor necrosis factor-alpha, lipopolysaccharide, and interleukin-1 beta. Am J Respir Cell Mol Biol. 1990 Apr;2(4):321–326. [Abstract] [Google Scholar]
Associated Data
Articles from The Journal of Clinical Investigation are provided here courtesy of American Society for Clinical Investigation
Full text links
Read article at publisher's site: https://doi.org/10.1172/jci115950
Read article for free, from open access legal sources, via Unpaywall:
http://www.jci.org/articles/view/115950/files/pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1172/jci115950
Article citations
Targeting PAR2-mediated inflammation in osteoarthritis: a comprehensive in vitro evaluation of oleocanthal's potential as a functional food intervention for chondrocyte protection and anti-inflammatory effects.
BMC Musculoskelet Disord, 25(1):769, 01 Oct 2024
Cited by: 0 articles | PMID: 39354427 | PMCID: PMC11446003
The chromatin landscape of pathogenic transcriptional cell states in rheumatoid arthritis.
Nat Commun, 15(1):4650, 31 May 2024
Cited by: 6 articles | PMID: 38821936 | PMCID: PMC11143375
IGF-1 Genome-Edited Human MSCs Exhibit Robust Anti-Arthritogenicity in Collagen-Induced Arthritis.
Int J Mol Sci, 25(8):4442, 18 Apr 2024
Cited by: 0 articles | PMID: 38674027 | PMCID: PMC11050354
Targeting proprotein convertase subtilisin/kexin type 9 (PCSK9): from bench to bedside.
Signal Transduct Target Ther, 9(1):13, 08 Jan 2024
Cited by: 15 articles | PMID: 38185721 | PMCID: PMC10772138
Review Free full text in Europe PMC
Sialic-Acid-Related Enzymes of B Cells and Monocytes as Novel Markers to Discriminate Improvement Categories and to Fulfill Two Remission Definitions in Rheumatoid Arthritis.
Int J Mol Sci, 24(16):12998, 20 Aug 2023
Cited by: 1 article | PMID: 37629178 | PMCID: PMC10455111
Go to all (393) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Synovial tissue macrophage as a source of the chemotactic cytokine IL-8.
J Immunol, 147(7):2187-2195, 01 Oct 1991
Cited by: 178 articles | PMID: 1918955
Epithelial neutrophil activating peptide-78: a novel chemotactic cytokine for neutrophils in arthritis.
J Clin Invest, 94(3):1012-1018, 01 Sep 1994
Cited by: 128 articles | PMID: 8083342 | PMCID: PMC295150
Monocyte chemoattractant protein-1 (MCP-1) in inflammatory joint diseases and its involvement in the cytokine network of rheumatoid synovium.
Clin Immunol Immunopathol, 69(1):83-91, 01 Oct 1993
Cited by: 86 articles | PMID: 8403545
Arthritic and non-arthritic synovial fluids modulate IL10 and IL1RA gene expression in differentially activated primary human monocytes.
Osteoarthritis Cartilage, 23(11):1853-1857, 01 Nov 2015
Cited by: 37 articles | PMID: 26521731
Review
Funding
Funders who supported this work.
NHLBI NIH HHS (1)
Grant ID: HL-02401
NIAMS NIH HHS (2)
Grant ID: AR30692
Grant ID: AR41492