Abstract
Introduction
Dietary patterns are associated with dementia risk, but the underlying molecular mechanisms are largely unknown.Methods
We used RNA sequencing data from post mortem prefrontal cortex tissue and annual cognitive evaluations from 1204 participants in the Religious Orders Study and Memory and Aging Project. We identified a transcriptomic profile correlated with the MIND diet (Mediterranean-Dietary Approaches to Stop Hypertension Intervention for Neurodegenerative Delay) among 482 individuals who completed ante mortem food frequency questionnaires; and examined its associations with cognitive health in the remaining 722 participants.Results
We identified a transcriptomic profile, consisting of 50 genes, correlated with the MIND diet score (p = 0.001). Each standard deviation increase in the transcriptomic profile score was associated with a slower annual rate of decline in global cognition (β = 0.011, p = 0.003) and lower odds of dementia (odds ratio = 0.76, p = 0.0002). Expressions of several genes (including TCIM and IGSF5) appeared to mediate the association between MIND diet and dementia.Discussion
A brain transcriptomic profile for healthy diets revealed novel genes potentially associated with cognitive health.Highlights
Why healthy dietary patterns are associated with lower dementia risk are unknown. We integrated dietary, brain transcriptomic, and cognitive data in older adults. Mediterranean-Dietary Approaches to Stop Hypertension Intervention for Neurodegenerative Delay (MIND) diet intake is correlated with a specific brain transcriptomic profile. This brain transcriptomic profile score is associated with better cognitive health. More data are needed to elucidate the causality and functionality of identified genes.Free full text

The MIND diet, brain transcriptomic alterations, and dementia
Associated Data
Abstract
INTRODUCTION
Dietary patterns are associated with dementia risk, but the underlying molecular mechanisms are largely unknown.
METHODS
We used RNA sequencing data from post mortem prefrontal cortex tissue and annual cognitive evaluations from 1204 participants in the Religious Orders Study and Memory and Aging Project. We identified a transcriptomic profile correlated with the MIND diet (Mediterranean‐Dietary Approaches to Stop Hypertension Intervention for Neurodegenerative Delay) among 482 individuals who completed ante mortem food frequency questionnaires; and examined its associations with cognitive health in the remaining 722 participants.
RESULTS
We identified a transcriptomic profile, consisting of 50 genes, correlated with the MIND diet score (p = 0.001). Each standard deviation increase in the transcriptomic profile score was associated with a slower annual rate of decline in global cognition (β = 0.011, p = 0.003) and lower odds of dementia (odds ratio = 0.76, p = 0.0002). Expressions of several genes (including TCIM and IGSF5) appeared to mediate the association between MIND diet and dementia.
DISCUSSION
A brain transcriptomic profile for healthy diets revealed novel genes potentially associated with cognitive health.
Highlights
Why healthy dietary patterns are associated with lower dementia risk are unknown.
We integrated dietary, brain transcriptomic, and cognitive data in older adults.
Mediterranean‐Dietary Approaches to Stop Hypertension Intervention for Neurodegenerative Delay (MIND) diet intake is correlated with a specific brain transcriptomic profile.
This brain transcriptomic profile score is associated with better cognitive health.
More data are needed to elucidate the causality and functionality of identified genes.
1. BACKGROUND
Identifying pathways underlying dementia is critical to improve prevention and treatment, which are public health priorities. 1 Initial evidence from some randomized clinical trials indicates that the Mediterranean diet and the Dietary Approaches to Stop Hypertension (DASH) may protect against cognitive decline. 2 , 3 , 4 The MIND diet (Mediterranean‐DASH Diet Intervention for Neurodegenerative Delay) was specifically designed by tailoring the Mediterranean and DASH diets to emphasize foods and nutrients associated with dementia prevention. 5 , 6 In multiple large‐scale prospective cohorts, a higher MIND diet score is robustly associated with lower dementia risk and slower cognitive decline. 5 , 6 , 7 , 8 , 9 , 10 , 11 A recent trial reported that both the MIND diet intervention and a calorie‐restricted control diet for weight loss maintained cognition to a similar extent over 3 years, while no differences were found between the two diets 12 ; this additionally suggests that a variety of healthy behaviors (including diet and weight loss) may contribute to the preservation of cognition during aging. This notion is further supported by findings from the FINGER trial, in which a multidomain intervention consisting of healthy diets, exercise, and other healthy behaviors, benefited cognitive health versus the control group. 13
A better understanding of the biological underpinnings linking behavioral risk factors (such as diet) to dementia may facilitate the identification of novel biologic targets for dementia prevention. Accumulating evidence from animal studies shows that calorie restriction or experimental feeding of specific nutrients or foods (such as polyphenols and polyunsaturated fatty acids) alters hippocampus and cortex expression of genes implicated in neurofunction and neuroinflammation, and improves brain plasticity and memory. 14 , 15 , 16 However, molecular mechanisms underlying the association between healthy diets and dementia risk in humans are poorly studied.
We previously examined large‐scale RNA sequencing (RNA‐Seq) data from post mortem cortex tissue in two community‐based, longitudinal cohorts—the Religious Orders Study (ROS) and Rush Memory and Aging Project (MAP); we found that the dorsolateral pre‐frontal cortex (DLPFC) expression levels of several gene clusters—including genes broadly involved in metabolism, immune response, and synaptic transmission—were associated with dementia, cognitive decline, and Alzheimer's disease (AD) pathology. 17 , 18 Further, in ROSMAP, we previously found that cortical expression of specific genes might be on the pathway from dementia risk factors (such as neuroticism) to dementia. 19 Here, to extend this prior work, we leveraged the DLPFC RNA‐Seq and cognitive data generated from 1204 ROSMAP participants, and further integrated dietary data available in a subset of 482 MAP participants. We identified a cortical transcriptomic profile correlated with the MIND diet score and tested whether such a transcriptomic profile was associated with cognitive trajectories and the likelihood of dementia.
2. METHODS
2.1. Study participants
Data and biospecimens for this study were drawn from ROS and MAP, two ongoing, prospective studies of aging and dementia, with a purposefully similar design. ROS started enrolling Catholic nuns, priests, and brothers from across the United States in 1994. MAP began in 1997, enrolling residents from retirement communities and senior public housing from across the Chicago metropolitan area. At enrollment, participants were free of known dementia, 20 and agreed to annual clinical evaluations and organ donation at the time of death. 21 Structured neuropathologic examination was also performed after death. 21 At the time of this study, > 3700 participants were enrolled in ROSMAP and > 2000 had died, with an overall follow‐up rate > 95% and an autopsy rate > 80%. Our study included the 1204 ROSMAP participants with complete clinical and DLPFC RNA‐Seq data 18 , 22 (Figure 1). The institutional review board of Rush University Medical Center approved the study, and all participants signed an informed consent and anatomical gift act to donate tissue at the time of death.
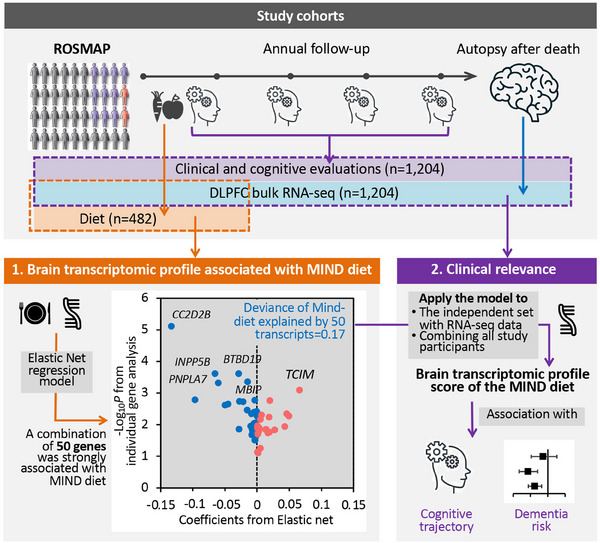
The flowchart of the study. We integrated dietary and clinical data, and bulk RNA‐Seq in DLPFC in ROSMAP. First, among 482 participants with dietary and RNA‐Seq data, we applied an elastic net regression to regress the MIND diet score on all gene transcripts. The elastic net model identified a brain transcriptomic profile—constituting 50 genes—modestly but significantly associated with the MIND diet score in cross‐validation analysis. Then, we applied this model to participants with RNA‐Seq data (n = 722 for the independent set, and n = 1204 combining all study participants) to calculate a brain transcriptomic profile score for the MIND diet and examined its association with cognitive trajectory (from baseline through death) and dementia status as of death. DLPFC, dorsolateral prefrontal cortex; MIND, Mediterranean‐Dietary Approaches to Stop Hypertension Intervention for Neurodegenerative Delay; RNA‐Seq, RNA sequencing; ROSMAP, Religious Orders Study and Memory and Aging Project.
2.2. Assessment of cognitive health
Uniform structured clinical evaluations were performed at baseline and annually thereafter until death. 21 Briefly, detailed neuropsychological data from 17 harmonized tests across ROS and MAP were used to measure cognition in five cognitive domains. 5 , 21 Raw scores from each test were first standardized based on means and standard deviations (SDs) of the total group at baseline. A global composite score was calculated by averaging standardized scores across all tests. Individual trajectories of cognitive decline have been estimated among individuals with at least one follow‐up cognitive assessment after baseline. 23 Briefly, mixed‐effects models were applied to regress the global cognitive score on age, sex, and years of education as fixed effects (indicating average cognitive change over time in a group), together with a random intercept (indicating individual variation in cognition at baseline) and a random slope (indicating individual variation in cognitive changes over time). Person‐specific slopes, adjusted for age, sex, and education, were extracted from the models to quantify individual cognitive decline rates. 23 This cognitive slope was used in our analysis to estimate cognitive trajectories.
At the time of death, all available clinical data were reviewed by a neurologist with expertise in dementia to determine the most likely cognitive diagnosis as of death, while blinded to post mortem data. 20 , 21 , 24 , 25 Dementia was classified based on criteria of the joint working group of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association, which require a history of cognitive decline with impairment in memory and at least one other cognitive domain. 20 , 24 Mild cognitive impairment (MCI) was defined by the presence of cognitive impairment but no dementia and determined by clinicians as described in prior papers. 20 , 24
2.3. Dietary assessments and the MIND diet score
Beginning in 2004, MAP participants were invited to complete a food frequency questionnaire (FFQ) at the time of their annual examinations. 5 , 6 The FFQ inquired about participants’ usual intake frequencies for > 144 food items in the past year and has been validated in older community residents. 5 , 6 , 26 The MIND diet score ranges from 0 to 15 (higher score represents healthier diet), summarizing 15 dietary components including 10 brain‐healthy food groups (i.e., green leafy vegetables, other vegetables, nuts, berries, beans, whole grains, fish, poultry, olive oil, and wine) and five unhealthy food groups (i.e., red meats, butter and stick margarine, cheese, pastries/sweets, and fried/fast food). 6 MIND diet score in MAP was highly stable over time based on repeated FFQ assessments. 5 As such, we used the MIND diet score calculated based on the first FFQ to represent long‐term dietary quality, as has been done in prior publications. 5 , 6
2.4. RNA sequencing and data processing
For RNA‐Seq of frozen DLPFC bulk tissues, laboratory procedures and data processing are described in detail in previous publications.
17
,
22
,
27
Briefly, the gray matter was separated from white matter and vasculature and was homogenized to extract RNA using standard commercial kits. Quality‐controlled RNA samples were sequenced using the Illumina HiSeq or NovaSeq platforms with an average sequencing depth of 50 million reads.
22
,
27
In ROSMAP, bulk RNA‐Seq was conducted by multiple projects,
17
,
22
,
27
and all available data were reprocessed and harmonized for combined use.
27
In data reprocessing, pair‐end RNA‐Seq data were aligned to the GRCh38 reference genome (GENCODE release v27) using STAR v2.6,
28
and Picard was used to assess the quality of the aligned data.
29
Then, transcript raw counts were calculated by Kallisto (v0.46)
30
and aggregated at the gene level to obtain gene counts separately in mRNAs and pre‐mRNAs. Samples were removed if the total reads mapped were < 5 million; had sex mismatch, single nucleotide polymorphism mismatch, or regional swap; were in a sequencing batch with only a few samples; or were identified as outliers based on multidimensional scaling analysis.
27
Genes with > 10 read in > 50% of samples were retained.
27
Conditional quantile normalization was applied to account for bias from gene length and guanine–cytosine content; the adjusted gene counts matrix was then converted to log2‐CPM (counts per million), followed by quantile normalization using voom implemented in the R package limma.
31
Finally, a linear regression model was applied to remove major technical confounders (i.e., sequencing batch, post mortem interval, RNA quality number, total spliced reads reported by STAR aligner, and metrics reported by Picard and Kallisto) while preserving regional differences.
27
In total, 17,255 quality‐controlled and annotated genes were included in analyses.
kits. Quality‐controlled RNA samples were sequenced using the Illumina HiSeq or NovaSeq platforms with an average sequencing depth of 50 million reads.
22
,
27
In ROSMAP, bulk RNA‐Seq was conducted by multiple projects,
17
,
22
,
27
and all available data were reprocessed and harmonized for combined use.
27
In data reprocessing, pair‐end RNA‐Seq data were aligned to the GRCh38 reference genome (GENCODE release v27) using STAR v2.6,
28
and Picard was used to assess the quality of the aligned data.
29
Then, transcript raw counts were calculated by Kallisto (v0.46)
30
and aggregated at the gene level to obtain gene counts separately in mRNAs and pre‐mRNAs. Samples were removed if the total reads mapped were < 5 million; had sex mismatch, single nucleotide polymorphism mismatch, or regional swap; were in a sequencing batch with only a few samples; or were identified as outliers based on multidimensional scaling analysis.
27
Genes with > 10 read in > 50% of samples were retained.
27
Conditional quantile normalization was applied to account for bias from gene length and guanine–cytosine content; the adjusted gene counts matrix was then converted to log2‐CPM (counts per million), followed by quantile normalization using voom implemented in the R package limma.
31
Finally, a linear regression model was applied to remove major technical confounders (i.e., sequencing batch, post mortem interval, RNA quality number, total spliced reads reported by STAR aligner, and metrics reported by Picard and Kallisto) while preserving regional differences.
27
In total, 17,255 quality‐controlled and annotated genes were included in analyses.
In secondary analysis, we included single‐nucleus RNA‐Seq data from a subset of 424 ROSMAP individuals. Methods have been described elsewhere. 32 , 33 Briefly, gray matter was processed in batches of eight individuals; and 5000 nuclei from each batch were pooled, prepared, and sequenced using either Illumina HiSeqX at the Broad Institute's Genomics Platform or Illumina NovaSeq 6000 at the New York Genome Center, with a target coverage of 1 million reads per channel. 32 , 33 Data were first processed by the CellRanger software (v6.0.0; 10x Genomics) and quality controlled. Each nucleus was assigned back to its participants based on its genotypes. Cell types of nuclei were determined, and pseudo‐bulk matrices were created by summing counts per individual. 32 , 33 , 34 , 35 Within each cell type, genes with CPM > 1 in 80% of samples were retained and normalized using tmm.voom. 36 Our analysis included expression of 32 genes from seven cell types.
2.5. Assessment of covariates and potential confounders
Information on age, sex, race, ethnicity, self‐reported years of school education, and smoking history was collected from a structured interview administered at baseline. For analysis of the MIND diet score, potential confounders including physical activity and body mass index (BMI) were acquired from the annual visit when FFQs were administrated. 5 , 6 Physical activity (hours per week) was measured based on self‐reported minutes spent within the previous 2 weeks on five activities: walking for exercise, yard work, calisthenics, biking, and water exercise. BMI was calculated from weight and height measured by trained staff at clinical evaluations.
2.6. Statistical analysis
To identify a brain transcriptomic profile correlated with the MIND diet, we applied an elastic net regression—a variable selection model 37 —to regress the MIND diet score on all 17,255 genes (all standardized to mean = 0 and SD = 1), in 482 individuals with both DLPFC RNA‐Seq and FFQ data (Figure 1). The model lambda was chosen using a 10‐fold cross‐validation (CV) approach. From the whole transcriptome, the elastic net model selected a group of genes whose weighted combination (i.e., a transcriptomic profile) showed the strongest correlation with the MIND diet score while removing genes unlikely to be related. We evaluated model performance based on the fraction of (null) deviance explained and the mean cross‐validated error. Further, to calculate the Pearson correlation between the MIND diet score and its transcriptomic profile in the same 482 individuals without overfitting, we calculate an unbiased transcriptomic profile score for each individual using an external 10‐fold CV approach, that is, to acquire the transcriptomic profile score for each 10% of the individuals, a prediction model was trained using the rest of the independent 90% of individuals. Associations of the MIND diet score and its food components with individual genes were analyzed using linear regression, adjusting for age at death, sex, education, total energy intake, and years from the first FFQ to death. In sensitivity analyses, we further adjusted for BMI, physical activity, smoking, or APOE genotype; and further excluded individuals with dementia at the time of FFQ. False discovery rate (FDR) was used to correct for multiple testing. Genes with FDR < 0.05 were considered statistically significant.
We then applied the elastic net model (trained in all 482 individuals with diet and transcriptomic data) to an independent set of 722 individuals with bulk DLPFC RNA‐Seq data in ROSMAP (but without diet data), to calculate the transcriptomic profile score for the MIND diet (Figure 1). In each individual, the transcriptomic profile score was calculated as the weighted sum of the selected genes with weights equal to the genes’ coefficients from the elastic net model. We analyzed associations between the transcriptomic profile score and (1) cognitive trajectories using linear regression and (2) final consensus diagnosis of dementia or MCI at death (vs. no cognitive impairment [NCI]) using multinomial logistic regression. Models were adjusted for age at death, sex, education, and study cohort. Considering the development of the transcriptomic profile in the first 482 individuals (i.e., those with diet data) is agnostic to participants’ cognitive health, to enhance statistical power, we secondarily applied the model to the 482 individuals with diet data and conducted joined analyses of all participants (n = 1204), to test associations between transcriptomic profile score and cognitive outcomes. We also analyzed associations between the DLPFC expression levels of the selected genes and cognitive outcomes in all 1204 participants. For genes showing FDR < 0.05, we further assessed the associations between their cell type–specific expression levels and the corresponding cognitive outcome in 424 individuals with single‐nucleus RNA‐Seq data.
As a simplified approach to control for confounding by cell composition in all study samples, we estimated the proportions of neurons, astrocytes, microglia, oligodendrocytes, and endothelial cells, based on bulk RNA‐seq data using a deconvolution digital sorting algorithm (DSA) implemented in the R package CellMix. 38 Two sets of cell type marker genes were used (200 top marker genes per cell type, 39 derived based on a human brain single‐cell RNA‐seq data, 40 and then separately, a cell‐sorted RNA‐seq data 41 ). We conducted sensitivity analyses: first to examine whether cell compositions confounded associations between gene expressions and MIND diet score and between the transcriptomic profile score and cognitive outcomes; we also examined whether APOE genotype and physical activity modified the association between the transcriptomic profile score with cognitive outcomes.
Among 444 individuals with FFQ, RNA‐Seq data, and free of dementia at the time of the FFQ, we verified previously reported associations between the MIND diet score (based on FFQ) and cognitive outcomes, 5 , 6 after adjusting for age, sex, education, smoking, physical activity, BMI, and total energy intake. We then examined whether any selected genes in the transcriptomic profile mediated associations between MIND diet score and cognitive outcomes, using multiple mediation analysis implemented in R package mma. 42
To assist with the interpretation of the potential temporal relationship between the transcriptomic profile score of the MIND diet and dementia, we conducted a secondary Mendelian randomization (MR) analysis using an inverse‐variance weighted method, 43 based on genome‐wide association study (GWAS) summary data obtained separately for the transcriptomic profile score and AD. GWAS of the transcriptomic profile score was conducted in 670 independent set individuals with genetic data, using RVTESTS, 44 adjusting for sex, age at death, and the first four genetic principal components. Given the limited sample size, independent genetic variants (linkage disequilibrium clumping with r 2 < 0.1 in a 1000 kbp window) with p < 1×10−6 were used as the genetic instrumental variable (IV) for the transcriptomic profile score. To maximize statistical power, we acquired the summary statistics from a large‐scale GWAS of clinically diagnosed AD (21,982 confirmed cases and 41,944 cognitively normal controls). 45 Independent genetic variants with p < 5×10−8 were used as IV of AD. All analyses, except for GWAS, were conducted using R version 4.2.1.
3. RESULTS
A flow chart of our study and analytic populations is presented in Figure 1. Participants were largely female (68%), with average age at enrollment approximately 81 years old (Table 1). The mean age at death was 90 years; 525 had dementia (of whom 96% were diagnosed as AD dementia) and 285 had MCI, as of death. The subgroup of 482 MAP participants with FFQ data was demographically comparable to the total study sample (Table 1); diet was assessed approximately 6 years before death and the mean MIND diet score was 7.5 (SD = 1.5).
TABLE 1
Characteristics of ROSMAP participants included in this study.
| Variables | Subset of participants with dietary and RNA‐Seq data | Independent set of participants with RNA‐Seq data | All participants with RNA‐Seq data |
|---|---|---|---|
| n = 482 | n = 722 | N = 1204 | |
| Female, n (%) | 340 (70.5%) | 479 (66.3%) | 819 (68.0%) |
| Education, years | 15.0 (12.0, 16.0) | 18.0 (15.0, 20.0) | 16.0 (13.0, 18.0) |
| Cognitive slope across study span a | 0.021 (−0.050, 0.060) | 0.005 (−0.065, 0.044) | 0.010 (−0.062, 0.050) |
| Age at study entry, years | 82.5 (6.0) | 79.7 (7.2) | 80.8 (6.9) |
| At the first FFQ: | |||
| Age, years | 84.7 (5.9) | – | – |
| MIND diet score | 7.5 (1.5) | – | – |
| Total energy intake | 1806.1 (554.0) | – | – |
| Ever smoking, n (%) | 193 (40.0%) | – | – |
| Body mass index, kg/m2 | 26.5 (4.9) | – | – |
| Physical activity, hours/week | 2.2 (0.5, 4.0) | – | – |
| At the time of death: | |||
| Age, years | 90.8 (6.1) | 88.8 (6.7) | 89.6 (6.6) |
| MCI, n (%) | 124 (25.7%) | 161 (22.3%) | 285 (23.7%) |
| Dementia, n (%) | 183 (38.0%) | 342 (47.4%) | 525 (43.6%) |
Note: Continuous variables were presented as mean (standard deviation) except for education years and cognitive slope, which were presented as median (25th and 75th percentiles) as they were not normally distributed. Categorical variables were presented as n (%).
Abbreviations: FFQ, food frequency questionnaire; MCI, mild cognitive impairment; MIND, Mediterranean‐Dietary Approaches to Stop Hypertension Intervention for Neurodegenerative Delay; RNA‐Seq, RNA sequencing; ROSMAP, Religious Orders Study and Rush Memory and Aging Project.
3.1. A DLPFC transcriptomic profile of the MIND diet
To identify a brain transcriptomic profile for the MIND diet, we applied an elastic net regression on all 17,255 genes simultaneously in 482 individuals with diet data. The model selected a combination of 50 genes showing the strongest association coefficients with the MIND diet score, while robust to the effects of collinearity between genes (Figure 2 and Figure S1 in supporting information). The model's fraction of null deviance explained was 0.17, and the cross‐validated r‐square was 0.035. An unbiased transcriptomic profile score of the MIND diet calculated in the same 482 individuals using a 10‐fold CV approach (to avoid overfitting) was modestly but significantly correlated with the MIND diet score (r = 0.15; p = 0.001; Figure S1); this modest correlation was expected given the complex pathway from baseline diet to brain molecular changes identified post mortem. 14 , 15 , 46
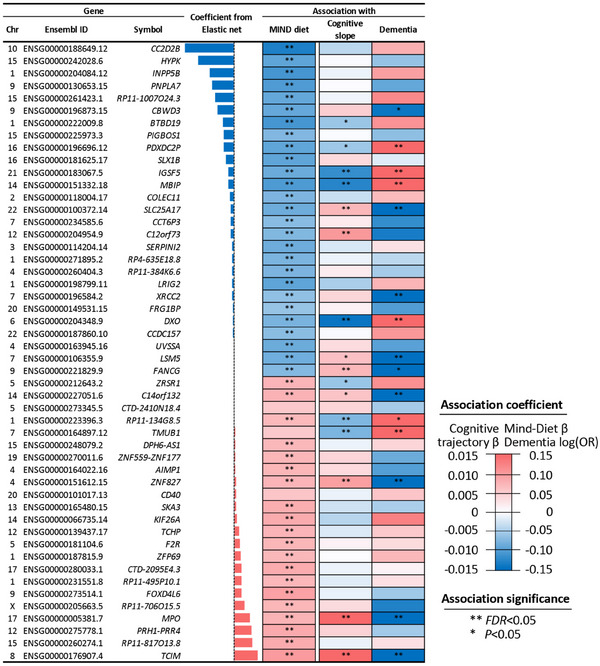
The 50 genes selected into the transcriptomic profile score of the MIND diet. The three columns indicate chromosomes, Ensembl ID, and symbol. For elastic net coefficients, blue and red bars indicate negative and positive coefficients, respectively, and bar heights indicate coefficient magnitudes. The three columns to the right are associations between each gene with MIND diet score (n = 482), cognitive slope (n = 1129), and dementia (n = 1204). Colors indicate association directions, and color depths depict magnitudes (linear regression β for MIND diet score and cognitive slope; log [OR] for dementia). Blue color represents a negative β (i.e., a negative association) for MIND diet and cognitive decline, and an OR below 1 (i.e., lower odds) for dementia. Red color represents a positive β for MIND diet and cognitive slope, and an above 1 OR for dementia. Analyses were adjusted for age at death, sex, education, cohort, total energy intake, and years from first FFQ to death (only for MIND diet score). FDR, false discovery rate; FFQ, food frequency questionnaire; MIND, Mediterranean‐Dietary Approaches to Stop Hypertension Intervention for Neurodegenerative Delay; OR, odds ratio.
Of these 50 selected genes, 23 were positively weighted in the transcriptomic profile (of which 21 genes were positively associated with MIND diet score at FDR < 0.05 in individual gene analysis), and the other 27 genes were negatively weighted (all showed negative associations with MIND diet score at FDR < 0.05; Figure 2). Genes showing the strongest positive associations with MIND diet included TCIM and MPO, which have been related to educational attainment, and cognitive performance in depression in previous GWAS. 47 , 48 Genes showing the strongest negative associations included CC2D2B, PDXDC2P, and MBIP, which are linked to AD, brain structure (e.g., white matter hyperintensities), and educational attainment; 47 , 49 , 50 , 51 and INPP5B and IGSF5, which have been related to cardiovascular outcomes 52 , 53 in prior genetic studies (Figure 2, Table S1 in supporting information).
In sensitivity analyses, associations between the MIND diet score and the 50 genes did not materially change after further adjusting for estimated cell type proportions, APOE genotypes, or other lifestyle factors, or excluding individuals classified with dementia at baseline (Figure S2 in supporting information). The transcriptomic profile score for the MIND diet was not associated with APOE genotypes (p > 0.05). Secondary analysis of food components constituting the MIND diet suggested that the correlation between the MIND diet and transcriptomic profile score was likely driven by the collective associations between all food components and the selected genes (Figure S3 in supporting information).
3.2. Gene expression and cognitive outcomes
We applied the 50‐gene elastic net model to the remaining independent set of 722 individuals with RNA‐Seq data, to acquire a transcriptomic profile score for the MIND diet and assessed its clinical relevance. In multivariable‐adjusted analysis, a higher transcriptomic profile score was associated with a slower rate of cognitive decline (β of cognitive slope per SD increment in transcriptomic profile score = 0.011, p = 3.5 × 10−3) and lower odds of dementia (odds ratio [OR] = 0.76 vs. NCI, p = 0.003); but was not significantly associated with MCI (OR = 0.89 vs. NCI, p = 0.3; Figure 3). A joint analysis including the 482 individuals with diet data and the independent set (total N = 1204) yielded highly consistent and more statistically significant findings (Figure 3 and Table S2 in supporting information). In sensitivity analyses, these associations did not materially change after further adjusting for the estimated cell type proportions; and were not modified by APOE genotype or physical activity (Tables S3, S4 in supporting information).
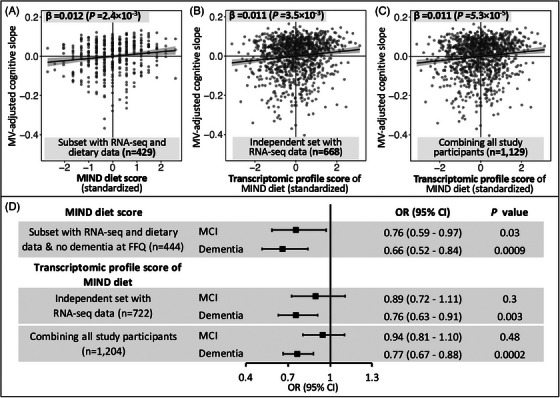
Associations of the MIND diet score and its cortical transcriptomic profile score with cognitive trajectory and dementia. The upper panel presents the associations with cognitive decline trajectory, for the MIND diet score (A), and for the transcriptomic profile score of the MIND diet in the independent set (B) and in the combined analysis of all participants (C). In the scatter plots, the solid lines are linear trend, and the gray areas are 95% CI. D, Associations between the MIND diet score and its transcriptomic profile score with dementia and MCI as of death (vs. no cognitive impairment). Black squares and gray lines indicate ORs and 95% CI, respectively. Analyses of the MIND diet score were conducted in participants with dietary data and free of dementia at the time of the FFQ; and were adjusted for age, sex, years of education, BMI, physical activity, smoking, and total energy intake. Associations between the transcriptomic scores were adjusted for age at death, sex, education years, and cohorts. Sample sizes were smaller in the upper panel versus in (D) because some participants had only one cognitive assessment and were thus excluded from cognitive trajectory analysis. BMI, body mass index; CI, confidence interval; FFQ, food frequency questionnaire; MCI, mild cognitive impairment; MIND, Mediterranean‐Dietary Approaches to Stop Hypertension Intervention for Neurodegenerative Delay; MV, multivariable; OR, odds ratio; RNA‐seq, RNA sequencing.
Of the 50 genes constituting the transcriptomic profile, cortical expression levels of 11 genes were significantly associated with cognitive slope and 12 were associated with dementia (FDR < 0.05), with highly consistent results from the independent set and the combined analysis of all participants (Figure 2 and Figure S4 in supporting information). Notably, four individual genes (TCIM, MPO, ZNF827, and C14orf132) whose expression levels were associated with higher MIND diet scores, were also associated with lower odds of dementia and/or a slower rate of cognitive decline. Another four genes (namely PDXDC2P, IGSF5, MBIP, and DXO) whose expression levels were associated with lower MIND diet scores were associated with worse cognitive outcomes (Figure 2 and Table S1).
3.3. MIND diet, gene expression, and cognitive outcomes
In prior publications, MAP participants in the highest versus lowest tertile of MIND diet score had a slower rate of cognitive decline with β = 0.0092 (equivalent to being 7.5 years younger in age) 5 and 53% lower risk of AD dementia. 6 In our study, among the 482 individuals with diet and RNA‐Seq data, we first confirmed that a higher MIND diet score was significantly associated with a slower rate of cognitive decline (β per SD increment in MIND score = 0.012, p = 0.002) and lower odds of dementia (OR = 0.66, p = 0.0009; vs. NCI) and MCI (OR = 0.76, p = 0.03; vs. NCI) as of death (Figure 3), consistent with the previous findings. 5 , 6 Further, a multiple mediation analysis suggested that three genes potentially mediated the association between the MIND diet and cognitive trajectory (TCIM, DXO, and MPO, accounting for 30% of the total association), and five genes mediated the association with dementia (TCIM, DXO, MPO, PDXDC2P, and IGSF5, accounting for 41% of the total association; Figure S5 in supporting information).
3.4. Single nucleus RNA‐Seq data
We then examined genes constituting the MIND diet transcriptomic profiles in single‐nucleus RNA‐Seq data (n = 424; 32 genes out of 50 passed the expression threshold in at least one cell type). We found that many of these genes were broadly expressed in all seven DLPFC cell types (e.g., LSM5, C14orf132, and TMUB1), with some restricted to specific cell types (e.g., IGSF5 and TCIM; Figure S6 in supporting information). Further, of the 15 genes whose expression levels in bulk tissue were associated with cognitive trajectory and/or dementia, we observed consistent associations between eight genes with cognitive outcomes in at least one of seven brain cell types. For example, expressions of TCIM in inhibitory neurons and oligodendrocytes were associated with slower cognitive decline and lower odds of dementia, whereas expressions of IGSF5 in astrocytes and excitatory neurons were associated with higher odds of dementia (Figure S7 in supporting information).
3.5. Secondary Mendelian randomization analysis
As RNA was sequenced in post mortem brain tissue, and thus it is possible that cognitive decline or dementia might cause alterations in gene expression rather than the reverse, we conducted a secondary MR analysis to assist interpretation of the potential temporal relationships between transcriptomic profile for the MIND diet and cognitive health. We found that the genetic IV of the transcriptomic profile score was marginally associated with lower odds of AD (OR = 0.93 per SD increment in genetically inferred transcriptomic profile score, p = 0.04), whereas genetically inferred AD was not associated with the transcriptomic profile score (p = 0.32).
4. DISCUSSION
Leveraging DLPFC RNA‐Seq data in two longitudinal cohorts of aging, we identified a transcriptomic profile in the prefrontal cortex—consisting of 50 genes—correlated with the MIND diet score and significantly associated with a slower rate of cognitive decline and lower odds of dementia. Given the substantial contribution of modifiable risk factors to dementia risk, 54 our study investigating molecular pathways through which risk factors (e.g., diet) are associated with dementia showcases a novel approach to facilitate the discovery of biological mechanisms in dementia and to inform novel targets for dementia prevention.
Approximately one third of AD dementia cases are estimated to be attributable to seven potentially modifiable risk factors, including diabetes, midlife hypertension, and obesity, that are all directly related to diet. 54 Evidence from clinical trials and prospective cohort studies also supports that healthy diets can improve cardiometabolic risk factors underlying dementia, including reducing hypertension, obesity, diabetes, and stroke. 55 However, little research has explored molecular mechanisms in the brain linking diets to dementia in humans. Prior studies suggested a potential role of brain transcriptional regulation in dementia. 17 , 18 , 27 , 56 , 57 For example, in ROSMAP, brain methylation levels of several CpGs were associated with the burden of AD pathology. 56 Cortical expressions of an extensive list of genes were correlated with cognitive decline, dementia, and neuropathology; 17 , 18 and a transcriptomic network module, consisting of 390 genes with diverse functions, was strongly associated with cognitive decline. 17 Recent studies further identified novel cortical proteomic modules associated with dementia, including some proteins potentially altered from the transcriptomic level. 27 , 57 However, evidence on transcriptomic changes linking diet to neurofunction has been mostly from animal studies. Feeding studies in animal models observed altered levels of hippocampal or cortical expressions of candidate genes related to metabolism, neuroinflammation, neurotransmission, and changes in brain plasticity and function. 14 , 15 , 16 In line with prior evidence, our findings suggest that in humans, behavioral risk factors such as complex dietary patterns are connected with brain molecular profiles at the gene expression level.
In particular, our findings from multiple types of analyses all pointed to several candidate genes of interest worthy of future investigation. Notably, cortical expression of TCIM showed the strongest positive correlation with the MIND diet score and was a top mediator for the association of the MIND diet with slower cognitive decline and less dementia. TCIM encodes a transcriptional and immune response regulator that positively regulates the Wnt/β‐catenin signaling pathway—a crucial pathway for neurogenesis, neuronal survival, and regulation of synaptic plasticity and blood–brain barrier integrity. 58 TCIM was previously identified as a genetic locus for educational attainment. 47 Interestingly, inhibitory neurons and oligodendrocytes—the cell types in which TCIM expression was also associated with slower cognitive decline and lower odds of dementia in our study—have been functionally implicated in learning and memory. 59 , 60 Our data, together with consistent prior evidence, suggested that healthy diets may upregulate cortical TCIM expression, and thus may help maintain cognition during aging.
Another noteworthy candidate gene is IGSF5, whose cortical expression was negatively correlated with the MIND diet score, and also appeared to be a top mediator for the association between the MIND diet and dementia. IGSF5 encodes a junctional adhesion molecule, and its genetic variants have been associated with blood pressure, hypertension, and worse short‐term memory. 53 , 61 In animal studies, “runner plasma,” collected from voluntarily running mice and infused into sedentary mice, downregulated brain IGSF5 (among other genes) and correspondingly reduced neuroinflammation by targeting cerebrovasculature. 62 Furthermore, astrocytes—the cell type in which IGSF5 expression showed the strongest association with higher odds of dementia in our study—may play a pivotal role in cerebral perfusion and brain blood flow. 63 It is possible that healthy diets may exert similar effects as exercise on reducing brain IGSF5 expression, and function via cardiovascular and/or cerebrovascular mechanisms to improve brain aging.
More broadly, for several genes whose brain expression was associated with both MIND diet and dementia, previous GWAS have linked them to inflammation (e.g., DXO and MPO) 64 and cardiovascular disease (e.g., INPP5B and ZNF827). 52 , 65 Inflammatory and vascular mechanisms have been associated with dementia risk, supporting the validity of our findings and further emphasizing the importance of targeting these pathways for dementia prevention.
Our study has important strengths. We uniquely leveraged a large sample of brain specimens with bulk RNA‐Seq data (including a subset with single‐nuclei RNA‐Seq data) and clinical and cognitive assessments, in two longitudinal aging cohorts. The additional availability of dietary data in MAP further provided us with an unprecedented opportunity to offer novel molecular insights into the association between the MIND diet and dementia.
Our study has several limitations. First, our primarily analysis used RNA‐seq in bulk DLPFC tissue that includes a mixture of cells. We estimated cell type proportions and showed that our primary results remained unchanged after adjusting for cell compositions; however, we cannot eliminate confounding by cell compositions. Although we examined single‐nuclei RNA‐Seq data in a subsample for selected genes, our statistical power was very limited, and we were unable to examine associations with diet due to little overlap with dietary data. Future studies investigating cell type–specific gene expressions in larger sample sizes will be important. Second, due to the use of post mortem samples, the temporal relationship between DLPFC gene expression and cognitive outcomes remains uncertain. Although our secondary MR analysis suggested that gene expression likely preceded dementia rather than the reverse, our statistical power for this analysis was limited due to the small sample size for GWAS of the brain transcriptomic profile score (although testing of the “reverse” directionality from dementia to the transcriptomic profile used 40 independent AD‐associated variants as a strong instrument variable, and the result was null). In addition, although our analyses were adjusted for a wide range of potential confounders, unmeasured or residual confounding is possible. Finally, our study participants were primarily non‐Hispanic White older adults who consented to brain autopsy and donation, whereas diet may differ in diverse populations. Future studies are warranted to validate our findings in diverse populations and elucidate the functionality and cell specificity of the identified genes in cognitive health.
In conclusion, we identified a brain transcriptomic profile correlated with the MIND diet, and this profile was significantly associated with slower cognitive decline and lower odds of dementia. Our study offers new insights into brain molecular mechanisms through which healthy behaviors may be related to cognitive health and dementia, and these findings may inform the development of novel molecular targets for dementia prevention.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts to declare. Author disclosures are available in the supporting information.
CONSENT STATEMENT
This study was conducted in two community‐based, longitudinal cohorts—the Religious Orders Study and the Rush Memory and Aging Project. The institutional review board of Rush University Medical Center approved the study, and all participants signed an informed consent and anatomical gift act to donate tissue at the time of death.
Supporting information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
We thank the study staff and participants of ROS and MAP for their essential contributions and donations of their brains to these projects. The ROS and MAP cohorts and multi‐omics data were funded by National Institute on Aging R01AG017917, P30AG010161, U01AG061356, R01NS084965, RF1AG059621, RF1AG074549, and R01AG069904. Dr. Li was funded by National Institute of Diabetes and Digestive and Kidney Diseases R00DK122128. The funders had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Notes
Li J, Capuano AW, Agarwal P, et al. The MIND diet, brain transcriptomic alterations, and dementia. Alzheimer's Dement. 2024;20:5996–6007. 10.1002/alz.14062 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
REFERENCES
Articles from Alzheimer's & Dementia are provided here courtesy of Wiley
Citations & impact
Impact metrics
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/166231144
Article citations
The ROSMAP project: aging and neurodegenerative diseases through omic sciences.
Front Neuroinform, 18:1443865, 16 Sep 2024
Cited by: 0 articles | PMID: 39351424 | PMCID: PMC11439699
Review Free full text in Europe PMC
The MIND diet, brain transcriptomic alterations, and dementia.
Alzheimers Dement, 20(9):5996-6007, 11 Aug 2024
Cited by: 2 articles | PMID: 39129336 | PMCID: PMC11497672
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The Mediterranean-Dietary Approaches to Stop Hypertension Intervention for Neurodegenerative Delay (MIND) Diet for the Aging Brain: A Systematic Review.
Adv Nutr, 15(3):100184, 03 Feb 2024
Cited by: 4 articles | PMID: 38311314 | PMCID: PMC10942868
Review Free full text in Europe PMC
Association of adherence to high-intensity physical activity and the Mediterranean-dietary approaches to stop hypertension intervention for neurodegenerative delay diet with cognition: A cross-sectional study.
Int J Nurs Stud, 131:104243, 20 Apr 2022
Cited by: 4 articles | PMID: 35550515
MIND not Mediterranean diet related to 12-year incidence of cognitive impairment in an Australian longitudinal cohort study.
Alzheimers Dement, 15(4):581-589, 28 Feb 2019
Cited by: 84 articles | PMID: 30826160
Review
Funding
Funders who supported this work.
NIA NIH HHS (14)
Grant ID: RF1AG059621
Grant ID: R01AG017917
Grant ID: U01 AG061356
Grant ID: R01 AG017917
Grant ID: R01AG069904
Grant ID: P30 AG010161
Grant ID: R01 AG054476
Grant ID: R01NS084965
Grant ID: RF1 AG059621
Grant ID: RF1 AG074549
Grant ID: U01AG061356
Grant ID: RF1AG074549
Grant ID: P30AG010161
Grant ID: R01 AG069904
NIDDK NIH HHS (2)
Grant ID: R00DK122128
Grant ID: R00 DK122128
NINDS NIH HHS (1)
Grant ID: R01 NS084965
National Institute of Diabetes and Digestive and Kidney Diseases (1)
Grant ID: R00DK122128
National Institute on Aging (7)
Grant ID: R01AG017917
Grant ID: R01NS084965
Grant ID: RF1AG074549
Grant ID: RF1AG059621
Grant ID: U01AG061356
Grant ID: R01AG069904
Grant ID: P30AG010161

 1
,
2
1
,
2


