Abstract
Free full text

A checkpoint for autoreactivity in human IgM+ memory B cell development
Associated Data
Abstract
Autoantibodies are removed from the repertoire at two checkpoints during B cell development in the bone marrow and the periphery. Despite these checkpoints, up to 20% of the antibodies expressed by mature naive B cells in healthy humans show low levels of self-reactivity. To determine whether self-reactive antibodies are also part of the antigen-experienced memory B cell compartment, we analyzed recombinant antibodies cloned from single circulating human IgM+ memory B cells. Cells expressing antibodies specific for individual bacterial polysaccharides were expanded in the IgM+ memory compartment. In contrast, B cells expressing self-reactive and broadly bacterially reactive antibodies were removed from the repertoire in the transition from naive to IgM+ memory B cell. Selection against self-reactive antibodies was implemented before the onset of somatic hypermutation. We conclude that a third checkpoint selects against self-reactivity during IgM+ memory B cell development in humans.
The majority of developing human B cells in the bone marrow express polyreactive or self-reactive antibodies, but most of these potentially harmful autoantibodies are eliminated from the repertoire at two self-tolerance checkpoints during early B cell development in the bone marrow and the periphery (1, 2). Nevertheless, ~20% of mature naive B cells in peripheral blood of healthy donors express low-affinity self-reactive antibodies and ~5% produce antibodies with low levels of polyreactivity (2).
During immune responses, naive B cells undergo affinity maturation and selection before differentiating into either antibody-secreting plasma cells or memory B cells (3). In humans, circulating memory B cells express either IgM or secondary antibody isotypes and are distinguished from naive B cells by cell surface CD27 (4–7). IgM+ memory B cells participate in T cell–independent (T-I) immune responses to polysaccharide antigens and bacterial infections, whereas class-switched memory B cells are produced in germinal centers during T cell–dependent (T-D) immune responses (8, 9). Furthermore, IgM+, but not class-switched memory B cells require a functional spleen for their development and maintenance (10). Based on their cell surface phenotype and gene expression profiles, it has been proposed that IgM+ memory B cells are the circulating form of splenic marginal zone (MZ) B cells (11).
Marginal zone B cells were first identified in the mouse, where (as in humans) they participate in T-I immune responses to polysaccharide antigens and the initial defense against blood-borne pathogens (12–14). Experiments with Ig transgenic mice show that B cells expressing transgenic low-affinity auto- or polyreactive antibodies are selected into the MZ B cell compartment (15–20). These broadly reactive antibodies are believed to be especially important in the early phase of adaptive immune responses because of their ability to react with a large number of different pathogens (21, 22). However, the reactivity of antibodies expressed by MZ B cells or IgM+ memory B cells has not been studied in normal mice or humans.
Here, we describe the reactivity profiles of 105 recombinant antibodies cloned from single human IgM+ memory B cells isolated from peripheral blood of three healthy donors. We find that the IgM+ memory B cell compartment is depleted of self-reactive and polyreactive antibodies relative to the naive B cell pool.
RESULTS
Igs expressed by IgM+ memory B cells
To characterize the antibodies produced by IgM+ memory B cells, we purified single cells from peripheral blood of three healthy donors and analyzed their antibody heavy and light chains. In agreement with previous reports, we found that IgM+ memory B cells showed a significant increase in VH3 gene family representation and a decrease in JH6 gene usage when compared with mature naive B cells from the same donors (Fig. 1, A–C, and references 23–26). As expected, the decreased JH6 usage was associated with shorter CDR3s (Fig. 1, A–C and reference 25). The numbers of positively charged residues in IgH CDR3s, as well as Igκ and Igλ light chain V and J gene usage, were all comparable in naive and IgM+ memory B cells (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20052033/DC1). We conclude that antibodies expressed by IgM+ memory differ from naive B cells only in VH3 and JH6 usage. 94.7% of IgM+ memory antibodies were somatically mutated (124/131; Tables S1–S3, available at http://www.jem.org/cgi/content/full/jem.20052033/DC1) with a mutation frequency of 3.20% for IgVH, 1.62% for IgVκ, and 1.61% for IgVλ genes (Fig. 1, D and E). Replacement mutations were enriched over silent mutations in CDRs compared with framework regions (FWRs) 1–3 (Fig. 1 E). Thus, antibodies expressed by IgM+ memory B cells show signs of antigen-mediated selection.
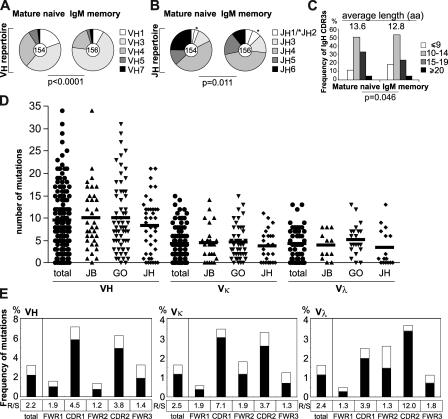
Ig gene features. (A) IgH V and (B) J gene repertoire and (C) IgH CDR3 length of antibodies from mature naive and IgM+ memory B cells. (D) The number of mutations and (E) frequency of mutations of VH, Vκ, and Vλ genes in antibodies from IgM+ memory B cells as calculatedby the number of replacement (R; black bar) and silent (S; white bar) nucleotide exchanges per base pair in FWRs and CDRs. The R/S ratio in each region is indicated.
Self-reactivity profiles of antibodies from IgM+ memory B cells
The self-reactivity profiles of IgM+ memory and naive B cell antibodies from the same donors were compared in ELISAs using lysates of the human cell line HEp-2 (Fig. 2 A and reference 2 for GO and JB). On average, 19.7% of the antibodies from mature naive B cells from three donors JH (17.6%; Fig. 2 A), GO (22.8% and reference 2), and JB (16.7% and reference 2) were self-reactive (Fig. 2 A and Tables S4–S6, available at http://www.jem.org/cgi/content/full/jem.20052033/DC1). In contrast, only 2.1% of antibodies expressed by IgM+ memory B cells from the same individuals were self-reactive (0% for JH and JB, 4.8% for GO; Fig. 2 A and Tables S1–S3). This result was confirmed by indirect immunofluorescence assay (IFA) with HEp-2 cell–coated slides (unpublished data). Thus, the frequency of self-reactive antibodies expressed by IgM+ memory B cells was significantly lower than that measured in mature naive B cells (Fig. 2 A; P < 0.0001 for all donors combined; P = 0.028 for JH, P = 0.033 for JB, and P = 0.021 for GO). We conclude that B cells expressing self-reactive antibodies are efficiently excluded from the IgM+ memory B cell pool.
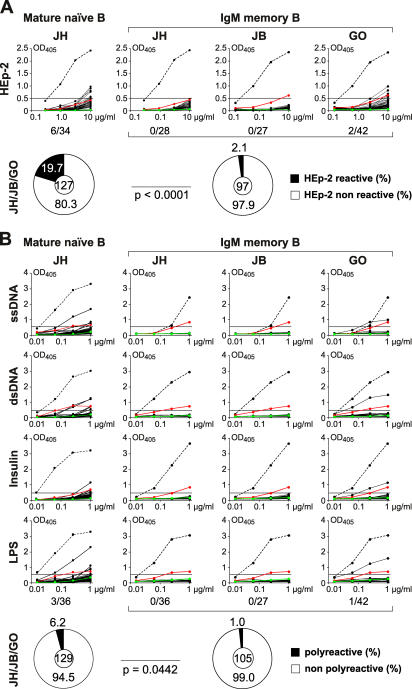
Polyreactive and self-reactive antibodies are excluded from the IgM+ memory B cell compartment. (A) Antibodies from mature naive (donor JH) and IgM+ memory B cells (donors JH, GO, JB) were tested for self-reactivity by HEp-2 cell ELISA. HEp-2 ELISA results for antibodies from mature naive B cells of donors GO and JB have been published previously (reference 2). Dotted line shows ED38 positive control antibody (references 2, 43). Horizontal line shows cut-off OD405 for positive reactivity as determined by comparison to low positive control serum (red line) and negative control serum (green line) from patients and healthy individuals, respectively. Pie charts summarize the frequency of HEp-2 self-reactive and nonself-reactive clones for all three donors. The number of tested antibodies is indicated in the pie chart center (reference 2). (B) Antibodies from mature naive (donor JH) and IgM+ memory B cells (donors JH, GO, JB) were tested for polyreactivity by ELISA with ssDNA, dsDNA, LPS, and insulin. Dotted lines represent the high positive control antibody ED38 (references 2, 43). Horizontal lines show cut-off OD405 for positive reactivity as determined by comparison to the previously published control antibodies mGO53 (negative control; green line) and eiJB40 (low positive control; red line) (reference 2). The frequency of polyreactive antibodies in mature naive B cells from donors GO and JB has been published (reference 2). Pie charts show the frequency of polyreactive clones in mature naive and IgM+ memory B cell antibodies from all three donors with the number of tested antibodies indicated in the center (reference 2).
To measure the frequency of polyreactive antibodies in mature naive and IgM+ memory B cells, we performed ELISAs on ss/dsDNA, LPS, or insulin (Fig. 2 B). As previously reported, 5.4% (5/93) of the antibodies cloned from mature naive B cells from donors GO and JB showed low levels of reactivity with at least two of these antigens (2). Antibodies cloned from mature naive B cells of JH were not significantly different from the previously published result showing 8.3% polyreactivity (3/36, P = 0.685; Table S6). On average, 6.2% of mature naive B cell antibodies were polyreactive (8/129; Fig. 2 B and reference 2). In contrast, only 1% of antibodies cloned from IgM+ memory B cells were polyreactive (1/105, P = 0.044; Fig. 2 B). In summary, polyreactive and self-reactive antibodies comprising 5% and 20% of the mature naive repertoire, respectively, are rarely found in the IgM+ memory antibody pool.
Antibody selection
Our experiments indicate that the transition to the IgM+ memory B cell compartment is associated with selection against self-reactivity. To determine whether selection occurs before or after somatic hypermutation, we reverted the somatic mutations of 27 IgM+ memory B cell antibodies to their unmutated germline form and tested the recombinant antibodies for self-reactivity and polyreactivity (Fig. 3, Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20052033/DC1, and Table S7, available at. http://www.jem.org/cgi/content/full/jem.20052033/DC1). Although an average of 19.7% of all antibodies produced by naive B cells were self-reactive (Fig. 2 A and reference 2), none of the 27 reverted antibodies displayed self-reactivity or polyreactivity, including the two self-reactive and one polyreactive IgM+ memory B cell antibodies (Fig. 3 and Table S7). We conclude that naive B cells expressing self-reactive antibodies do not contribute to the IgM+ memory B cell compartment. Furthermore, the rare IgM+ memory B cells that produce antibodies with low levels of self-reactivity acquire this reactivity by somatic hypermutation.
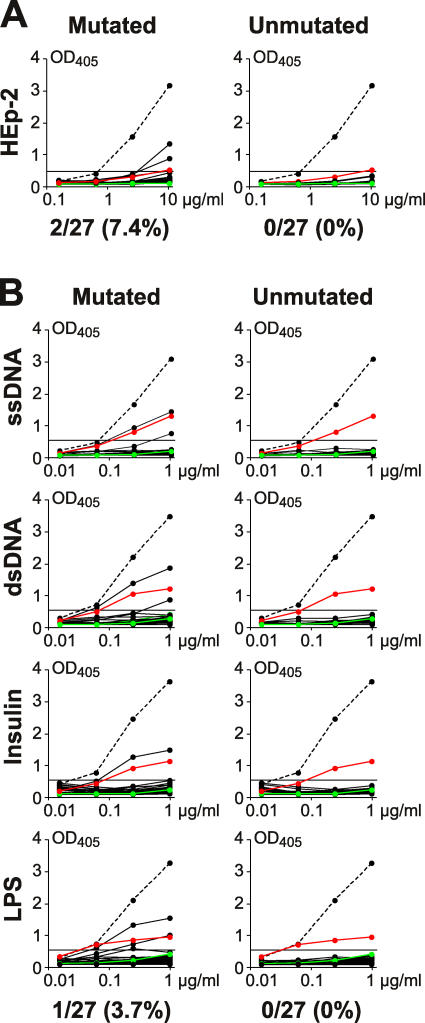
Self-reactivity and polyreactivity profiles of mutated IgM+ memory B cell antibodies in comparison with their unmutated counterparts. IgH and IgL chains from IgM+ memory B cell antibodies were reverted into their germline counterparts by PCR. (A) Recombinant antibodies were tested for self-reactivity by HEp-2 ELISA. Dotted line shows ED38 positive control antibody (references 2, 43). Horizontal line shows cut-off OD405 for positive reactivity as determined by comparison to low positive control serum (red line) and negative control serum (green line) from patients and healthy individuals, respectively. (B) Recombinant antibodies were tested for polyreactivity by ELISA with ss/dsDNA, insulin and LPS. Dotted lines represent the high positive control antibody ED38 (references 2, 43). Horizontal lines show cut-off OD405 for positive reactivity as determined by comparison to the previously published control antibodies mGO53 (negative control; green line) and eiJB40 (low positive control; red line) (reference 2).
Antibody reactivity with bacteria and bacterial polysaccharide antigens
To determine whether antibodies cloned from IgM+ memory B cells react with bacteria or T-I bacterial antigens, we performed ELISAs using Streptococcus pyogenes, a mixture of 10 Streptococcus pneumoniae serotypes (1, 3–6, 9V, 14, 18, 19, 23F); Enterococcus faecalis; Escherichia coli, a protein A mutant (SpA-) strain of Staphylococcus aureus (Fig. 4 A); or bacterial polysaccharide antigens in human vaccines PNEUMOVAX 23 (Streptococcus pneumoniae), PedvaxHIB (Haemophilus influenzae), and Menomune (Neisseria meningitidis) (Fig. 4 B).
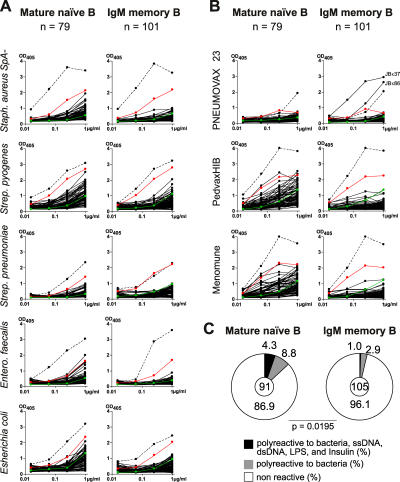
Whole bacteria and bacterial polysaccharide ELISAs. (A) Non-polyreactive antibodies from mature naive (n = 79) and IgM+ memory B cells (n = 101) from three healthy donors were tested for reactivity with whole cell bacteria (Staphylococcus aureus, Streptococcus pyogenes, Streptococcus pneumoniae, Enterococcus faecalis, Esherichia coli) or (B) bacterial polysaccharide vaccines (PNEUMOVAX 23, PedvaxHIB, Menomune) by ELISA. Dotted line shows the positive control antibody ED38 (references 2, 43). Horizontal line shows cut-off OD405 for positive reactivity as determined by comparison to the negative control antibody mGO53 (green line) and the low positive control antibody eiJB40 (red line) (reference 2). (C) Pie charts summarize the frequency of polyreactive antibodies that recognize ssDNA, dsDNA, LPS, insulin, bacteria and bacterial polysaccharides (black), the frequency of antibodies polyreactive with bacteria and bacterial polysaccharides (gray), and the frequency of non-polyreactive antibodies (white) in mature naive and IgM+ memory B cells in all three donors. The number of tested antibodies is indicated in the pie chart centers.
As expected, polyreactive antibodies from mature naive (4.3%, 4/91) and IgM+ memory B cells (1%, 1/105) showed high levels of reactivity with whole bacteria and bacterial antigens (Fig. 4 and Tables S1–S6). In addition, we found a new group of antibodies that showed broad reactivity with bacteria or bacterial polysaccharide antigens, but not with DNA, LPS, or insulin (Fig. 4 and Tables S1–S6). These antibodies comprised 8.8% (8/91) of the mature naive but only 2.9% (3/105) of the IgM+ memory B cell repertoire (Fig. 4 C). When combined, the broadly bacterially reactive antibodies comprised 13.1% of the mature naive and 3.9% of the IgM+ memory B cell compartment; overall, IgM+ memory B cells show a lower frequency of broadly bacterially reactive antibodies than mature naive B cells (P = 0.0195).
Among 91 antibodies from naive B cells and 105 antibodies from memory B cells tested, we found only two antibodies with high levels of specific reactivity against bacterial antigens. Both antibodies were cloned from IgM+ memory B cells from donor JB (2/27, 7.4%) who had previously been vaccinated and both recognized polysaccharides from the serotype 11A vaccine strain of Streptococcus pneumoniae contained in PNEUMOVAX 23 (Fig. 4 B, top right; Tables S1–S3, and Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20052033/DC1).
VH3 gene usage and SpA reactivity
VH3 genes are overrepresented in IgM+ memory B cells and several members of the VH3 gene family bind to modified Staphylococcus protein A (mSpA) in an Fc-independent manner (27, 28). This association led to the suggestion that SpA binding is positively selected in IgM+ memory B cells (29). To determine if SpA binding is involved in selection, we compared VH3 antibodies cloned from naive and IgM+ memory B cells for mSpA binding by ELISA (Fig. 5, A and B, and Fig. S4, available at http://www.jem.org/cgi/content/full/jem.20052033/DC1). We found that 70.0% of VH3-positive antibodies from mature naive B cells showed mSpA binding. In contrast, only 54.3% of VH3-positive antibodies from IgM+ memory B cells were SpA reactive (Fig. 5, A and B, Tables S1–S3, and Fig. S4).
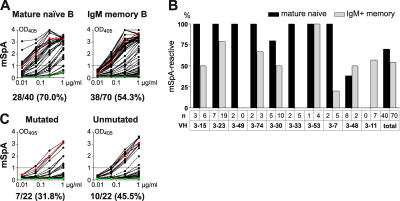
VH3 and modified SpA reactivity. (A) VH3-positive antibodies from mature naive and IgM+ memory B cells were tested for reactivity with mSpA by ELISA. Horizontal line shows cut-off OD405 for positive reactivity as determined by comparison to ED38 (dotted line, VH1-69 and references 2, 43), the negative control antibody mGO53 (green line, VH4-39, and reference 2) and the low positive control antibody eiJB40 (red line, VH3-23 and reference 2). The frequency of mSpA-reactive clones and the number of antibodies tested are indicated below the graphs. (B) The frequency of mSpA-reactive antibodies for individual VH3-family members is shown for mature naive (black bars) and IgM+ memory (gray bars) B cell antibodies with the number of antibodies tested indicated. (C) mSpA-binding as measured by ELISA for mutated IgM+ memory B cell antibodies (n = 22) and their unmutated counterparts after reversion of IgM+ memory B cells antibody IgH and IgL chain genes into their germline forms by PCR.
To further determine if non–SpA-reactive antibodies from IgM+ memory B cells had lost SpA binding as a result of somatic mutation, we reverted 27 of these antibodies into their unmutated counterparts. After reversal of V gene mutations, 10 out of the 27 reverted antibodies became strongly mSpA reactive (Fig. 5 C). Our results suggest that SpA binding is not a selective advantage because it is frequently lost in the transition between the naive and IgM+ memory B cell compartments as a result of V gene somatic hypermutation.
DISCUSSION
Antibody genes in IgM+ memory B cells are somatically mutated, and show signs of antigen-mediated selection with increased numbers of replacement mutations in IgH and IgL chain CDRs (5, 11, 23–25, 30–34). However, the development of somatically mutated IgM+ memory B cells does not require CD40L or CD40 and, therefore, these cells appear to be germinal center independent (33). Phenotypically, IgM+ memory B cells resemble MZ B cells and are thought to be their circulating counterparts (11). In support of this idea, IgM+ memory B cells are strongly reduced in splenectomized and asplenic patients and cannot be detected in infants, where the marginal zone is still populated with mature naive B cells (10, 35). In all of these cases and in a sub-group of common variable immunodeficiency (CVID) patients, loss of circulating IgM+ memory B cells is associated with a specific increase in susceptibility to infection with encapsulated bacteria (10, 11). Consistent with the idea that circulating IgM+ memory B cells play an important role in these infections, we found antibodies with high specificity for streptococcal polysaccharides enriched in this B cell compartment after immunization with PNEUMOVAX 23 (9–11).
In mice, MZ B cell development is promoted by transgenic expression of B cell antigen receptors with low levels of self-reactivity or polyreactivity (15–20). For example, MZ B cells but few follicular B cells develop in transgenic mice expressing a polyreactive VH81x heavy chain (15, 16). Mouse MZ B cells are optimally anatomically positioned to react with blood-borne pathogens and expression of low affinity broadly reactive antibodies by these cells would be consistent with a protective role early in infection (13, 14).
Human IgM+ memory B cells are enriched in VH3 antibodies, some of which bind SpA (23–25, 27, 28). Based on sequence information, it was proposed that this might reflect selection into the IgM+ memory/MZ B cell compartment for cells reactive with common blood-borne pathogens (29). However, we find that VH3 antibodies cloned from IgM+ memory B cells are not more SpA reactive than VH3 antibodies from mature naive B cells and, in many cases, have lost SpA reactivity by somatic mutation. In addition, broadly bacterially reactive antibodies are selected against during IgM+ memory B cell development. These observations were surprising because they appear to be at odds with the transgenic mouse experiments showing that B cells expressing polyreactive antibodies are selected into the MZ B cell compartment (15–20). However, the reactivity of marginal zone B cells has never been measured in normal mice or humans and experiments with anti–hen egg lysozyme (HEL) transgenic mice show that the development, half-life, and anatomic distribution of self-reactive B cells is altered in the absence of competition (36, 37). For example, anti-HEL transgenic B cells develop normally in the presence of HEL and fill the follicular B cell compartments; however, the same B cells fail to enter the follicular compartment when exposed to HEL in the presence of normal B cells (36, 37). Thus, the repertoire of authentic MZ B cells developing in the presence of a complete B cell repertoire may differ from those that arise in transgenic mice in the absence of competition. Alternatively, MZ B cell selection might differ from mouse to human, or the circulating IgM+ memory B cell repertoire could represent a subset of cells entirely different from the phenotypically similar MZ B cells in the human spleen.
Most newly arising antibodies are self-reactive, but many of these are removed from the repertoire during B cell development in the bone marrow and the periphery (1, 2). Nevertheless, 20% of mature naive B cells in healthy humans express antibodies with low levels of self-reactivity (2). Despite the prevalence of self-reactive B cells in the naive B cell compartment, these potentially autotoxic antibodies are not likely to make a significant contribution to the circulating repertoire because naive B cells do not secrete high levels of antibodies. In contrast with naive B cells, IgM+ memory B cells are readily induced to proliferate in an Ig-independent manner and differentiate into antibody-secreting plasmablasts (38–41); therefore, any autoantibody producing IgM+ memory B cells would more likely be autotoxic. Our experiments suggest that, under physiologic conditions, this possibility is averted by a third checkpoint for B cell tolerance between the naive and IgM+ memory compartment imposed before B cells undergo somatic hypermutation.
MATERIALS AND METHODS
Single cell sorting.
The study was performed in accordance with institutional review board–reviewed protocols of The Rockefeller University and all samples were obtained after signed informed consent. Single cell sorting was performed as described previously (2, 42). In brief, peripheral blood B cells were enriched by incubation with RossetteSep (Stem Cell Technology, Inc.) followed by Ficoll-Paque Plus (GE Healthcare) gradient centrifugation. Enriched B cells were stained with anti–human CD19-allophycocyanin, anti–human CD10-PE, anti–human CD27-FITC, and anti–human IgM-biotin (BD Biosciences), and biotinylated anti–human IgM antibody was visualized using Streptavidin-PeCy7 (Caltag Laboratories). Single mature naive B cells (CD19+CD10−IgM+CD27−) and IgM+ memory B cells (CD19+CD10−IgM+CD27+) were isolated by FACS into 96-well plates containing 4 μl of 0.5 × PBS, 10 mM DTT, 8 U RNAsin (Promega), and 3 U Prime RNase Inhibitor (Eppendorf) using a FACSvantage (Becton Dickinson). All samples were immediately frozen on dry ice and stored at −70°C.
Ig gene RT-PCR amplification and expression vector cloning.
Antibodies were cloned as described previously (2, 42). In brief, single cell cDNA was synthesized in the original sort plates in a total volume of 14.5 μl (4 μl sort lysis solution + 10.5 μl RT-PCR reaction mix containing 150 ng random hexamer primer [pd{N}6]; GE Healthcare), 0.5% vol/vol Igepal CA-630 (NP40; Sigma-Aldrich), 7 mM DTT, 4 U RNAsin (Promega), 7.5 U Primer RNase Inhibitor (Eppendorf), 1.2 mM each dNTP, and 50 U Superscript II RT (Invitrogen). cDNA was synthesized at 42°C for 55 min. Individual IgH (μ) and IgL chain (κ or λ) gene rearrangements were amplified in two successive rounds of PCR (50 cycles each) using primers as described previously (2, 42). Ig genes were amplified from 3.5 μl cDNA or first PCR product in 40 μl of total reaction volume with 0.2 μM 5′ and 3′ primers or primer mixes, 312.5 μM each dNTP and 1 U Hotstar Taq DNA polymerase (QIAGEN). All PCR products were purified (Qiaquick; QIAGEN), sequenced, and analyzed by Ig BLAST comparison with GenBank. Digested PCR products were purified and cloned into human IgG1, Igκ, or Igλ expression vectors (2, 42). Plasmids were sequenced to select clones with inserts identical to the original PCR product.
Antibody production and purification.
Recombinant antibodies were produced as described previously (2, 42). In brief, 293A human embryonic kidney cells were cultured in DMEM supplemented with 10% ultra-low IgG FCS (Invitrogen) and cotransfected with 12.5 μg of IgH and IgL chain encoding plasmid DNA by calcium phosphate precipitation. Cells were washed with serum-free DMEM for 8-12 h after transfection and thereafter cultured in DMEM supplemented with 1% Nutridoma SP (Roche). Supernatants were collected after 7 d of culture. For self-reactive HEp-2 ELISAs, antibodies were purified with protein G–Sepharose (GE Healthcare).
Bacteria strains and media.
Streptococcus pneumoniae strains DCC1355 (serotype 19), DCC1335 (9V), DCC1420 (23F), DCC1490 (14), DCC1714 (3), DCC1850 (6), AR314 (5), AR620 (1), GB2017 (18), GB2092 (4), and Enterococcus faecalis V12 were grown in Brain Heart Infusion broth (Sigma-Aldrich). Streptococcus pyogenes D471 was grown in Todd-Hewitt broth (Sigma-Aldrich). Staphylococcus aureus protein A-negative Foster strain 8325–4 spa::Ebr was grown in super broth (10 g/liter MOPS, 30 g/liter tryptone, 20 g/liter yeast extract). Escherichia coli was grown in Luria-Bertani medium. Cell counts from log-phase bacteria cultures were determined before treatment with 50 μg/ml mitomycin-C for 1 h at 37°C. Whole bacteria were suspended at a concentration of 108/ml PBS for ELISA.
ELISA.
To measure reactivity of antibodies with individual antigens, microtiter plates (COSTAR 9018) were coated with 50 μl/well of 10 μg/ml of ssDNA, dsDNA, LPS (Sigma-Aldrich), or 5 μg/ml of recombinant human insulin (Sigma-Aldrich) or 10 μg/ml of human bacterial polysaccharide vaccines PNEUMOVAX 23 (Merck & Co.), PedvaxHIB (Merck & Co.), or Menomune (Aventis) or 10 μg/ml of modified protein A. To measure antibody reactivity with specific bacteria, microtiter plates were coated with 100 μl/well of 100 μg/ml poly-l-lysine, 25 μl/well of bacteria solution, and 25 μl/well of 0.2% gluteraldehyde were added at room temperature for 20 min. Plates were centrifuged at 1,500 g for 20 min and washed with PBS. To block aldehydes, 50 μl/well of 0.1 M l- lysine in PBS was added and wells were washed with PBS.
Antibody concentrations in supernatants and after purification were determined by anti–human IgG1 ELISA using human monoclonal IgG1 as standard (Sigma-Aldrich). For polyreactivity and bacterial ELISAs, supernatants were adjusted to a starting antibody concentration of 1 μg/ml and with three subsequent 1:4 dilutions in PBS. Samples were considered negative if the OD405 did not exceed a threshold value at any of the four dilutions in at least two independent experiments as indicated in each graph. In all assays, threshold values were set using the negative control antibodies mGO53, low positive control eiJB40, and high polyreactive antibody ED38 (2, 43). HEp-2 cell self-reactivity ELISAs used QUANTA Lite ANA ELISA plates (INOVA Diagnostics) and purified antibodies at a concentration of 10 μg/ml with three subsequent 1:4 dilutions in PBS. Positive and negative controls for HEp-2 ELISAs included control sera from patients and healthy individuals (INOVA Diagnostics) as well as ED38 and were included in every experiment. Consistent with our previously published results, the negative and low positive serum was used to determine the cut-off OD405 for reactivity in each experiment (2). Cut-off OD405 levels vary among experiments depending on the absolute OD405 values and antibody concentrations, but allow us to compare the data among individual experiments. Antibodies were considered reactive if they showed reactivity levels above the cut-off OD405 in at least two independent experiments and if the results could be confirmed by IFA on HEp-2 cell–coated slides (unpublished data). All ELISAs were developed with horseradish peroxidase–labeled goat anti–human IgG Fc antibodies (Jackson ImmunoResearch Laboratories) and horseradish peroxidase substrates (Bio-Rad Laboratories). OD405 was measured with a microplate reader (Molecular Devices).
Reversion of somatic hypermutations into germline sequences.
Antibodies for reversion were chosen randomly and are listed in Table S7. Mutated IgH and IgL chain genes were reverted to their germline sequence by two separate PCR reactions for the V gene and the (D)J gene followed by a third overlap PCR to fuse the two PCR products (44). Germline V genes were amplified from previously cloned Ig genes from unmutated naive B cell templates using FWR1 sense and FWR3 antisense primers. Germline (D)J sequences were generated with sense primers spanning the 3′ end of the V gene and the CDR3 region and antisense primers in the J gene. A second 20-cycle PCR reaction fused the two overlapping V and (D)J PCR fragments to generate unmutated germline antibody sequences (Fig. S2). All unmutated IgH and IgL chain PCR products were sequenced before and after cloning into the corresponding expression vectors to confirm the lack of mutations. For all clones' mutated and unmutated IgH and IgL chain CDR3 regions, Ig gene repertoire and reactivity profiles are summarized in Table S7.
Statistics.
p-values for Ig gene repertoire analysis, analysis of positive charges in IgH CDR3, and antibody reactive were calculated by 2 × 2 of 2 × 5 Fisher's Exact test or Chi-square test. p-values for IgH CDR3 length were calculated by two-tailed Student's t test.
Online supplemental material.
Fig. S1 shows positive charges in IgH CDR3, IgV, and IgJ gene usage for Igκ and Igλ light chains from mature naive and IgM+ memory B cell antibodies. Fig. S2 shows the PCR-mediated reversion strategy of mutated IgM+ memory B cell antibodies into their unmutated germline forms. Fig. S3 shows specificity with serotype 11A polysaccharides from Strep. pneumoniae for PNEUMOVAX 23–reactive antibodies from donor JB. Fig. S4 shows ELISA reactivity with mSpA for VH3 family antibodies from IgM+ memory B cells and their unmutated counterparts. Tables S1–S6 show IgH and IgL chain characteristics and antibody reactivities with ssDNA, dsDNA, LPS, insulin, HEp-2, mSpA, Staph. aureus (A-), Strep. pyogenes, Strep. pneumoniae, Ent. faecalis, E. coli, PNEUMOVAX 23, PedvaxHIB, and Menomune from IgM+ memory B cells and mature naive B cells of donors JB, GO, and JH. Table S7 shows IgH and IgL chain gene repertoire, reactivity profiles, and mutated and unmutated CDR3 sequence of antibodies selected for reversion. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20052033/DC1.
Acknowledgments
We thank Dr. G.J. Silverman, Dr. C.S. Goodyear, Dr. V.A. Fischetti, Dr. J.M. Loeffler, Dr. D. Ho, and Dr. Y. Huang for reagents and advice, and all members of the Nussenzweig laboratory and Dr. E. Besmer for discussion and critical reading of the manuscript.
This work was supported by grants from the National Institutes of Health (to M.C. Nussenzweig and S. Yurasov), the Dana Foundation (to H. Wardemann), and the Naito Foundation (to M. Tsuiji). M.C. Nussenzweig is a Howard Hughes Medical Investigator and S. Yurasov is a New York Chapter Arthritis Foundation Young Scholar.
The authors have no conflicting financial interests.
Notes
Abbreviations used: ANA, antinuclear antibody; CVID, common variable immunodeficiency; FWR, framework region; HEL, Hen egg lysozyme; IFA, indirect immunofluorescence assay; MZ, marginal zone; mSpA, modified Staphylococcus aureus protein A; SpA, Staphylococcus aureus protein A; T-I, T cell–independent; T-D, T cell–dependent.
M.C. Nussenzweig and H. Wardemann contributed equally to this work.
References
Articles from The Journal of Experimental Medicine are provided here courtesy of The Rockefeller University Press
Full text links
Read article at publisher's site: https://doi.org/10.1084/jem.20052033
Read article for free, from open access legal sources, via Unpaywall:
http://jem.rupress.org/content/203/2/393.full.pdf
Citations & impact
Impact metrics
Article citations
Expression Cloning of Antibodies from Single Human B Cells.
Methods Mol Biol, 2865:103-124, 01 Jan 2025
Cited by: 0 articles | PMID: 39424722
Impaired proliferation and migration of HUVEC and melanoma cells by human anti-FGF2 mAbs derived from a murine hybridoma by guided selection.
Bioengineered, 14(1):2252667, 01 Dec 2023
Cited by: 0 articles | PMID: 37661761 | PMCID: PMC10478743
Activation and pro-inflammatory cytokine production by unswitched memory B cells during SARS-CoV-2 infection.
Front Immunol, 14:1213344, 10 Aug 2023
Cited by: 5 articles | PMID: 37638016 | PMCID: PMC10449608
Selective Recognition of Carbohydrate Antigens by Germline Antibodies Isolated from AID Knockout Mice.
J Am Chem Soc, 144(11):4925-4941, 12 Mar 2022
Cited by: 2 articles | PMID: 35282679 | PMCID: PMC10506689
Loss of immune homeostasis in patients with idiopathic pulmonary arterial hypertension.
Thorax, 76(12):1209-1218, 07 May 2021
Cited by: 14 articles | PMID: 33963088 | PMCID: PMC8606455
Go to all (126) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Autoreactivity in human IgG+ memory B cells.
Immunity, 26(2):205-213, 01 Feb 2007
Cited by: 313 articles | PMID: 17306569 | PMCID: PMC1839941
Accumulation of self-reactive naïve and memory B cell reveals sequential defects in B cell tolerance checkpoints in Sjögren's syndrome.
PLoS One, 9(12):e114575, 23 Dec 2014
Cited by: 23 articles | PMID: 25535746 | PMCID: PMC4275206
The dual phases of the response to neonatal exposure to a VH family-restricted staphylococcal B cell superantigen.
J Immunol, 161(10):5720-5732, 01 Nov 1998
Cited by: 19 articles | PMID: 9820554
Immunoglobulin genes in chronic lymphocytic leukemia.
Blood Cells, 19(3):615-25; discussion 631-2, 01 Jan 1993
Cited by: 22 articles | PMID: 8018942
Review





