Abstract
Free full text

Orbitofrontal cortex, decision-making and drug addiction
Abstract
The orbitofrontal cortex, as a part of prefrontal cortex, is implicated in executive function. However, within this broad region, the orbitofrontal cortex is distinguished by its unique pattern of connections with crucial subcortical associative learning nodes, such as basolateral amygdala and nucleus accumbens. By virtue of these connections, the orbitofrontal cortex is uniquely positioned to use associative information to project into the future, and to use the value of perceived or expected outcomes to guide decisions. This review will discuss recent evidence that supports this proposal and will examine evidence that loss of this signal, as the result of drug-induced changes in these brain circuits, might account for the maladaptive decision-making that characterizes drug addiction.
Introduction
Our ability to form expectations about the desirability or value of impending events underlies much of our emotion and behavior. In fact, two broad functions are crucially subserved by the formation of such expectations. On the one hand, expectations guide our immediate behavior, allowing us to pursue goals and avoid potential harm. On the other hand, expectations can be compared with actual outcomes to facilitate learning so that future behavior can become more adaptive. Both of these functions require that information about expected outcomes be maintained in memory so that it can be compared and integrated with information about internal state and current goals. Such an integrative process generates a signal that we will refer to as an outcome expectancy, a term long-used by learning theorists to refer to an internal representation of the consequences likely to follow a specific act [1]. The disruption of such a signal would be expected to create a myriad of difficulties, in the ability both to make adaptive decisions and to learn from negative consequences of decisions. In this review, we first describe recent evidence that the orbitofrontal cortex (OFC) plays a crucial role in the generation and use of outcome expectancies. Subsequently, we will discuss recent evidence that the maladaptive decisions that characterize drug addiction reflect, in part, a disruption of this signal as a result of drug-induced changes in the OFC and related brain areas.
Neural activity in the OFC and OFC-dependent behavior reflect a crucial role of the OFC in the generation of outcome expectancies
The ability to maintain information so that it can be manipulated, integrated with other information and then used to guide behavior has been variously described as working, scratchpad or representational memory, and it depends crucially on the prefrontal cortex [2]. Within the prefrontal cortex, the OFC, by its connections with limbic areas, is uniquely positioned to enable associative information regarding outcomes or consequences to access representational memory (Box 1). Indeed a growing number of studies suggest that a neural correlate of the expected value of outcomes is present and perhaps generated in the OFC. For example, human neuroimaging studies show that blood flow changes in the OFC during anticipation of expected outcomes and also when the value of an expected outcome is modified or not delivered [3-6]. This activation appears to reflect the incentive value of these items and is observed when that information is being used to guide decisions [7]. These results suggest that neurons in the OFC increase activity when such information is processed. Accordingly, neural activity in the OFC that precedes predicted rewards or punishments increases, typically reflecting the incentive values of these outcomes [8-11]. For example, when monkeys are presented with visual cues paired with differently preferred rewards, neurons in the OFC fire selectively according to whether the anticipated outcome is the preferred or non-preferred reward within that trial block [10]. Moreover, Roesch and Olson [11] have recently demonstrated that firing in the OFC tracks several other specific metrics of outcome value. For example, neurons fire differently for a reward depending on its expected size, the anticipated time required to obtain it and the possible aversive consequences associated with inappropriate behavior [11,12].
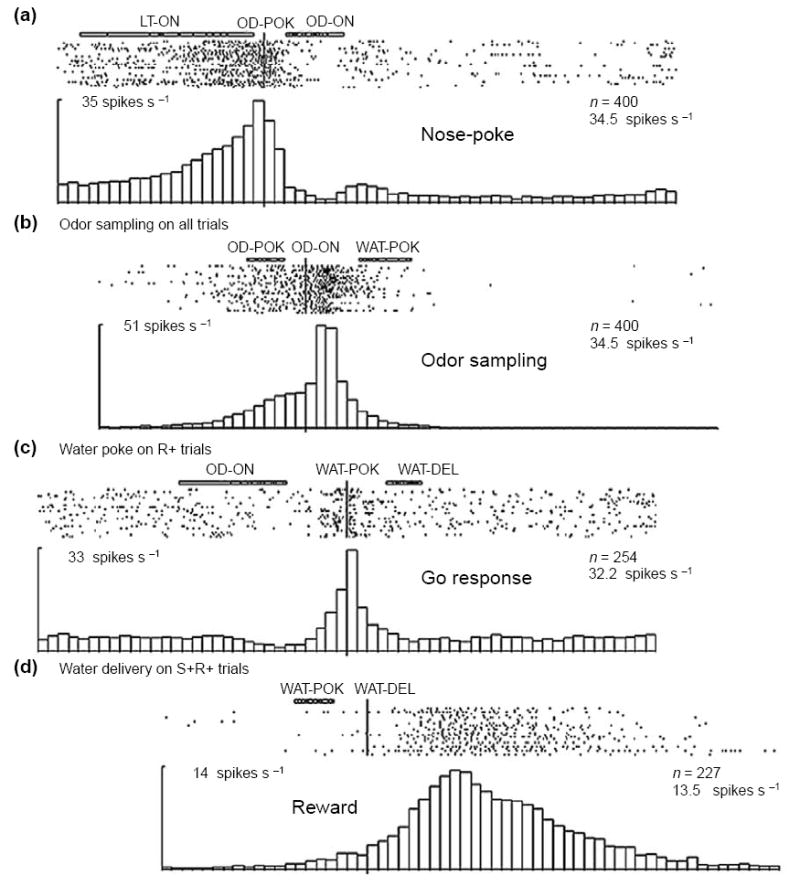
Such anticipatory activity appears to be a common feature of firing activity in the OFC across many tasks in which events occur in a sequential, and thus predictable, order (Box 2). Importantly, however, these selective responses can be observed in the absence of any signaling cues, and they are acquired as animals learn that particular cues predict a specific outcome. In other words, this selective activity represents the expectation of an animal, based on experience, of likely outcomes. These features are illustrated in Figure 1, which shows the population response of OFC neurons recorded in rats as they learn and reverse novel odor-discrimination problems [8,9,13]. In this simple task, the rat must learn that one odor predicts reward in a nearby fluid well, whereas the other odor predicts punishment. Early in learning, neurons in the OFC respond to one but not to the other outcome. At the same time, the neurons also begin to respond in anticipation of their preferred outcome. Over a number of studies, 15–20% of the neurons in the OFC developed such activity in this task, firing in anticipation of either sucrose or quinine presentation [8,9,13]. The activity in this neural population reflects the value of the expected outcomes, maintained in what we have defined here as representational memory.
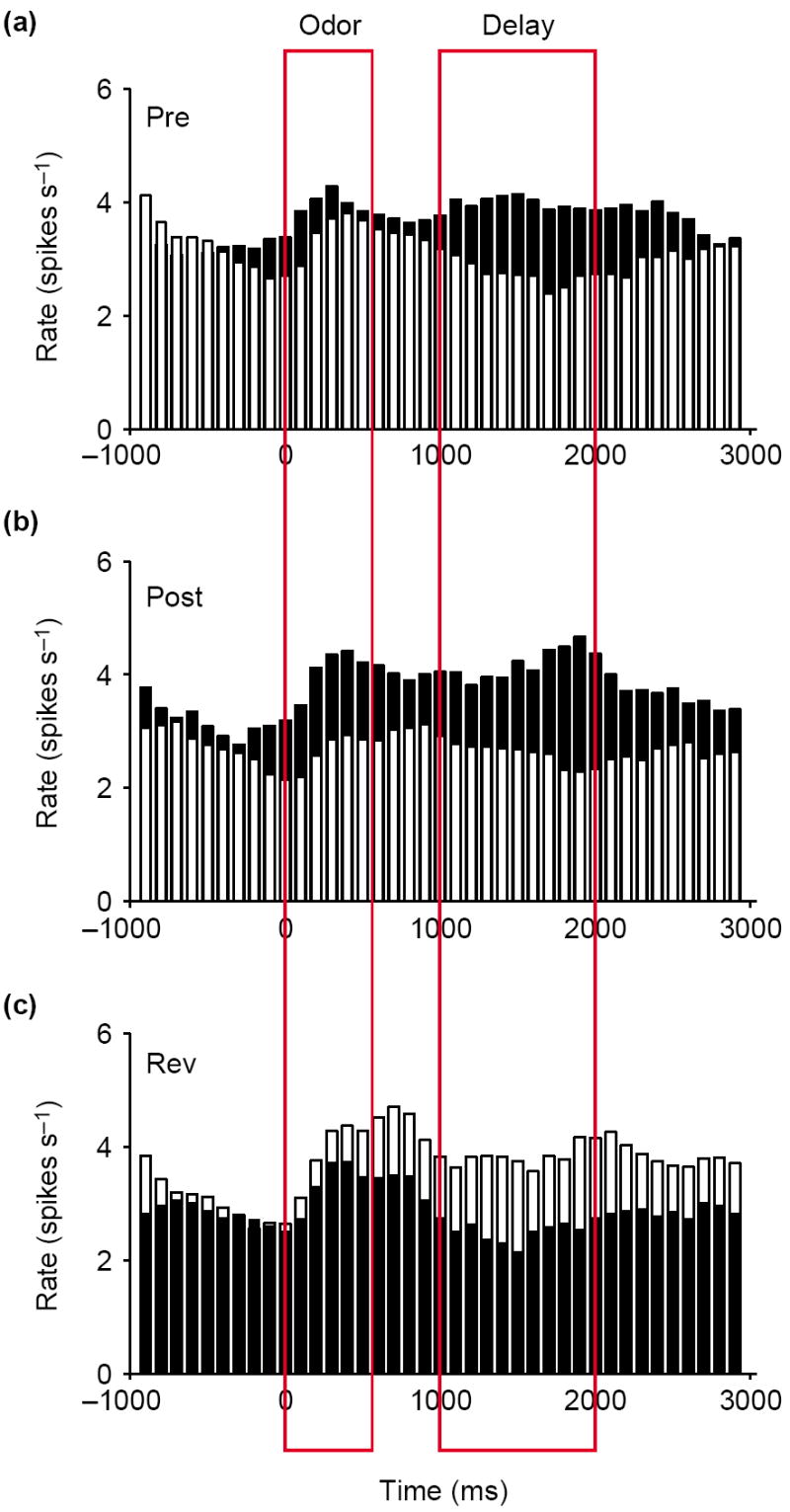
Signaling of outcome expectancies in the orbitofrontal cortex. Black bars show the response on trials involving the preferred outcome of the neurons in the post-criterion phase. White bars show the response to the non-preferred outcome. Activity is synchronized to odor onset and displayed in 100 ms bins; red boxes indicate the average duration of odor sampling and of the delay between responding and reinforcement when a response was made at the fluid well. (a) The average response of neurons that responded selectively during a delay period early in learning, after the rat went to the fluid well but before delivery of one of the outcomes (Pre). This activity signals the expected outcome after responding. (b) Later (Post), these neurons became activated by the odor cue that predicted the preferred outcomes of the neurons after learning, thereby signaling the expected outcome at the time the decision to respond is made. (c) This pattern switched after reversal of the odor–outcome associations (Rev). Average firing rate is shown on the y-axis in spikes per second. Adapted, with permission, from [13].
After learning, these neurons come to be activated by the cues that predict their preferred outcomes, thereby signaling the expected outcome even before a response is made. This is evident in the population response presented in Figure 1, which exhibits higher activity, after learning, in response to the odor cue that predicts the preferred outcome of the neuronal population. These signals would allow an animal to use expectations of likely outcomes to guide responses to cues and to facilitate learning when expectations are violated.
The notion that the OFC guides behavior by signaling outcome expectancies is consistent with the effects of OFC damage on behavior. These effects are typically evident when the appropriate response cannot be selected using simple associations, but instead requires outcome expectancies to be integrated over time or to be compared between alternative responses. For example, humans with damage to the OFC are unable to guide behavior appropriately based on the consequences of their actions in the Iowa gambling task [14]. In this task, subjects must choose from decks of cards with varying rewards and penalties represented on the cards. To make advantageous choices, subjects must be able to integrate the value of these varying rewards and penalties over time. Individuals with OFC damage initially choose decks that yield higher rewards, indicating that they can use simple associations to direct behavior according to reward size; however, they fail to modify their responses to reflect occasional large penalties in those decks. Integrating information about the occasional, probabilistic penalties would be facilitated by an ability to maintain information about the value of the expected outcome in representational memory after a choice is made, so that violations of this expectation (occasional penalties) could be recognized. This deficit is analogous to the reversal deficits demonstrated in rats, monkeys and humans after damage to the OFC [15-21].
This ability to hold information about expected outcomes in representational memory has also been probed in a recent study in which subjects made choices between two stimuli that predicted punishment or reward at varying levels of probability [22]. In one part of this study, subjects were given feedback about the value of the outcome that they had not selected. Normal subjects were able to use this feedback to modulate their emotion about their choice and to learn to make better choices in future trials. For example, a small reward made them happier when they knew that they had avoided a large penalty. Individuals with OFC damage showed normal emotional responses to the rewards and punishments that they selected; however, feedback about the unselected outcome had no effect on either their emotions or on their subsequent performance. That is, they were happy when they received a reward, but they were no happier if they were informed that they had also avoided a large penalty. This impairment is consistent with a role for the OFC in maintaining associative information in representational memory to compare different outcome expectancies. Without this signal, individuals cannot compare the relative value of the selected and unselected outcomes and thus fail to use this comparative information to modulate emotional reactions and facilitate learning.
Although these examples are revealing, a more-direct demonstration of the crucial role of the OFC in generating outcome expectancies to guide decision-making comes from reinforcer devaluation tasks. These tasks assess the control of behavior by an internal representation of the value of an expected outcome. For example, in a Pavlovian version of this procedure (Figure 2), rats are first trained to associate a light cue with food. After conditioned responding is established to the light, the value of the food is reduced by pairing it with illness. Subsequently, in the probe test, the light cue is presented again in a non-rewarded extinction session. Animals that have received food-illness pairings respond less to the light cue than do non-devalued controls. Importantly, this decrease in responding is evident from the start of the session and is superimposed on the normal decreases in responding that result from extinction learning during the session. This initial decrease in responding must reflect the use of an internal representation of the current value of the food in combination with the original light-food association. Thus, reinforcer devaluation tasks provide a direct measure of the ability to manipulate and use outcome expectancies to guide behavior.
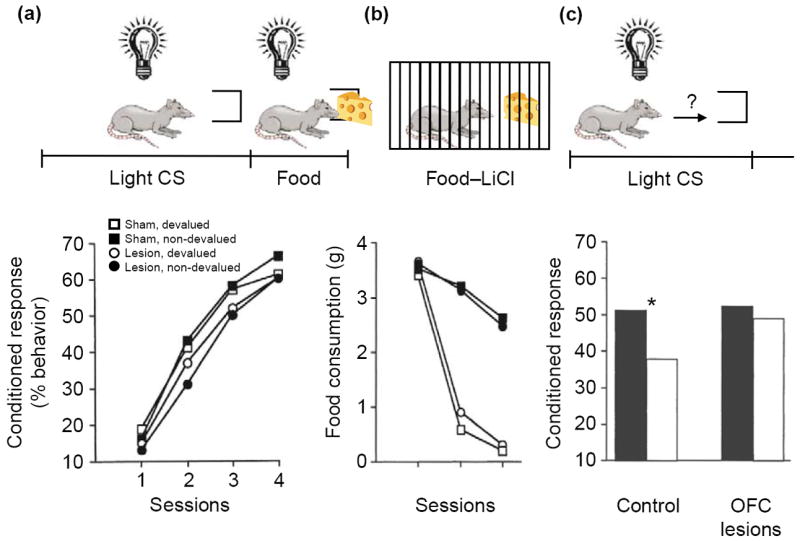
Effects of neurotoxic lesions of the orbitofrontal cortex (OFC) on performance in a reinforcer devaluation task. (a) Control rats and rats with bilateral neurotoxic lesions of the OFC were trained to associate a conditioned stimulus (CS, light) with an unconditioned stimulus (US, food). Over four sessions, both lesioned and control rats developed a conditioned responding at the food cup during light presentation. This food-cup response is represented as the percentage of total behavior. The lesion had no effect on the development of the food-cup response. (b) The rats then received presentations of the food item in their home cages followed by illness induced by lithium chloride (LiCl) injection. Some rats in each group received paired presentations of food and illness (black circles and squares); others received unpaired presentations (white circles and squares). Rats that received paired presentations stopped consuming the food item (i.e. the food stimulus was ‘devalued’). Again, no effect of lesion was observed. (c) The following day, the rats were returned to the training environment, and conditioned responses to the light cue were measured. When exposed to the light CS, control rats that had received paired presentations of food and illness (for which the food had been devalued; white bars on the left) showed reduced conditioned responses to the food cup compared with unpaired controls (white bars on the right; asterisk indicates P<0.05). Rats with OFC lesions did not show this decrease in conditioned response as a result of reinforcer devaluation. Lower panels adapted, with permission, from [23] © (1999) the Society for Neuroscience.
Rats with OFC lesions fail to show any effect of devaluation on conditioned responding in this paradigm, despite normal conditioning and devaluation of the outcome [23]. In other words, they continue to respond to the light cue and attempt to obtain the food, even though they will not consume it if it is presented (Figure 2). Importantly, OFC-lesioned rats display a normal ability to extinguish their responses within the test session, demonstrating that their deficit does not reflect a general inability to inhibit conditioned responses [24]. Rather, the OFC has a specific role in controlling conditioned responses according to internal representations of the new value of the expected outcome. Accordingly, OFC lesions made after learning continue to affect behavior in this task [25]. Similar results have been reported in monkeys trained to perform an instrumental version of this task [19].
Rats with OFC lesions also show neurophysiological changes in downstream regions that are consistent with the loss of outcome expectancies. In one study [26], responses were recorded from single units in the basolateral amygdala, an area that receives projections from OFC, in rats learning and reversing novel odor discriminations in the task described earlier. Under these conditions, OFC lesions disrupted outcome-expectant firing normally observed in the basolateral amygdala. Furthermore, without OFC input, neurons of the basolateral amygdala became cue-selective much more slowly, particularly after cue-outcome associations were reversed. Slower associative encoding in the basolateral amygdala as a result of OFC lesions, particularly during reversal, is consistent with the idea that outcome expectancies facilitate learning in other structures, especially when expectations are violated as they are in reversals. Thus, OFC appears to generate and represent outcome expectancies that are critical not only to the guidance of behavior according to expectations about the future, but also to the ability to learn from violations of those expectations. Without this signal, animals engage in maladaptive behavior, driven by antecedent cues and stimulus-response habits, rather than by a cognitive representation of an outcome or goal.
Addictive behavior and outcome expectancies
Recent findings suggest that this conceptualization of OFC function has much to offer an understanding of drug addiction. According to the Diagnostic and Statistical Manual of Mental Disorders [27], a diagnosis of substance dependence requires that an individual display an inability to control his or her drug-seeking behavior, despite adverse consequences. Such addictive behavior is characterized variously as compulsive, impulsive, perseverative or under the control of drug-associated cues. Moreover, it is often observed despite a stated desire on the part of addicts to stop. Thus, a diagnosis of substance dependence requires a pattern of behavior similar to that of OFC-lesioned rats, monkeys and humans.
Accordingly, drug addiction is associated with changes in OFC structure and function. For example, imaging studies of addicts have consistently revealed abnormalities in blood flow in the OFC [28-33] (for an excellent review, see [34]). Alcohol and cocaine addicts display reductions in baseline measurements of OFC activation during acute withdrawal and even after long periods of abstinence. Conversely, during exposure to drug-related cues, addicts show an overactivation of OFC that correlates with the degree of craving that they experience. These changes are associated with impairments to OFC-dependent behaviors in drug addicts [35-39]. For example, alcohol and cocaine abusers display similar, although not as severe on average, impairments on the gambling task described earlier, as do individuals with lesions of the OFC. Similarly, other laboratory tests of decision-making have revealed that amphetamine abusers take longer and are less likely to choose the most rewarding option than are controls. But do these deficits reflect a pre-existing vulnerability to addiction in some people? Or are they a result of long-term drug-induced neuroadaptations? And if so, do they reflect changes in structure and/or function within OFC, or are they the result of changes elsewhere in corticolimbic networks that mimic the effects of OFC lesions?
To answer these questions, it is necessary to turn to animal models, in which addictive drugs can be delivered in a controlled manner against a relatively fixed genetic and environmental background. A growing number of such studies now demonstrate that prolonged exposure to addictive drugs – and particularly psychostimulants – results in relatively long-lasting brain and behavioral changes [40-50]. Importantly these effects are typically observed months after cessation of and in behavioral settings that are unrelated to drug exposure, consistent with the hypothesis that addictive drugs modify brain circuits that are crucial for the normal control of behavior. Recently, several studies have demonstrated effects on the OFC. For example, rats trained to self-administer amphetamine for several weeks have been reported to show a reduction in dendritic spine density in the OFC one month later [46]. Furthermore, these drug-experienced rats exhibited less remodeling of their dendrites in response to appetitive instrumental training. These findings are particularly noteworthy in light of the increased spine density that has been previously reported in the medial prefrontal cortex, nucleus accumbens and elsewhere after treatment with psychostimulants [41]. Thus, among these corticolimbic regions, the OFC appears to be unique in showing evidence of decreased synaptic plasticity after drug exposure.
A decrease in plasticity in the OFC might be expected to impact on OFC-dependent functions. Consistent with this conjecture, rats given a two-week course of treatment with cocaine show long-lasting impairments in OFC-dependent behavior. Specifically, these animals are unable to use the value of predicted outcomes to guide their behavior. In one experiment [51], rats were given daily injections of cocaine for two weeks. Over one month later, these rats were tested in a Go–NoGo odor discrimination task. In this task, rats learn to go to a fluid port to obtain sucrose after smelling one odor and withhold going to the same fluid port to avoid quinine after smelling a second odor. Rats treated with cocaine learned these discriminations at the same rate as did saline-treated controls, but were unable to acquire reversals of the discriminations as rapidly as were the controls. Similar reversal deficits have also been demonstrated in primates that are given intermittent chronic access to cocaine [43]. Such reversal deficits are characteristic of OFC-lesioned animals and humans [15-21], where they are thought to reflect an inability to change established behaviors rapidly. We propose that the role of OFC in supporting this rapid flexibility relates to its importance in signaling outcome expectancies [26]. During reversal learning, the comparison of this signal with the actual, reversed outcome would generate error signals crucial to new learning [1]. Without this signal, OFC-lesioned rats would learn more slowly. As we have already discussed, a neurophysiological correlate of this slow learning has recently been demonstrated in the inflexible associative encoding of basolateral amygdala neurons in OFC-lesioned rats [26].
The loss of this signal is also evident in a second experiment in which rats were treated with cocaine for two weeks and then tested in the Pavlovian reinforcer devaluation task described earlier [24]. Again, testing was conducted about one month after the last cocaine treatment. These rats exhibited normal conditioning and devaluation, and also extinguished responding normally in the final test phase; however, devalued cocaine-treated rats did not show the normal spontaneous reduction in response to the predictive cue. This deficit (Figure 3) is identical to the deficit after OFC lesions in this task (Figure 2). These findings are consistent with an inability to signal the value of the expected outcome. Indeed, because in this task there is no ambiguity regarding the representations required to mediate normal performance, the deficits described here point unequivocally towards a loss of outcome expectancies in cocaine-treated rats.
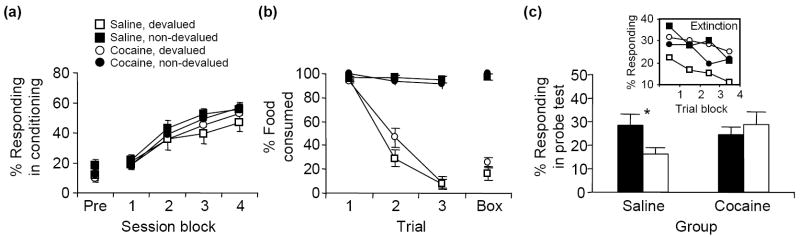
Effects of cocaine treatment on performance in the reinforcer devaluation task (Figure 2). Saline- and cocaine-treated rats were trained to associate a conditioned stimulus (CS, light) with an unconditioned stimulus (US, food). (a) Over four session blocks, both cocaine- and saline-treated rats developed a conditioned responding at the food cup during light presentation. This food-cup response is represented as the percentage of total behavior. There was no effect of the lesion on the development of the food-cup response. (b) The rats then received presentations of the food item in their home cages followed by illness induced by LiCl injection. Some rats in each group received paired presentations of food and illness (black circles and squares) while others received unpaired presentations (white circles and squares). Rats that received paired presentations stopped consuming the food item, both in their home cage and in the testing chamber (‘Box’). Again, no effect of lesion was observed. (c) The following day, the rats were returned to the training environment, and conditioned responses to the light cue were measured. Control rats that had received paired presentations of food and illness showed reduced conditioned responses to the food cup compared with unpaired controls. Rats with orbitofrontal lesions did not show this decrease in conditioned response as a result of reinforcer devaluation. This effect was observed while all four groups showed normal extinction of responding during the probe test (inset). Adapted, with permission of Oxford University Press, from [24].
Loss of this signaling mechanism would account for the propensity of addicts to continue to seek drugs, despite the almost inevitable negative consequences of such behavior, because it would render them unable to incorporate this predictive information into their decision-making and perhaps unable to learn from even repeated experience of these negative consequences. Although other brain systems might also be involved, drug-induced changes to this OFC-dependent signal would by themselves contribute powerfully to a transition from normal goal-directed behavior to compulsive habitual responding. This transition would reflect a change in the balance between these competing mechanisms of behavioral control. Such an explanation would hold for the drug-seeking behavior of addicts, and also for recent findings in several animal models of addiction in which rats are unable to withhold drug-seeking behavior, even when adverse outcomes are made contingent upon that behavior [45,47].
Concluding remarks
We have reviewed recent findings to support the proposal that the OFC is crucial for signaling the value of expected outcomes or consequences. We have also discussed how this idea might be important for understanding the pathology that underlies drug addiction. Of course these ideas raise many more questions. If the OFC generates signals regarding expected outcomes, it becomes crucial to understand how downstream areas use these signals – in normal animals, in addition to those exposed to addictive drugs. We have suggested how the basolateral amygdala might be involved [26]; however, understanding the role these signals have in the nucleus accumbens – and how they interact with other ‘limbic’ inputs – might be far more relevant for understanding addiction. Several laboratories are working hard to resolve these important issues. In addition, it will be important to demonstrate whether changes in OFC-dependent behavior after drug exposure actually reflect altered molecular or neurophysiological function in the OFC, as suggested by preliminary recording data [52], or alternatively whether they might reflect changes elsewhere in the circuit, such as in the nucleus accumbens, an area long implicated in addiction. And, of course, any animal model of disease is only of value if it suggests a remedy for the pathological changes. This is difficult in the case of lesions but could be possible for deficits stemming from drug exposure. However, it remains to be seen whether manipulations might be undertaken to normalize the behavior and perhaps any molecular or neurophysiological correlates that are identified in drug-treated animals. We expect that these and many more issues will be addressed in the coming years (Box 3).
Acknowledgments
Our research was supported by grants from the NIDA (R01-DA015718 to G.S.), NINDS (T32-NS07375 to M.R.R.) and NIDCD (T32-DC00054 to T.A.S.).
References
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.tins.2005.12.006
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc2430629?pdf=render
Citations & impact
Impact metrics
Article citations
Urgency Theory in the context of broader emotion theories: a conceptual review.
Front Psychiatry, 15:1403639, 05 Jul 2024
Cited by: 0 articles | PMID: 39035607 | PMCID: PMC11257906
Review Free full text in Europe PMC
Cocaine self-administration behavior is associated with subcortical and cortical morphometry measures in individuals with cocaine use disorder.
Am J Drug Alcohol Abuse, 50(3):345-356, 29 Mar 2024
Cited by: 0 articles | PMID: 38551365 | PMCID: PMC11305926
Examination of reward processing dysfunctions in the left dorsal striatum and other brain regions among psychiatric inpatients with substance use.
Drug Alcohol Depend, 256:111097, 20 Jan 2024
Cited by: 1 article | PMID: 38266574
Craving dynamics and related cerebral substrates predict timing of use in alcohol, tobacco, and cannabis use disorders.
Addict Neurosci, 9:100138, 17 Nov 2023
Cited by: 0 articles | PMID: 38389954 | PMCID: PMC10883348
Recent Opioid Use Impedes Range Adaptation in Reinforcement Learning in Human Addiction.
Biol Psychiatry, 95(10):974-984, 13 Dec 2023
Cited by: 0 articles | PMID: 38101503
Go to all (256) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Choose your path: Divergent basolateral amygdala efferents differentially mediate incentive motivation, flexibility and decision-making.
Behav Brain Res, 409:113306, 19 Apr 2021
Cited by: 8 articles | PMID: 33887310 | PMCID: PMC8189324
Review Free full text in Europe PMC
Orbitofrontal cortex, associative learning, and expectancies.
Neuron, 47(5):633-636, 01 Sep 2005
Cited by: 269 articles | PMID: 16129393 | PMCID: PMC2628809
Review Free full text in Europe PMC
How the orbitofrontal cortex contributes to decision making - a view from neuroscience.
Prog Brain Res, 174:61-71, 01 Jan 2009
Cited by: 14 articles | PMID: 19477330
Orbitofrontal cortex and its contribution to decision-making.
Annu Rev Neurosci, 30:31-56, 01 Jan 2007
Cited by: 454 articles | PMID: 17417936
Review
Funding
Funders who supported this work.
NIDA NIH HHS (3)
Grant ID: R01 DA015718
Grant ID: R01-DA015718
Grant ID: R01 DA015718-03
NIDCD NIH HHS (2)
Grant ID: T32-DC00054
Grant ID: T32 DC000054
NINDS NIH HHS (2)
Grant ID: T32-NS07375
Grant ID: T32 NS007375