Abstract
Free full text

Effects of dietary omega-3 polyunsaturated fatty acids on brain gene expression
Abstract
Polyunsaturated fatty acids (PUFA) are essential structural components of the central nervous system. Their role in controlling learning and memory has been well documented. A nutrigenomic approach with high-density microarrays was used to reveal brain gene-expression changes in response to different PUFA-enriched diets in rats. In aged rats fed throughout life with PUFA-enriched diets, genes with altered expressions included transthyretin, α-synuclein, and calmodulins, which play important roles in synaptic plasticity and learning. The effect of perinatal omega-3 PUFA supply on gene expression later in life also was studied. Several genes showed similar changes in expression in rats fed omega-3-deficient diets in the perinatal period, regardless of whether they or their mothers were fed omega-3 PUFA-sufficient diets after giving birth. In this experiment, among the down-regulated genes were a kainate glutamate receptor and a DEAD-box polypeptide. Among the up-regulated genes were a chemokine-like factor, a tumor necrosis factor receptor, and cytochrome c. The possible involvement of the genes with altered expression attributable to different diets in different brain regions in young and aged rats and the possible mode of regulatory action of PUFA also are discussed. We conclude that PUFA-enriched diets lead to significant changes in expression of several genes in the central nervous tissue, and these effects appear to be mainly independent of their effects on membrane composition. The direct effects of PUFA on transcriptional modulators, the downstream developmentally and tissue-specifically activated elements might be one of the clues to understanding the beneficial effects of the omega-3 PUFA on the nervous system.
Two types of polyunsaturated fatty acids (PUFA), omega-6 (n-6) and omega-3 (n-3) PUFA, are essential for the vertebrate body, because they cannot be formed de novo and need to be ingested from the diet. Linoleic acid (LA; 18:2 omega-6) is the precursor of omega-6 PUFA, and α-linolenic acid (ALA; 18:3 omega-3) is the precursor for the omega-3 PUFA. These fatty acids (FA), and the complex lipids formed from them, are important constituents of biological membranes and contribute to maintain the structural and functional integrity of cells and cellular components (1–3). The long-chain PUFA arachidonic acid (AA; 20:4 omega-6) is also a specific precursor of hormone-like compounds called eicosanoids, which are involved in inflammation and in several homeostatic biological functions (4). The list of PUFA-derived eicosanoids is expanding beyond classically studied leukotrienes and prostaglandins and includes endocannabinoids, N-acyl-linked amino acids and dopamine, and various oxygenated and epoxylated bioactive derivatives of AA and docosahexaenoic acid (DHA) (5). Although these PUFA occur in various proportions in all organs, the nervous tissue is characterized by very low levels of LA, ALA, and eicosapentaenoic acid (EPA; 20:5 omega-3) and high concentrations of DHA (22:6 omega-3), AA, and docosatetraenoic acid (22:4 omega-6). DHA and AA not only are the main FA in gray matter phospholipids of neural membranes, where they account for 6% of the dry matter of the cerebral cortex (6), but also are required for the development of the central nervous system (7–11).
The level of DHA appears to be strictly controlled, because any deviation from the physiological level results in disturbance of cognitive functions (12, 13). In several different species, animals raised on ALA-deficient diets had decreased levels of DHA in the brain and retina that were associated with impairments in neural and visual functions (14–18). Both the omega-6 and the omega-3 PUFA have essential roles in the growth and function of the brain, and these effects likely are mediated by their action on gene expression, electrophysiological responses, eicosanoid synthesis, and membrane structures. The exact mode of action of DHA and DHA-containing phospholipids, especially in the ethanolamine and serine phosphoglycerides, is not known, but numerous mechanisms have been suggested (19–21).
DHA plays a crucial role in diverse functions at multiple levels. At the membrane level, it can influence the function of the blood–brain barrier (22); it can alter membrane receptors such as rhodopsin (23); it can regulate the activity of membrane-bound enzymes (Na/K-dependent ATPase) (24), ionic channels (25), and dopaminergic and serotoninergic neurotransmission (26, 27), most probably by changing membrane fluidity (28); and it can alter signal transduction by means of effects on inositol phosphates, diacylglycerol, and protein kinase C (29). At the cellular level, DHA can protect neural cells from apoptotic death (30), stimulate neurite outgrowth in PC12 cells (31, 32), induce synaptic growth cones during neuronal development (9, 33, 34), enhance synaptic functions (35), regulate nerve growth factor (36), and influence neuron size (37, 38). Recent studies in Caenorhabditis elegans depleted of the delta-6 desaturase have provided pharmacological, ultrastructural, and electrophysiological evidence that the worms became depleted of synaptic vesicles and released low levels of neurotransmitter at cholinergic and serotonergic neuromuscular junctions (39). These data suggest that long-chain PUFA thus are essential for efficient neurotransmission in C. elegans and possibly other organisms. At the gene activity level, it has been shown that PUFA of both omega-3 and omega-6 families control gene expression in a variety of tissues (40–45). Several studies have confirmed the modulatory action of PUFA on gene expression in the brain (46–51).
Dietary omega-3 PUFA and mixtures of omega-3 and omega-6 PUFA exert complex changes in gene expression in the brain (47, 49) and in certain brain regions such as cerebrum (51) and hippocampus (48, 50) as assessed by a high-throughput analysis of the transcriptome by DNA microarray analysis.
Regulation of gene expression by PUFA can occur through interactions with specific or nonspecific ligands that bind to response factors acting on cis-regulatory elements of the gene, which finally turn on or off mRNA synthesis. For example, PUFA can directly interact with transcription factors, like peroxisome proliferator-activated receptors (PPAR), that directly modulate the expression of target genes (46, 52, 53).
Positive effects of DHA on learning and memory in animal and human models have been demonstrated (13, 17, 54–57). The amount of DHA in human milk is positively correlated with visual and language development in breast-fed infants, and DHA supplementation led to better visual function later in childhood than that shown by infants fed commercial formula with AA and DHA (58–60). However, the question remains unanswered as to whether these highly complex and sophisticated processes can be explained by an indirect effect of long-chain PUFA on the biophysical properties and molecular architecture of neural membranes and/or, as more recent evidence suggests, by direct control of transcription of several pivotal genes in the brain. The present article will focus primarily on PUFA in the mammalian brain from the aspects of their regulatory roles in gene expression related to the genetic machinery of neural systems.
Effect of Dietary Omega-3 PUFA on Gene Expression in the Brain
The final FA composition of brain is determined during embryogenesis, particularly in times of rapid brain growth and in rats 12–15 days after delivery (10, 61, 62). It is important that, during this time, the brain is supplied with adequate intakes of PUFA for its functions. Several genes have been reported to be activated by dietary long-chain PUFA, and some gene products, alone or in combination with the membrane effects of these PUFA, exert their beneficial effect on neural functions such as learning and memory. The fact that ALA and DHA activate several genes in other tissues, like liver or adipose tissue, is well known (40–45), but the underlying molecular mechanisms of the direct effects of PUFA diet-induced gene-expression changes in the brain have been addressed by very few studies (46–51, 63).
One of the first observations that dietary fat influenced brain gene expression was reported by DeWille and Farmer (64). They found that mRNA level of several genes involved in myelination, such as those coding for proteolipid protein and myelin basic protein, were affected by a diet lacking essential FA.
Novel techniques, such as DNA microarrays or real-time PCR, enabled our group to study brain gene-expression changes in response to dietary FA in a global way. We conducted two separate experiments where different PUFA were added to the diet of rats. In our first report, essential FA-sufficient rats were fed from conception with a diet containing perilla oil, which is rich in ALA (39% ALA/28% LA), compared with the control rat chow containing 6% ALA and 51% LA or a fish oil rich in DHA (27% DHA/23% LA/3% ALA/12% EPA) compared with the control chow containing very low levels of long-chain omega-3 PUFA (1.2% DHA and 0.7% EPA) for one generation. Transcriptional changes in brains of adult rats were monitored (47).
In the second experiment, rats were supplied from conception until adulthood with a diet in which the fat was a mixture of sunflower oil and fish oil (LA plus DHA) (45% LA/18% DHA/4% ALA/7% EPA) or a mixture of soybean oil and perilla oil (LA plus ALA) (49% LA/10% ALA) (49). We showed that the level of DHA-containing phosphatidylethanolamine molecular species, especially of 18:0/22:6, was higher in rat brains after consuming ALA or EPA plus DHA compared with the levels in the control chow-fed rats, indicating that even in essential FA adequate rats, it is possible to alter the level of DHA in their neural membranes (47, 49). Besides the changes of membrane composition, gene-expression changes were followed by using high-density DNA microarrays to identify previously uncharacterized cellular pathways involved in the direct neural effects of PUFA (47, 49).
The expression levels of 102 cDNAs, representing 3.4% of the total 3,200 DNA elements on the array, were significantly altered in brains of rats fed with the omega-3-enriched experimental diets. It was found that 55 genes were up-regulated and 47 were down-regulated relative to controls by the four dietary regimens. The altered genes included those involved in synaptic plasticity, cytoskeleton, signal transduction, ion channel formation, energy metabolism, and regulatory proteins (Table 1). It was interesting to observe that 15 genes responded more intensively to the dietary FA mixtures, LA plus ALA and LA plus DHA, than to the diets where there was one main dietary PUFA (DHA or ALA) (49). These genes included those that encode a clathrinassociated adaptor protein, farnesyl pyrophosphatase synthetase, Sec24 protein, NADH dehydrogenase/cytochrome c oxydase, cytochrome b, cytochrome c oxidase subunit II, ubiquitin-protein ligase Nedd42, and transcription factor-like protein. Several genes participating in signal transduction, like RAB6B small GTPase and calmodulins, were up-regulated.
Table 1.
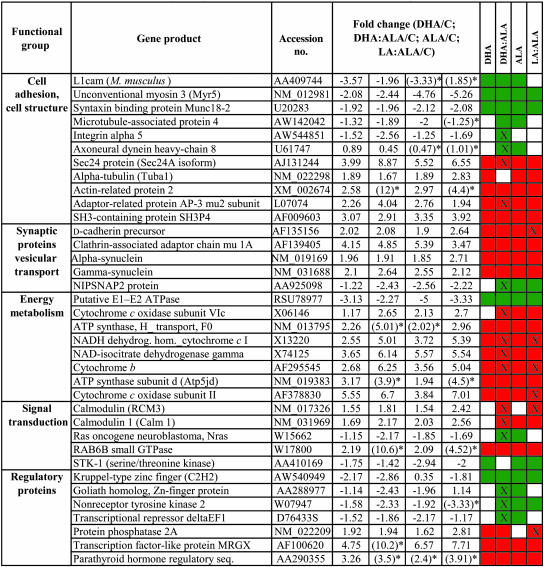
It is noteworthy that genes coding for α- and γ-synuclein also were overexpressed and that the d-cadherin gene exhibited up-regulation in response to the diets rich in ALA and DHA. d-cadherin and α-synuclein have been reported to be specifically enriched at synaptic contacts (65, 66). These proteins play a role in neural plasticity and are increased in the brains of songbirds when learning to sing (67). In addition, synucleins may play a role in the development and maturation of some neurons (68). In avian species, sex steroids have been shown to induce the transcription of α-synuclein (69).
We found that diets rich in omega-3 PUFA (ALA and DHA) and rich in the mixture of omega-3 and omega-6 PUFA significantly affected neural energy metabolism and ATP-generating machinery, as determined by the level of gene expression, because four different subunits of cytochrome c oxidase, cytochrome b, and ATP synthases were markedly up-regulated as evidenced by microarray data (Table 1). The observation that some mitochondrial enzymes were overexpressed by dietary PUFA or their mixtures suggests that the brain was in an elevated metabolic state and thus required additional ATP.
The transthyretin gene was down-regulated in all four dietary conditions (ALA, DHA, LA plus ALA, and LA plus DHA) in young animal brains. This gene also was shown to be repressed by omega-3-rich fish oil (51), whereas in other studies it has been reported that diets rich in AA induced its transcription in mouse hippocampus (50). Transthyretin binds thyroid hormones, and it has been shown that thyroid hormone deficiency during brain development impairs cognitive functions (70). In a transgenic mouse model, a low level of calmodulin-dependent protein kinase II activation resulted in enhanced long-term potentiation and a modest learning and memory impairment, which was accompanied by an increased transthyretin transcription (71). These observations suggest a role of transthyretin in synaptic plasticity, learning, and memory. In contrast, elevated transthyretin mRNA level has been documented in response to an omega-3-rich fish oil in aged rat hippocampus, which suggests age-related gene-expression alteration caused by dietary PUFA and their different roles during ontogeny (48, 51).
The transcript levels of three copies of calmodulin also were up-regulated to the same extent by the four dietary conditions tested (ALA, DHA, LA plus ALA, and LA plus DHA) (48). Ca2+/calmodulin signaling recently has been suggested to have a special role in the stimulant-induced plasticity of the central nervous system (72).
The products of several genes involved in cellular architecture, such as axoneural dynein heavy-chain 8, microtubule-associated protein 4, α-tubulin, and actin-related protein 2, were differentially expressed in the brain of animals in all four dietary conditions. Modulation of the cytoskeleton because of PUFA can influence a wide range of neural functions, including stabilization of axons and dendrites, cell shape, neuronal plasticity, polarity, vesicle formation, and transport.
Effect of Omega-3 PUFA on Gene Expression in the Aging Brain
Advanced age is accompanied with reduced levels of long-chain PUFA (AA and DHA) in the brain (73, 74). Aging may specifically affect the activity of enzymes responsible for acylation of phospholipids, such as glycerol-3-phosphate acyltransferase (75). The levels of PUFA in the senescence-accelerated mouse hippocampus also were significantly lowered in parallel with reduced delta 9-desaturase transcription, which causes alterations in membrane biophysical state and affects diverse cellular pathways (76). A reduced level of DHA was detected in the brains of 2-year-old rats compared with young animals (51). Impairments, such as loss of memory and learning disabilities, are also a common phenomenon in aged animals. The reduced DHA level of the aged brain could be elevated to the level of 3-month-old (control, young) rats by feeding the aged rat on a diet containing fish oil (11% DHA) for 1 month. This diet resulted in an increase of brain diacyl 18:0/22:6 phosphatidylethanolamine species from 29.0 ± 1.9% to 33.0 ± 1.25% (P < 0.05) in young rats and from 25.1 ± 1.4% to 29.6 ± 1.5% (P < 0.05) in old rats (51).
In 3-month-old rats receiving a diet containing omega-3-rich fish oil for 1 month, it was found by gene-expression profiling that only six genes were overexpressed and eight genes were repressed. Responses to this diet (DHA) were much weaker compared with those animals that were fed for longer periods from the time of conception (47). Of the overexpressed genes, transthyretin was slightly and α-synuclein was dramatically up-regulated. These inductions would indicate that a diet containing DHA might have a beneficial effect on learning and memory of these rats. Indeed, we found that these rats performed better in the Morris water maze test than did the rats kept on the control diet (51).
To extend this study, different brain regions (hippocampus and cerebellum) of 3-month-old and 2-year-old rats, receiving fish oil for 1 month, were investigated (48). The genes coding for α-synuclein and 90-kDa heat shock protein (Hsp90) responded in a similar way in different brain regions of young rats receiving the diet with the omega-3-rich fish oil for 1 month, whereas other genes responded in a different way in the different brain regions (data not shown). It has been published that Rab-αGDI activity is regulated by a Hsp90 chaperone complex, which controls vesicle membrane traffic and neurotransmitter release (77). It was found that only 7 genes were overexpressed and 11 genes were repressed in the hippocampus of old rats fed on a diet containing omega-3-rich fish oil. In contrast, more genes responded with transcriptional changes in young mouse hippocampus from animals fed with long-chain PUFA (50).
Although transthyretin was significantly down-regulated in young murine hippocampus (50) and slightly overexpressed in young rat brain, in old rat hippocampus from rats fed on an omega-3-rich fish oil diet for 1 month, its expression was dramatically induced (48). These age- and region-related expression changes indicate that transthyretin fulfills different functions in different brain regions and different periods of aging. In the old rat hippocampus, the major function of transthyretin might be to sequester amyloid β polypeptide, thus protecting the brain from developing Alzheimer's disease (AD). AD is characterized by the accumulation of fibrillar amyloid β peptide (Aβ) formed from the soluble form of Aβ, which is a normal metabolic product detectable in the ventricular cerebro-spinal fluid (CSF) and plasma of healthy and AD subjects. Understanding the balance of production and clearance of Aβ peptides is the key to elucidating amyloid plaque homeostasis. Different extracellular proteins such as α1-antichymotrypsin, ApoJ, ApoE, and transthyretin found in CSF are implicated in deposition and clearance of Aβ (78). Purified transthyretin has been shown to bind Aβ in vitro (79, 80), and the overexpressed protein prevented the formation of insoluble amyloid in vivo (81). Transthyretin is known to transport thyroxine and retinol-binding protein in CSF (82). In this context, it is interesting to note that an inverse relationship has been found between transthyretin level in CSF and the severity of dementia in AD patients (83). We suggest that natural transthyretin inducers such as omega-3-rich fish oil or pure preparations of DHA could be of benefit in the prevention of AD.
However, gene-expression pattern may depend also on the experimental approach. Blalock et al. (84) studied gene-expression profiling in the hippocampus in relation to cognitive impairment of rats. They reported 29 genes down-regulated and 40 up-regulated; none of these were identical to those we found altered in old rat hippocampi preparations.
In the cerebellum of 2-year-old rats fed with an omega-3-rich fish oil for 1 month, 19 genes were overexpressed and 20 were repressed (51). Among the up-regulated genes were those that code for cytochrome P450 4a10, mad-related protein Smad7, integral membrane protein 2, microfibrillar 7c, Proteasome 28-kDa subunit 1, ajaba, ganglioside-induced differentiation-associated protein, stromal cell-derived factor 1, and heparan sulfate proteoglycan 1. Among the down-regulated genes, there were those coding for splicing factor C3, CD2-associated protein, myeloblastosis oncogene, α-1,6-mannosyl glycoprotein acetylglucose-aminotransferase, carbon catabolite repression 4, galectiomega-6, gap junction membrane channel protein a4, scavenger receptor class B type I, ferritin light-chain, and membrane protein TMS-2. However, these data suggest that certain genes respond differently in distinct brain regions to the same PUFA diet, which might be because of their specific functions, their different regulatory pathways, or different sensitivities of brain regions to external FA supply.
Although these experiments provide evidence that the brains of old rats respond to dietary omega-3 PUFA in an age-dependent manner, the possibility cannot be ruled out that length of feeding time might be a factor affecting the gene-expression profile. Moreover, expression of intracellular FA binding proteins (FABPs), such as heart H-FABP and the brain-specific B-FABP, were shown to be reduced in aged mouse brain (85). Age differences in brain H-FABP and B-FABP levels in synaptosomal plasma membranes and synaptosomal cytosol might have an impact in modulating neuronal differentiation and function and might be correlated with age-dependent response of the brain to diets rich in different PUFA.
Effect of Perinatal Omega-3 PUFA Supply on Gene Expression Later in Life
Adequate nutrition during the perinatal period is thought to be crucial for later health and well-being. A direct link between fetal growth and birth weight with the incidence of cardiovascular disease during adulthood has been published (86). Later work using a microarray approach by Napoli et al. (87) showed that maternal hypercholesterolemia affected atherogenesis in the offspring. A growing body of evidence has emerged to suggest that perinatal life is a critical period in lifespan during which an organism can be subjected to a number of permanent metabolic or genetic alterations that can influence the fate of an individual's health later in life (88, 89). The omega-3 PUFA are among various nutrients thought to be important in the perinatal period. For example, it has been shown that omega-3 FA during early life have an impact on the regulation of blood pressure later in life (90).
In a separate investigation, we examined whether the supply of omega-3 PUFA during the perinatal period can influence brain gene expression later in life. For that purpose, female Sprague–Dawley rats were mated with Sprague–Dawley males and allowed ad libitum access to an omega-3 PUFA-sufficient (10% ALA plus 69% LA; CON) or -deficient (0.2% ALA and 65% LA; DEF) diet during pregnancy. Four groups of male offspring derived from those mothers were studied: (i) pups maintained on CON diet from mothers maintained on CON diet; (ii) pups maintained on DEF diet from mothers maintained on DEF diet; (iii) pups maintained on CON diet from birth from mothers maintained on DEF diet until the birth of their pups; and (iv) pups maintained on CON diet from weaning from mothers maintained on DEF diet.
At necropsy of animals at 8 months of age, the whole brains were isolated and DNA microarray analysis was carried out. Among 1,600 genes examined, several showed altered expression caused by omega-3 PUFA deficiency during the perinatal period. Here, we list only those genes that showed up- or down-regulation in all of the three treated groups (ii–iv) compared with the control group (i).
A gene coding for cytochrome c having a role in mitochondrial energy metabolism was overexpressed. Release of cytochrome c from mitochondria could activate caspase-3, leading to apoptosis in neuronal cells and to neurodegeneration (91). Furthermore, cellular death is reported to be induced by an early mitochondrial activation and cytochrome c up-regulation (92). Another up-regulated gene, encoding the 55-kDa tumor necrosis factor receptor (TNFRSF1A) critical for the development of demyelination during immune-mediated central nervous system disease, was found in groups ii–iv (93). A chemokine-like factor super family 5 (CKLFSF5) gene involved in inflammatory response also showed elevated mRNA synthesis. Other hypothetical genes with unknown function also were raised in all of the animals having the omega-3 PUFA-deficient diet during the gestation period. The exact role of these genes in brain development and or neural activity remains to be revealed.
D-E-A-D (Asp-Glu-Ala-Asp)-box polypeptide 5 coding gene was repressed in all three treated groups (ii–iv). RNA helicases of the DEAD-box and related families have been found to be required for all processes involving RNA molecules including translation initiation. A protease and an exonuclease homolog also exhibited decreased mRNA level, similar to Escherichia coli protein fate protease II (PTRB) and a 3′–5′ exonuclease homolog. A glutamate receptor kainate GRIK5 gene also was repressed. Physiological studies have identified that kainate receptors have important roles both in post- and presynaptic plasticity. Kainate receptors, which are a subtype of the ionotropic glutamate receptors, contribute to excitatory postsynaptic currents in many regions of the central nervous system, including hippocampus, cortex, spinal cord, and retina (94).
Animals made deficient in omega-3 PUFA showed substantial decreases in the levels of DHA in their neural tissue (16, 18, 27), and this loss of DHA is accompanied by changes in neural function (membrane-related events, metabolic events, and cellular events) (1, 12, 14, 19, 21, 27). The present data reveal that deficiency of omega-3 PUFA during the perinatal period significantly alters the expression of several important genes. Future research will reveal whether the effects of omega-3 PUFA-deficient diets on neural function will be able to be explained by changes in the expression of genes and the proteins resulting from such changes.
Mechanisms of Gene-Expression Regulation by PUFA
The exact mechanism of action of omega-3 PUFA on gene-expression modulation is still far from being fully understood. Several lines of evidence, obtained from other tissues, suggest that gene-expression alteration can be directly realized involving specific FABPs and transcriptional regulators. PUFA regulate the activity or abundance of four families of transcription factor, including PPARs (α, β, and γ), liver X receptors (LXRs) (α and β), hepatic nuclear factor 4 α (HNF-4), and sterol regulatory element-binding proteins (SREBPs) 1 and 2 (38, 95–98). The very recent use of knockout mice, and laboratory animals treated with specific agonists of PPARs and LXRs, has greatly expanded the list of genes regulated by the above transcription factors (99, 100). Among these transcriptional regulators, both α and β LXRs are expressed in the brain, and it has been shown that they have important functions in lipid homeostasis and loss of their function results in neurodegenerative disorders (101). Expression of SREBP-1 also has been detected in hippocampus and neocortex (102). Of particular interest in this regard are the PPARs, which are ligand-dependent transcription factors belonging to the nuclear hormone receptor superfamily (53). They can modulate directly the gene activity by first being activated by specific ligands, and then they can modulate DNA transcription by binding to defined nucleotide sequences in the promoter region of target genes, so-called PPAR-responsive elements (103). PPARs form dimers, either homodimers or, more often, heterodimers, with the receptor for 9-cis retinoic acid known as retinoid X receptor (RXR), which is the obligate partner of PPARs (46). So far, three different isoforms of the PPARs have been isolated, showing differences either in the binding properties or in their distribution pattern (104). Recent studies have shown that PPARs present a promiscuous binding pattern, because they bind to unesterified saturated and unsaturated FA as well as to other lipid derivatives like eicosanoids and prostaglandins (105, 106). Interestingly, ALA had the highest binding constant among a wide range of different FA tested so far (105). On the other hand, DHA has been shown to be a natural ligand for the RXR in the brain (46). Recently, the role of FA controlling gene expression has been reviewed, and it has been suggested that FA can act also in PPAR-independent pathways (107). In contrast to PPAR activation, PUFA regulate the nuclear abundance of SREBPs by controlling the proteolytic processing of SREBP precursors or by regulating transcription of the SREBP-1c gene or turnover of mRNA (95, 108). PUFA are feed-forward activators of PPARs, whereas these same FA are feedback inhibitors of LXRs and SREBPs.
Additional transcription factors that can be affected by long-chain PUFA in the brain may be discovered by using nutrigenomic approaches that use global gene-expression analysis techniques (5, 47–51).
However, PUFA and their derivatives may use numerous other indirect mechanisms to regulate protein expression, such as modulation of posttranslational activation of regulators. EPA decreases lipopolysaccharide-induced phosphorylation and activation of mitogen-activated protein kinase (109). It also is plausible that free PUFA might alter gene activities by modifying the lifetime of RNA, and the distribution or the protein maturation of other regulatory elements, which further complicates the understanding of their action.
Conclusions and Future Remarks
Our data show that omega-3 PUFA affect the genetic machinery of the central nervous system in a complex way and thus may open up new ways of understanding how these FA control responses of the brain to different challenges. However, there is much to learn concerning the speed of the response, the minimal dose and the effects of different PUFA, their combinations, and their derivatives. Another exciting question is whether the different functional regions in the forebrain (like the visual and motor functions, etc.) and the hippocampus similar to the brain areas investigated here respond differently to DHA in particular or to omega-6 and omega-3 PUFA. In this respect, one can expect great plasticity and specialization within different brain areas and cellular structures.
As has been presented previously, PUFA induce various genes involved in diverse functions in different brain regions in an age- and time-dependent manner. Therefore, it seems that depending on the cell-specific context and the target gene, PUFA can take very different routes to alter transcription. Several novel transcription factors, different from PPARs and LXRs, are likely candidates to be identified (5). The recently available data highlight the complexity in studying the transcriptional effects of PUFA on a highly adaptive system such as the brain. We believe that new regulatory pathways soon will be discovered in this rapidly evolving field. This research will be facilitated with gene knockout and knock-in approaches, the use of specific antagonists to dissect signal transduction cascades, and the combined use of other “-omic” approaches (5). Moreover, genes induced by dietary FA also might serve as markers for association gene polymorphism studies in correlation with metabolic syndromes and brain disorders.
Acknowledgments
We dedicate this article in memory of Professor Tibor Farkas. We appreciate the diligent and thorough review by Dr. Alvin Berger (Paradigm Genetics, Research Triangle Park, NC), who made significant suggestions and reorganized the manuscript to condense and expedite findings and conclusions. This work was supported by Grants OTKA F 042850 and TS 044836 from the Hungarian Scientific Research Fund and grants from the Australian Research Council (DP0346830) and the Australian Health Management Group. K.K. and L.G.P. are supported by the János Bolyai fellowship of the Hungarian Ministry of Education.
Notes
Abbreviations: PUFA, polyunsaturated fatty acid; LA, linoleic acid; ALA, α-linolenic acid; FA, fatty acid; AA, arachidonic acid; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; PPAR, peroxisome proliferator-activated receptor; AD, Alzheimer's disease; Aβ, amyloid β peptide; FABP, FA binding protein; LXR, liver X receptor; SREBP, sterol regulatory element-binding protein.
References
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.0402342101
Read article for free, from open access legal sources, via Unpaywall:
http://www.pnas.org/content/101/30/10931.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1073/pnas.0402342101
Article citations
The MIND diet, brain transcriptomic alterations, and dementia.
Alzheimers Dement, 20(9):5996-6007, 11 Aug 2024
Cited by: 2 articles | PMID: 39129336 | PMCID: PMC11497672
Effects of Dietary Fiber, Phenolic Compounds, and Fatty Acids on Mental Health: Possible Interactions with Genetic and Epigenetic Aspects.
Nutrients, 16(16):2578, 06 Aug 2024
Cited by: 0 articles | PMID: 39203714 | PMCID: PMC11356825
Review Free full text in Europe PMC
Fatty Acid-Binding Protein 4-Mediated Regulation Is Pivotally Involved in Retinal Pathophysiology: A Review.
Int J Mol Sci, 25(14):7717, 14 Jul 2024
Cited by: 0 articles | PMID: 39062961 | PMCID: PMC11277531
Review Free full text in Europe PMC
Feeding flaxseed to chicken hens changes the size and fatty acid composition of their chicks' brains.
Front Physiol, 15:1400611, 07 Jun 2024
Cited by: 0 articles | PMID: 38911324 | PMCID: PMC11190958
Navigating my career in lipid research.
Eur J Clin Nutr, 27 May 2024
Cited by: 0 articles | PMID: 38802606
Review
Go to all (157) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
A high ratio of dietary n-3/n-6 polyunsaturated fatty acids improves obesity-linked inflammation and insulin resistance through suppressing activation of TLR4 in SD rats.
Nutr Res, 33(10):849-858, 09 Aug 2013
Cited by: 88 articles | PMID: 24074743
Omega-3 polyunsaturated fatty acid supplementation attenuates microglial-induced inflammation by inhibiting the HMGB1/TLR4/NF-κB pathway following experimental traumatic brain injury.
J Neuroinflammation, 14(1):143, 24 Jul 2017
Cited by: 110 articles | PMID: 28738820 | PMCID: PMC5525354
Exon-intron split analysis reveals posttranscriptional regulatory signals induced by high and low n-6/n-3 polyunsaturated fatty acid ratio diets in piglets.
J Anim Sci, 101:skad271, 01 Jan 2023
Cited by: 1 article | PMID: 37561402 | PMCID: PMC10503648
Nutrigenomic approaches to study the effects of n-3 PUFA diet in the central nervous system.
Nutr Health, 18(3):227-232, 01 Jan 2006
Cited by: 8 articles | PMID: 17180868
Review






